High quality sulfur-doped titanium dioxide nanocatalysts with visible light photocatalytic activity from non-hydrolytic thermolysis synthesis†
Na
Li
ab,
Xinyu
Zhang
a,
Weijia
Zhou
c,
Zhengqing
Liu
a,
Gang
Xie
b,
Yaoyu
Wang
b and
Yaping
Du
*ab
aFrontier Institute of Chemistry, Frontier Institute of Science and Technology Jointly with College of Science, Xi'an Jiaotong University, 99 Yanxiang Road, Yanta District, Xi'an Shaanxi Province 710054, China. E-mail: ypdu2013@mail.xjtu.edu.cn; ypdupku@gmail.com; Tel: +86-29-83395385
bKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi 710069, China
cNew Energy Research Institute, School of Environment and Energy, South China University of Technology, Guangzhou Higher Education Center, Guangzhou 510006, China
First published on 13th June 2014
Abstract
A facile non-hydrolytic thermolysis route for monodisperse sulfur doped TiO2 nanocatalysts is demonstrated. Compared with the as-obtained undoped TiO2 nanocatalysts, the sulfur doped TiO2 nanocatalysts present obvious enhanced visible light activation for the degradation of rhodamine B and methylene blue dyes under the artificial visible light (λ = 420–770 nm) irradiation.
Titanium dioxide (TiO2) is the most widely used photocatalyst in environmental cleaning.1–4 However, because of its large band gap (Eg = 3.20 eV for anatase), TiO2 can solely be activated in the ultraviolet (UV) region. The limited UV-driven activity decreases its overall efficiency under natural sunlight irradiation, thus limiting its practical applications. Engineering the band gap of TiO2 is one of the robust strategies for improving its photocatalytic efficiency, since it can availably extend the absorption from the UV to the visible-light region. Up to now, many attempts have been made in the field of visible-light-active TiO2 by introducing various dopants into its crystal lattice, including metal5 and nonmetal elements.6 Although metal dopants can give the desired shift of the TiO2 absorption from the UV to the visible light region, many of them also serve as recombination centers, which notably reduce the photocatalytic efficiency.5a,7 Band-gap narrowing by doping nonmetals (such as N, C, S and F) into TiO2 was recently found to be a more efficient way to yield photocatalysts with high catalytic activity under visible light irradiation.6 As an important sort of visible light activated doped TiO2 photocatalysts, S-doped TiO2 photocatalysts have been receiving extensive attention due to their high photocatalytic activity, structural stability and efficient band gap manipulation.6c,8,12b
Various doping strategies have been adopted to achieve S-doped TiO2 photocatalysts; while most of the S-doped TiO2 photocatalysts were synthesized by oxidative annealing of TiS2 or by treating the titanium contained precursors in a hydrogen sulfide (H2S) atmosphere at high temperatures,6c,9 besides the energy consumption and intricate setup, the high temperature treatment usually leads to low surface areas and photocatalytic activities due to the aggregation of catalysts. It is widely recognized that nanosized materials have opened the doors for discovering new properties and applications with respect to their macroscopic counterparts.10 In recent years, several methods have been developed to prepare S-doped TiO2 nanocatalysts by a number of research groups. Examples include the catalyzed hydrolysis,11 hydrothermal,12 solvothermal synthesis,13 sol–gel,14 co-precipitation,15 ball milling,16 and supercritical fluid-assisted method.17
However, until now, a facile method for the synthesis of high quality (monodisperse, single crystalline, well shaped, and phase pure) dispersible colloidal S-doped TiO2 nanocatalysts has not been demonstrated, which inspires the continuous and systematic exploration. In this paper, for the first time, we report a facile and effective non-hydrolytic thermolysis method for obtaining high quality S-doped TiO2 nanocatalysts in high boiling solvents, and for in-depth investigation of the photocatalytic properties of as-obtained nanocatalysts. In comparison with the pure TiO2 nanocatalysts, the absorption edge shifts to the visible light region on doping S into the TiO2 crystal lattice, thus exhibiting an obvious visible light activation for the degradation of rhodamine B and methylene blue dyes.
