Magneto-chiral dichroism in chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker SMMs†
Kang
Wang
a,
Suyuan
Zeng
b,
Hailong
Wang
a,
Jianmin
Dou
b and
Jianzhuang
Jiang
*a
aBeijing Key Laboratory for Science and Application of Functional Molecular and Crystalline Materials, Department of Chemistry, University of Science and Technology Beijing, Beijing 100083, China. E-mail: jianzhuang@ustb.edu.cn
bDepartment of Chemistry, Liaocheng University, Liaocheng, China
First published on 27th January 2014
Abstract
Two new chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker complexes with both (R)- and (S)-enantiomers were synthesized and structurally characterized. The significant magneto-chiral cross effect revealed for the dysprosium compound suggests the great potential of sandwich-type tetrapyrrole rare earth systems in the research field of magneto-chiral dichroism.
Chirality is one of the most fascinating and complicated features in nature.1 Molecule-based materials with chirality have also been intensively studied owing to their specific physical and chemical properties in optical materials, chiral medicine, and asymmetric catalysis.2 In recent years, multifunctional chiral molecular materials including chiral molecular magnets have attracted a great deal of attention due to the observation of the magneto-chiral dichroism effect from chiral compounds.3,4 However, despite the significant progress achieved in either the chiral bis/tris(tetrapyrrole) rare earth compounds5,6 or sandwich tetrapyrrole lanthanide-based molecular magnets7–9 in the past decade, investigation of the magneto-chiral dichroism effect for chiral sandwich systems still remains undeveloped at present.
In the present paper, two new chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker complexes with both (R)- and (S)-enantiomers of M2[Pc(OBNP)4](TClPP)2 [M = Y (1), Dy (2)] have been synthesized [TClPP = 5,10,15,20-tetrakis(4-chlorophenyl)porphyrinate, Pc(OBNP)4 = tetrakis(dinaphtho[1,2-e:1′,2′-g]-1,4-dioxocine)[2,3-b;2′,3′-k;2′′,3′′-t;2′′′,3′′′-c′]phthalocyaninate], Scheme 1. The structures of both (R)- and (S)-enantiomers for 1 and 2 have been determined by single-crystal X-ray diffraction analysis. Study of the magnetic properties of the dysprosium species revealed its magnetic field-induced single-molecule magnet (SMM) nature. Nevertheless, the magneto-chiral dichroism spectroscopic measurements over both enantiomers of 2 disclose a significant magneto-chiral cross effect, which represents the first example of sandwich-type tetrapyrrole rare earth systems that have been revealed to exhibit a magneto-chiral dichroism effect.
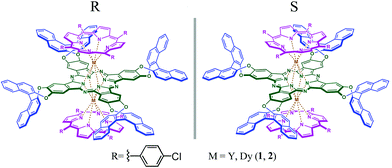 | ||
| Scheme 1 Schematic molecular structures of chiral sandwich-type mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker complexes (R)/(S)-{M2[Pc(OBNP)4](TClPP)2} (M = Y, Dy) (1, 2). | ||
As clarified previously, several synthetic pathways have been developed for the synthesis of sandwich-type mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker complexes.10,11 In the present case, the target triple-decker rare earth complexes involving Pc(OBNP)4 and TClPP ligands (R)/(S)-{M2[Pc(OBNP)4](TClPP)2} (M = Y, Dy) (1, 2) were isolated in good yield by a one pot reaction starting from (R)/(S)-H2[Pc(OBNP)4] and H2TClPP in the presence of M(acac)3·nH2O (M = Y, Dy) in refluxing n-octanol. Actually, in addition to the main triple-decker target compounds, a very small amount of another triple-decker complex (R)/(S)-{M2[Pc(OBNP)4]2(TClPP)} (M = Y, Dy) was also obtained as a side product. It is worth pointing out that racemization of the chiral phthalocyanine at such high temperatures over 200 °C under the present reaction conditions did not occur. A satisfactory elemental analysis result was obtained for both rare earth complexes after repeated column chromatography followed by recrystallization. The MALDI-TOF mass spectra of these compounds clearly showed intense signals for the molecular ion [M]+. The isotopic pattern closely resembled that of the simulated one as exemplified by the spectrum of 1 given in Fig. S1 in the ESI.† These new chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker complexes were also characterized by a range of spectroscopic methods including NMR, electronic absorption, and in particular circular dichroism (CD) spectroscopy.