The powder X-ray diffraction (PXRD) patterns of as-prepared pure TiO2 and S-doped TiO2 nanocrystals are shown in Fig. 1a. The as-synthesized samples exhibit the well-defined diffraction peaks, indicative of the high crystallinity of the sample. All the diffraction peaks are exclusively indexed to the anatase phase of TiO2 (tetragonal structure; JCPDS card no. 21-1272, space group I41/amd) for both pure and S-doped TiO2 nanocrystals. No diffraction peaks from any other chemical species such as the rutile and brookite phase of TiO2 are detectable. Compared with the pure TiO2, a systematical peak shift towards low diffraction angles is unambiguously detected in the S-doped TiO2 nanocrystals (Fig. S1†), suggesting a lattice expansion when introducing S into the TiO2 crystal lattice. Using the least squares refinement of cell dimensions from PXRD data, the calculated crystal lattice parameters are as follows: a = b = 3.7852 Å, c = 9.5139 Å, V = 136.3127 Å3 for TiO2 nanocrystals; a = b = 3.8008 Å, c = 9.5212 Å, V = 137.5440 Å3 for S-doped TiO2 nanocrystals.
In order to obtain more information about the microstructure of as-obtained S-doped TiO2 nanocrystals, their room temperature Raman spectra were measured. Group theory predicts that the typical anatase TiO2 has six Raman active modes: Γ = A1g + 2B1g + 3Eg.18Fig. 1b displays the Raman spectrum of as-obtained undoped TiO2 and S-doped TiO2 nanocrystals excited with a 514.5 nm beam of the Ar+ laser. The Raman spectra of as-obtained nanocrystals present four bands at 147 cm−1 (Eg), 392 cm−1 (B1g), 508 cm−1 (A1g) and 631 cm−1 (Eg). All samples exhibit the characteristic Raman-active modes of the anatase TiO2 phase, and show no detectable signals corresponding to any other titanium and sulfur containing species in the Raman spectra, demonstrating that the doped nanocrystals have the same structure as the anatase TiO2, in agreement with the phase composition determined by PXRD. The inset of Fig. 1b shows the expanded views of the Eg Raman band at 147 cm−1. It is found that the Eg band in undoped TiO2 shifts to higher wavenumber when S doped, also indicating the incorporation of S into the TiO2 crystal lattice.11c,12b
Transmission electron microscopy (TEM) measurements shown in Fig. S2† reveal that the TiO2 nanocrystals obtained from the thermo-decomposition of TiF4 in OA/OM/ODE solvents without S doping present irregular morphologies with an average size ranging from 11.8 nm to 25.7 nm, while the S-doped TiO2 nanocrystals are nearly monodisperse in the size of (14.5 ± 1.2) nm × (15.3 ± 1.1) nm (Fig. 2), indicating that S anions can act as a growth controlling agent to produce well-shaped nanocrystals in the current reaction system. However, the sole use of ODE in our synthesis can only yield irregular products (Fig. S3†). As demonstrated from the energy dispersive X-ray analysis (EDAX) (Fig. S4†), the atomic ratio of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O is calculated to be 0.49
O is calculated to be 0.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for undoped TiO2 and 0.53
1 for undoped TiO2 and 0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for S-doped TiO2, which is in agreement with the stoichiometric ratio of TiO2. The inset of Fig. 2 shows the representative high resolution transmission electron microscopy (HRTEM) image of S-doped TiO2 nanocrystals that display evident lattice fringes, indicating the high crystallinity of the as-obtained nanocrystals. The interplanar spacings for the S-doped TiO2 nanocrystals are calculated to be 0.35 nm, identical to the distances between the (101) crystal planes of anatase TiO2. It is also worth noting from Fig. 2 that the as-obtained S-doped TiO2 nanocrystals are highly dispersed and exhibit an ordered arrangement, indicative of the retentivity of capping ligands on the surfaces of nanocrystals, as evidenced by Fourier transform infrared spectroscopy (FTIR) measurement results in Fig. S5.†
1 for S-doped TiO2, which is in agreement with the stoichiometric ratio of TiO2. The inset of Fig. 2 shows the representative high resolution transmission electron microscopy (HRTEM) image of S-doped TiO2 nanocrystals that display evident lattice fringes, indicating the high crystallinity of the as-obtained nanocrystals. The interplanar spacings for the S-doped TiO2 nanocrystals are calculated to be 0.35 nm, identical to the distances between the (101) crystal planes of anatase TiO2. It is also worth noting from Fig. 2 that the as-obtained S-doped TiO2 nanocrystals are highly dispersed and exhibit an ordered arrangement, indicative of the retentivity of capping ligands on the surfaces of nanocrystals, as evidenced by Fourier transform infrared spectroscopy (FTIR) measurement results in Fig. S5.†
 | ||
| Fig. 2 TEM images of as-obtained S-doped TiO2 nanocrystals (inset shows the corresponding HRTEM image). | ||
The chemical states of the S-doped TiO2 nanocrystals have been investigated with X-ray photoelectron spectra (XPS) as shown in Fig. 3. It can be seen from the XPS survey spectra in Fig. 3a that the S-doped TiO2 contained predominantly Ti, O, C and S elements. The sharp peaks attributed to the core levels of C 1s reveal the presence of oleic acid and oleylamine ligands on the surfaces of the doped nanocrystals.19 Panels b–d of Fig. 3 show XPS spectra recorded for the Ti 2p, O 1s, and S 2p regions of the S-doped TiO2 nanocrystals. As is seen from Fig. 3b, two intense peaks at 465.3 and 459.5 eV ascribed to the core levels of Ti 2p1/2 and Ti 2p3/2, respectively, demonstrate that the oxidation states of titanium ions are mainly quadrivalence for the S-doped TiO2 nanocrystals. The fitting of the O 1s region with two peaks shown in Fig. 3c indicates that at least two kinds of oxygen species existed in the near surface domain of the S-doped TiO2 nanocrystals. The peak located at about 530.3 eV is due to crystal lattice oxygen of S-doped TiO2 nanocrystals, while the peak at about 532.6 eV is due to chemisorbed oxygen on the nanocrystal surfaces. Fig. 3d depicts the XPS spectra obtained for the S 2p regions of S-doped TiO2 nanocrystals. There were two observable peaks at 162.4 and 161.4 eV attributable to the core levels of S 2p1/2 and S 2p3/2, respectively, which are separated by a spin–orbit splitting of 1.0 eV. No evidence of S6+ (binding energy at 169.0 eV) is detected, demonstrating that the sulfur atoms are all in the state of S2−.12a It corresponds to the Ti–S bond formed when some of the oxygen atoms in the TiO2 crystal lattice are replaced by sulfur atoms, as a result of band gap narrowing of TiO2 calculated by Umebayashi et al.6c It is also noted that in our XPS characterization, we employed the Al-Kα radiation as the X ray light source, and the escape depth was around 2 nm (Fig. S6 and S7†). Relative to the sizes of as-synthesized S-doped TiO2 nanocrystals, the XPS characterization information in our study is also from the total samples.
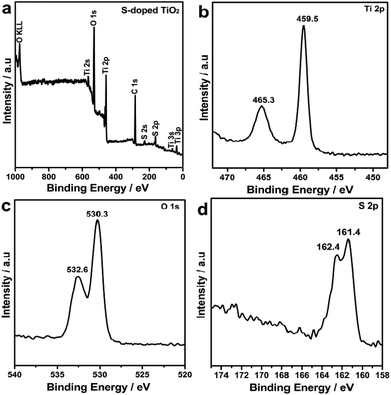 | ||
| Fig. 3 (a) XPS survey spectra of as-obtained S-doped TiO2 nanocrystals. (b) Ti 2p, (c) O 1s, and (d) S 2p signals taken from S-doped TiO2 nanocrystals. | ||
Fig. S5† shows the FTIR spectra of oleic acid, oleylamine, and the prepared S-doped TiO2 nanocrystals dispersed in toluene–hexane solutions. The strong C–H stretching vibrations at 2920 and 2849 cm−1 show the coexistence of free oleic acid and oleylamine. The observable peak located at 1456 cm−1 is ascribed to carboxylate (COO−) stretch, indicating that the COO− ligands existed on the surface of TiO2 and S-doped TiO2 nanocrystals. In addition, the discernible peak at 1375 cm−1 is attributed to the C–N stretch of oleylamine. Based on the FTIR analysis of TiO2 and S-doped TiO2 nanocrystals, it is validated that the as-obtained nanocrystals are possibly coated by two kinds of organic molecules, namely oleic acid and oleylamine.20 Moreover, compared with the pure TiO2, the new band located at 1045 and 860 cm−1 is the characteristic of Ti–S vibration, further indicating the successful doping of S anions into TiO2 nanocrystals.11b,13