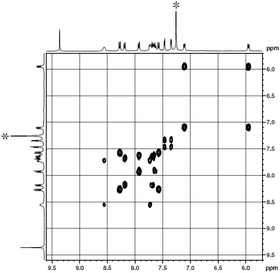
The 1H NMR spectra of 1 were recorded in CDCl3 and the data are summarized in Table S1 (ESI†). As can be seen in Fig. 1 and S2 (ESI†), singlet signal at δ = 9.36 ppm can be easily assigned to the Pc α-protons. The other signals can also be assigned unambiguously on the basis of 1H–1H COSY analysis and by reference to previous results of the corresponding analogues.11d The signals at δ = 8.19, 9.23, and 7.60–7.70 ppm, which are correlated with each other in the 1H–1H COSY spectrum, are assigned to the protons on the outer set of the benzene rings of the binaphthyl substituents, while the two doublet signals at δ = 7.72 and 7.57 ppm to the protons in the inter-benzene rings of the binaphthyl substituents are peripherally attached to the Pc π-system. The signals of the β protons of the porphyrin ligand are observed at δ = 7.46 and 7.35 ppm as two doublets. The signals at δ = 8.56, 8.27, 7.11, and 5.95 ppm are attributed to the four different types of the protons in the C6H4Cl moieties of TClPP ligands.
Single crystals of both enantiomers for the two complexes 1 and 2 suitable for X-ray diffraction analysis were obtained by slow diffusion of methanol into the corresponding toluene or CHCl3 solution. This represents the first example of chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker complexes that have ever been structurally characterized via single crystal X-ray diffraction analysis. Both enantiomers of these two compounds crystallize in the monoclinic system with a C2 space group with four triple-deckers per unit cell. The crystal data are summarized in Table S2 (ESI†).
Fig. 2 and S3 (ESI†) show the molecular structures of (R)/(S)-2 and (R)/(S)-1, respectively, in two different perspective views. As can be seen, the molecular structures of (R)- and (S)-enantiomers are perfectly mirror symmetrical. In (R)-2, each dysprosium center is octa-coordinated by the pyrrole and isoindole nitrogen atoms of an outer TClPP and the inner Pc rings, respectively, confirming the ligand arrangement of {(TClPP)Dy[Pc(OBNP)4]Dy(TClPP)} in the triple-decker molecules. The two dysprosium centers are not identical in terms of their coordination geometry and separation from the ligands. One dysprosium atom (Dy1) adopts a slightly distorted square-antiprismatic structure while the second one (Dy2) exhibits a slightly distorted cubic geometry. The average twist angle, defined as the rotation angle of one macrocycle away from the eclipsed conformation of the two macrocycles, between a Pc ring and a TClPP ring was found to be 42.32 and 7.02°, respectively. The Pc ring is slightly domed toward the Dy2 atom, resulting in different distances between the outer TClPP and the inner Pc rings, 2.879 and 3.208 Å. Both dysprosium centers lie closer to the TClPP ring due to its larger central cavity in comparison with the Pc ligand, 1.213 vs. 1.692 Å for Dy1 and 1.180 vs. 2.014 Å for Dy2. This is also true of the yttrium analogue 1, Fig. S3 and Table S3 (ESI†). It is worth noting that owing to the very much similar ionic radius between Y(III) and Dy(III),12 only a very slight difference can be observed in the molecular structural parameters between 1 and 2, Table S3 (ESI†).