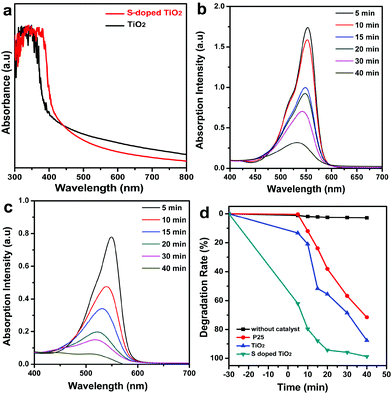
Fig. 4a shows the ultraviolet visible (UV-vis) absorption spectrum of as-prepared pure TiO2 and S-doped TiO2 nanocrystals dispersed in toluene–hexane solutions. A discernible absorption edge was detected at 385 and 415 nm, which is the lowest-energy excitonic absorption peak of TiO2 and S-doped TiO2 nanocrystals, respectively. In comparison with the band-gap energy of pure TiO2, the lowest-energy exciton transition peak of S-doped TiO2 shows a noticeable red shift, implying that the S dopants are incorporated into the lattice of TiO2, thus altering its crystal and electronic structures, which enables the S-doped TiO2 nanocrystals as effective visible-light photocatalysts.6c,12b
The photocatalytic properties of as-prepared nanocatalysts are studied by photodegradation of the rhodamine B (RhB) dye. Fig. 4b and c show the change of absorption spectra for the photocatalytic degradation of the RhB dye as a function of irradiation time under artificial visible light (λ = 420–770 nm) in the presence of synthesized TiO2 and S-doped TiO2 nanocatalysts. The intensity of the absorption band of the RhB dye located at λ = 553 nm gradually decreases when prolonging the irradiation time, showing that the decoloration of the RhB dye can be achieved by TiO2 and S-doped TiO2 nanocatalysts under visible-light irradiation. It should be pointed out that the visible-light photocatalytic activity of undoped TiO2 nanocatalysts may be caused by the concomitant oxygen deficiency.6f,12b Compared with the pure TiO2 shown in Fig. 4b, the RhB dye has almost completely degraded after 40 min under visible light irradiation for S-doped TiO2 nanocatalysts (Fig. 4c), indicating that the S-doped TiO2 nanocatalysts show enhanced photocatalytic activity under the same conditions (Fig. S8†).
Fig. 4d shows the photodegradation rate of the RhB dye at various time intervals during the visible light irradiation by pure TiO2 and S-doped TiO2 nanocatalysts. For comparison, the decomposition over commercial P25 was carried out under the same experimental conditions. As can be seen from Fig. 4d, S-doped TiO2 nanocrystals show a superior visible light photodegradation rate of the RhB dye to undoped TiO2 nanocatalysts. Compared with the pure TiO2 nanocrystals (the degradation rate is 87.5%), the photocatalytic activities and photodegradation rates increase for S-doped TiO2 nanocrystals with the maximum photocatalytic activity being 99%. In addition, both the as-obtained undoped TiO2 and S-doped TiO2 nanocrystals show the enhanced degradation rate of the RhB dye compared with the commercial P25 (the degradation rate is 70.1%). On the other hand, the adsorption experiments were performed on all catalysts in the absence of light for 30 min. The larger absorption ratio of S-doped TiO2 nanocrystals may be attributed to their higher surface area (Fig. 4d).11a The photocatalytic stability of the S-doped TiO2 nanocrystals under visible light irradiation is shown in Fig. S9.† Experimentally, the photocatalysts were used repeatedly six times after separation through filtration, and the activity was evaluated and compared. As can be seen from Fig. S9,† when irradiated by visible light for 15 min, the photocatalytic degradation efficiency of RhB was kept 80% after the sixth time, demonstrating a highly stable photocatalytic performance for as-obtained S-doped TiO2 nanocatalysts. In addition, we used the methylene blue (MB) dye as another model molecule to evaluate the photodegradation performance of as-synthesized S-doped TiO2 nanocatalysts; as can be seen from Fig. S10,† the S-doped TiO2 nanocrystals also show an enhanced visible light photodegradation rate of the MB dye compared to pure P25 and undoped TiO2 nanocatalysts.