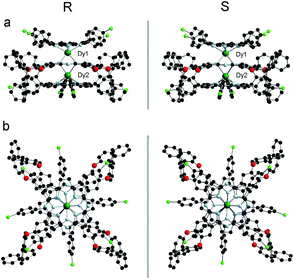 | ||
| Fig. 2 Molecular structures of (R)/(S)-2 in side view and top view with hydrogen atoms omitted [Dy(III) dark green, C black, N pale blue, O red, Cl light green]. | ||
The electronic absorption spectra of the (R)- and (S)-enantiomers of 1 and 2 were recorded in CHCl3 and the data are summarized in Table S4 (ESI†). As can be seen in Fig. 3 and S4 (ESI†), both 1 and 2 exhibit typical features of the electronic absorption spectra for mixed mono(phthalocyaninato)di(porphyrinato) rare earth triple-decker complexes with the tetrapyrrole Soret bands appearing at ca. 374 and 420 nm and Q bands at ca. 497, 612, and 1000 nm, respectively. The intense band observed at 300 nm is attributed to the absorption of binaphthyl groups. Owing to the binaphthyl substituents introduced at the periphery of a phthalocyanine ligand, Q bands of 1 and 2 are slightly red-shifted compared to M2(Pc)(TClPP)2 (M = Y and Dy).11d Associated with their electronic absorption peaks, the MCD spectra of 1 and 2 show apparently dispersion type Faraday A terms with crossover points corresponding to the band centers of the main absorption bands, Fig. 3, suggesting that these are corresponding to transitions to the nearly degenerate excited states.6,13
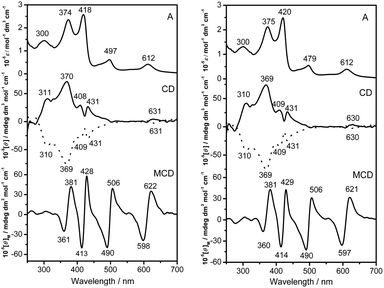 | ||
| Fig. 3 Electronic absorption, CD, and MCD spectra of 1 (left) and 2 (right). Solid and dot lines are used to plot the CD spectra of the (R)- and (S)-enantiomers, respectively. | ||
The CD spectra of the two chiral triple-decker complexes 1 and 2 recorded in CHCl3 are also shown in Fig. 3. As can be seen from this figure, nearly perfect mirror-image circular dichroism (CD) spectra are observed almost in the whole spectral region of the optically pure isomers of both yttrium and dysprosium compounds 1 and 2. This indicates the effective chiral information transfer from the peripheral chiral binaphthyl units to the porphyrin and phthalocyanine chromophores in the triple-decker molecules because of the intense intramolecular π–π interaction between porphyrin and phthalocyanine rings. With the exception of wavelengths at about 310 nm, the CD intensity is primarily due to induced CD (ICD) associated with the presence of the optically active binaphthyl moieties. Consistent with binaphthyl linked monomeric phthalocyanine systems including the chiral metal free phthalocyanine H2[Pc(OBNP)4] and its zinc complex Zn[Pc(OBNP)4],13 enantiomers with (R)-binaphthyl moieties (left-handed conformer) exhibited a positive ICD sign in the main electronic absorption region of 300–650 nm, while the enantiomers with (S)-binaphthyl moieties (right-handed conformer) exhibited the opposite sign pattern.
Fig. S5 (ESI†) shows the temperature dependence of the magnetic susceptibility χmT of the dysprosium compound 2. As can be seen, the χmT value of 2 at 300 K is 28.21 cm3 K mol−1, which is consistent with the expected value of 28.34 cm3 K mol−1 for two Dy(III) ions [6H15/2, S = 5/2, L = 5, g = 4/3].9 The magnetic susceptibility of 2 obeyed the Curie–Weiss law over the entire temperature range with a Curie constant (C) of 28.1 cm3 K mol−1. When the temperature is lowered, the χmT value of this compound decreases slowly until about 50 K, then decreasing quickly to a minimum value of 21.38 cm3 K mol−1 at 2 K due to the crystal-field effect and possible intramolecular antiferromagnetic dipole–dipole interactions.8,9 Fig. S6 (ESI†) displays the M vs. H curves for 2 at the temperature of 2 K, which show a rapid increase tendency at low field and eventually reach the maximum value of 9.66μB without achieving the magnetization saturation in terms of the expected saturation value of 20μB for two dysprosium ions [10μB for each Dy(III) ion]. This indicates the presence of magnetic anisotropy and the crystal-field effect for the Dy(III) ions.