As indicated from the XRD, XPS, FTIR, and UV-vis results, it can be concluded that S was incorporated into the O site of the TiO2 crystal lattice. In the undoped TiO2 crystal, the valence band (VB) and conduction band (CB) consist of the Ti 3d and O 2p orbital. According to the crystal field theory, the Ti 3d orbital is split into two parts of the t2g and eg states in an octahedral field with Oh symmetry; thus the CB is separated into the lower and upper parts. When S doped into TiO2 crystals, the S 3p states are delocalized, thus playing a significant role in the formation of the VB with the O 2p and Ti 3d states. As a consequence, the mixing of the S 3p states with VB increases the width of the VB, which accounts for the band gap narrowing due to S doping, as illustrated in Fig. S11.†6c,21
In summary, in this paper, we have demonstrated for the first time a facile synthesis of monodisperse S-doped TiO2 nanocatalysts in hot boiling organic solvents of oleic/oleylamine/1-octadecene. The as-obtained S-doped TiO2 nanocatalysts are predominately composed of quadrilateral plate-like shapes, and have an average size in the range of (14.5 ± 1.2) nm × (15.3 ± 1.1) nm. Compared with the as-obtained undoped TiO2 nanocatalysts, the S-doped TiO2 nanocatalysts present obvious enhanced visible light activation for the degradation of rhodamine B and methylene blue dyes under the artificial visible light (λ = 420–770 nm) irradiation. We believe that our strategy could be broadly applicable for the facile production of other TiO2 nanocatalysts doped with nonmetals with great promise for various applications.
Acknowledgements
We gratefully acknowledge the financial aid from the start-up funding from Xi'an Jiaotong University (XJTU) and the National Nature Science Foundation of China (NSFC, grant no. 21371140).Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- D. F. Ollis and H. Al-Ekabi, Photocatalytic Purification and Treatment of Water and Air, Elsevier Science, 1993, pp. 719–725 Search PubMed.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332 CrossRef CAS PubMed.
- (a) W. Choi, A. Termin and M. R. Hoffmann, Angew. Chem., Int. Ed. Engl., 1994, 33, 1091 CrossRef; (b) J. A. Wang, R. Limas-Ballesteros, T. Lopez, A. Moreno, R. Gomez, O. Novaro and X. J. Bokhimi, J. Phys. Chem. B, 2001, 105, 9692 CrossRef CAS; (c) I. S. Shah, W. Li, P. C. Huang, O. Jung and C. Ni, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 6482 CrossRef PubMed; (d) H. Haick and Y. Paz, J. Phys. Chem. B, 2003, 107, 2319 CrossRef CAS; (e) S. Kim, S. J. Hwang and W. J. Choi, Phys. Chem. B, 2005, 109, 24260 CrossRef CAS PubMed.
- (a) R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS PubMed; (b) S. U. M. Khan, M. Al-Shahry and W. B. Ingler Jr., Science, 2002, 297, 2243 CrossRef CAS PubMed; (c) T. Umebayashi, T. Yamaki, H. Itoh and K. Asai, Appl. Phys. Lett., 2002, 81, 454 CrossRef CAS PubMed; (d) S. Sakthivel and H. Kisch, Angew. Chem., Int. Ed., 2003, 42, 4908 CrossRef CAS PubMed; (e) J. C. Yu, W. K. Ho, J. G. Yu, H. Y. Yip, P. K. Wong and J. C. Zhao, Environ. Sci. Technol., 2005, 39, 1175 CrossRef CAS; (f) W. Ho, J. C. Yu and S. Lee, Chem. Commun., 2006, 1115 RSC; (g) H. Tian, J. F. Ma, K. Li and J. J. Li, Ceram. Int., 2009, 35, 1289 CrossRef CAS PubMed; (h) B. Naik, K. M. Parida and C. S. Gopinath, J. Phys. Chem. C, 2010, 114, 19473 CrossRef CAS.
- C. Wang, D. W. Bahnemann and J. K. Dohrmann, Chem. Commun., 2000, 1539 RSC.
- T. Ohno, T. Mitsui and M. Matsumura, Chem. Lett., 2003, 32, 364 CrossRef CAS.
- T. Umebayashi, T. Yamaki, S. Tanaka and K. Asai, Chem. Lett., 2003, 32, 330 CrossRef CAS.
- L. Gao, S. Zheng and Q. H. Zhang, Nanometer Titania Photocatalytic Materials and Their Applications, Chemical Industry Press, 2002, p. 104 Search PubMed.
- (a) S. X. Liu and X. Y. Chen, J. Hazard. Mater., 2008, 152, 48 CrossRef CAS PubMed; (b) Y. M. Liu, J. Z. Liu, Y. L. Lin, Y. F. Zhang and Y. Wei, Ceram. Int., 2009, 35, 3061 CrossRef CAS PubMed; (c) S. H. Nam, T. K. Kim and J. H. Boo, Catal. Today, 2012, 185, 259 CrossRef CAS PubMed.
- (a) W. Ho, J. C. Yu and S. C. Lee, J. Solid State Chem., 2006, 179, 1171 CrossRef CAS PubMed; (b) G. Liu, C. H. Sun, S. C. Smith, L. Z. Wang, G. Q. Lu and H. M. Cheng, J. Colloid Interface Sci., 2010, 349, 477 CAS.
- G. D. Yang, Z. F. Yan and T. C. Xiao, Appl. Surf. Sci., 2012, 258, 4016 CrossRef CAS PubMed.
- (a) X. W. Wu, D. J. Wu and X. J. Liu, Appl. Phys. A, 2009, 97, 243 CrossRef CAS; (b) M. V. Dozzi, S. Livraghi, E. Giamellob and E. Selli, Photochem. Photobiol. Sci., 2011, 10, 343 RSC.
- (a) E. M. Rockafellow, L. K. Stewart and W. S. Jenks, Appl. Catal., B, 2009, 91, 554 CrossRef CAS PubMed; (b) L. Szatmáry, S. Bakardjieva, J. Šubrt, P. Bezdička, J. Jirkovský, Z. Bastl, V. Brezová and M. Korenko, Catal. Today, 2011, 161, 23 CrossRef PubMed.
- M. Jalalah, M. Faisal, H. Bouzid, A. A. Ismai and S. A. Al-Sayari, Mater. Res. Bull., 2013, 48, 3351 CrossRef CAS PubMed.
- H. X. Li, X. Y. Zhang, Y. N. Huo and J. Zhu, Environ. Sci. Technol., 2007, 41, 4410 CrossRef CAS.
- (a) T. Ohsaka, F. Izumi and Y. Fujiki, J. Raman Spectrosc., 1978, 7, 321 CrossRef; (b) C. Han, M. Pelaez, V. Likodimos, A. G. Kontos, P. Falaras, K. O'Shea and D. D. Dionysiou, Appl. Catal., B, 2011, 107, 77 CrossRef CAS PubMed.
- Y. P. Du, Z. Y. Yin, J. X. Zhu, X. Huang, X. J. Wu, Z. Y. Zeng, Q. Y. Yan and H. Zhang, Nat. Commun., 2012, 3, 1177 CrossRef PubMed.
- Y. P. Du, Z. Y. Yin, X. H. Rui, Z. Y. Zeng, X. J. Wu, J. Q. Liu, Y. Y. Zhu, J. X. Zhu, X. Huang, Q. Y. Yan and H. Zhang, Nanoscale, 2013, 5, 1456 RSC.
- (a) T. Umebayashi, T. Yamaki, S. Yamamoto, A. Miyashita, S. Tanaka, T. Sumita and K. Asai, J. Appl. Phys., 2003, 93, 5156 CrossRef CAS PubMed; (b) T. Ohno, M. Akiyoshi, T. Umebayashi, K. Asai, T. Mitsui and M. Matsumura, Appl. Catal., A, 2004, 265, 115 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental details, result of FTIR is provided. See DOI: 10.1039/c4qi00027g |
| This journal is © the Partner Organisations 2014 |