Different from the tris(phthalocyaninato) dysprosium analogue Dy2[Pc(OC4H9)8]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8b and mixed (phthalocyaninato)(porphyrinato) dysprosium(III) double-decker Dy(Pc)(TClPP),9b measurement of alternating-current (ac) magnetic susceptibility over triple-decker 2 carried out in a 3.0 Oe ac field in the range of 1.0–780 Hz, Fig. S7 (ESI†), does not show the frequency-dependent character of the in-phase signal (χ′) and the out-of-phase signal (χ′′), revealing the non-SMM nature under zero applied dc magnetic field. For the purpose of further understanding the magnetic behavior of this tetrapyrrole dysprosium triple-decker complex, dynamic magnetic measurement of 2 has been conducted under an external direct current (dc) of 2000 Oe magnetic field. The frequency-dependent character in both the in-phase signal (χ′) and the out-of-phase signal (χ′′) in the whole oscillating range of 1–780 Hz employed is observed for 2, Fig. 4, indicating the field-induced SMM nature of this chiral compound.14
8b and mixed (phthalocyaninato)(porphyrinato) dysprosium(III) double-decker Dy(Pc)(TClPP),9b measurement of alternating-current (ac) magnetic susceptibility over triple-decker 2 carried out in a 3.0 Oe ac field in the range of 1.0–780 Hz, Fig. S7 (ESI†), does not show the frequency-dependent character of the in-phase signal (χ′) and the out-of-phase signal (χ′′), revealing the non-SMM nature under zero applied dc magnetic field. For the purpose of further understanding the magnetic behavior of this tetrapyrrole dysprosium triple-decker complex, dynamic magnetic measurement of 2 has been conducted under an external direct current (dc) of 2000 Oe magnetic field. The frequency-dependent character in both the in-phase signal (χ′) and the out-of-phase signal (χ′′) in the whole oscillating range of 1–780 Hz employed is observed for 2, Fig. 4, indicating the field-induced SMM nature of this chiral compound.14
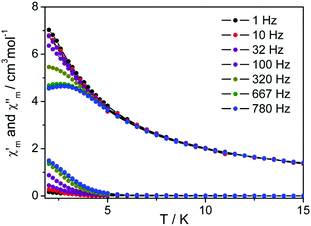 | ||
| Fig. 4 Temperature dependence of the out-of-phase (χ′′) and the in-phase (χ′) ac susceptibility of 2 in dc fields of 2000 Oe. | ||
The two enantiomers available together with the field-induced SMM nature revealed for the dysprosium triple-decker compound 2 render it possible to investigate the magneto-chiral dichroism effect of sandwich-type tetrapyrrole rare earth systems. The absorbance of both (R)- and (S)-enantiomers of 2 in CHCl3 was measured under magnetic fields of up to 1.6 T (1 T = 1 tesla) with both parallel and antiparallel fields. As exhibited in Fig. 5, the difference between the absorbance in parallel and antiparallel fields for both (R)- and (S)-2 clearly reveals the magneto-chiral dichroism effect existing in the chiral triple-decker complex 2. This, to the best of our knowledge, represents the first sandwich-type tetrapyrrole rare earth compounds that have been revealed to show the magneto-chiral dichroism effect despite the relatively rich studies over either the chiral or magnetic properties of such systems. In detail, the magneto-chiral dichroism signal is significant in the region at ca. 380 and 426 nm where strong ICD and MCD signs were observed. It is worth noting that the value of Δε (which indicates the intensity of the magneto-chiral dichroism effect) revealed for 2, 102 mol−1 dm3 cm−1 T−1, is actually comparable to those reported so far in this field,3,4 suggesting the great potential of sandwich-type tetrapyrrole rare earth complexes in the field of magneto-chiral dichroism.
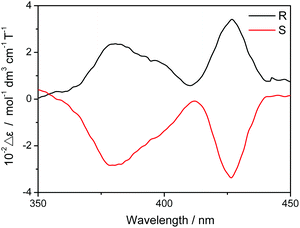 | ||
| Fig. 5 Magneto-chiral dichroism spectra of (R)- and (S)-enantiomers for the chiral mixed (phthalocyaninato)(porphyrinato) dysprosium triple-decker 2. | ||
In summary, two new chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker complexes with both (R)- and (S)-enantiomers were prepared, and spectroscopically, electrochemically, and structurally characterized. Investigation into the magnetic properties of the dysprosium triple-decker revealed its magnetic field-induced SMM nature. Nevertheless, a significant magneto-chiral cross effect was revealed for the chiral dysprosium triple-decker compounds, rendering them the first chiral sandwich-type tetrapyrrole rare earth compounds to exhibit the magneto-chiral cross effect.
Financial support from the Natural Science Foundation of China, National Key Basic Research Program of China (grant no. 2013CB933402 and 2012CB224801), National Ministry of Education of China, and Beijing Municipal Commission of Education is gratefully acknowledged.
Notes and references
- (a) R. A. Garoff, E. A. Litzinger, R. E. Connor, I. Fishman and B. A. Armitage, Langmuir, 2002, 18, 6330–6337 CrossRef CAS; (b) M. Wang, G. L. Silva and B. A. Armitage, J. Am. Chem. Soc., 2000, 122, 9977–9986 CrossRef CAS; (c) R. F. Pasternack, A. Giannetto, P. Pagano and E. J. Gibbs, J. Am. Chem. Soc., 1991, 113, 7799–7780 CrossRef CAS; (d) K. C. Hannah and B. A. Armitage, Acc. Chem. Res., 2004, 37, 845–853 CrossRef CAS PubMed.
- (a) Q.-H. Xia, H.-Q. Ge, C.-P. Ye, Z.-M. Liu and K.-X. Su, Chem. Rev., 2005, 105, 1603–1662 CrossRef CAS PubMed; (b) W. Eerenstein, N. D. Mathur and J. F. Scott, Nature, 2006, 442, 759–765 CrossRef CAS PubMed; (c) J. Chin, S. S. Lee, K. J. Lee, S. Park and D. H. Kim, Nature, 1999, 401, 254–257 CrossRef CAS PubMed; (d) G. L. J. A. Rikken and E. Raupach, Nature, 2000, 405, 932–935 CrossRef CAS PubMed.
- (a) M. P. Groenewege, Mol. Phys., 1962, 5, 541–563 CrossRef CAS; (b) G. Wagnière and A. Meier, Chem. Phys. Lett., 1982, 93, 78–81 CrossRef; (c) L. D. Barron and J. Vrbancich, Mol. Phys., 1984, 51, 715–730 CrossRef CAS; (d) J. L. J. A. Rikken and E. Raupach, Nature, 1997, 390, 493–494 CrossRef PubMed; (e) L. D. Barron, Nature, 2000, 405, 895–896 CrossRef CAS; (f) C. Train, R. Gheorghe, V. Krstic, L. Chamoreau, N. S. Ovnesyan, G. L. J. A. Rikken, M. Gruselle and M. Verdaguer, Nat. Mater., 2008, 7, 729–734 CrossRef CAS PubMed.
- (a) G. L. J. A. Rikken and E. Raupach, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1998, 58, 5081–5084 CrossRef CAS; (b) C. Koerdt, G. Duchs and G. L. J. A. Rikken, Phys. Rev. Lett., 2003, 91, 073902 CrossRef CAS; (c) Y. Kitagawa, H. Segawa and K. Ishii, Angew. Chem., 2011, 123, 9299–9302 CrossRef; (d) Y. Kitagawa, T. Miyatakeb and K. Ishii, Chem. Commun., 2012, 48, 5091–5093 RSC; (e) S. Bordács, I. Kézsmárki, D. Szaller, L. Demkó, N. Kida, H. Murakawa and Y. Onose, Nat. Phys., 2012, 8, 734–738 CrossRef.
- (a) K. Tashiro, K. Konishi and T. Aida, Angew. Chem., Int. Ed. Engl., 1997, 36, 856–858 CrossRef CAS; (b) K. Tashiro, K. Konishi and T. Aida, J. Am. Chem. Soc., 2000, 122, 7921–7926 CrossRef CAS; (c) M. Takeuchi, T. Imada and S. Shinkai, Angew. Chem., Int. Ed., 1998, 37, 2096–2099 CrossRef CAS; (d) A. Sugasaki, M. Ikeda, M. Takeuchi and S. Shinkai, Angew. Chem., Int. Ed., 2000, 39, 3839–3842 CrossRef CAS; (e) M. Takeuchi, M. Ikeda, A. Sugasaki and S. Shinkai, Acc. Chem. Res., 2001, 34, 865–873 CrossRef CAS PubMed; (f) M. Ikeda, M. Takeuchi, S. Shinkai, F. Tani, Y. Naruta, S. Sakamoto and K. Yamaguchi, Chem.–Eur. J., 2002, 8, 5541–5550 CAS.
- (a) Y. Bian, R. Wang, D. Wang, P. Zhu, R. Li, J. Dou, W. Liu, C.-F. Choi, H.-S. Chan, C. Ma, D. K. P. Ng and J. Jiang, Helv. Chim. Acta, 2004, 87, 2581–2596 CrossRef CAS; (b) Y. Bian, R. Wang, J. Jiang, C.-H. Lee, J. Wang and D. K. P. Ng, Chem. Commun., 2003, 1194–1195 RSC; (c) W. Lv, P. Zhu, Y. Bian, C. Ma, X. Zhang and J. Jiang, Inorg. Chem., 2010, 49, 6628–6635 CrossRef CAS PubMed; (d) Y. Zhou, Y. Zhang, H. Wang, J. Jiang, Y. Bian, A. Muranaka and N. Kobayashi, Inorg. Chem., 2009, 48, 8925–8933 CrossRef CAS PubMed; (e) J. Lu, Y. Deng, X. Zhang, N. Kobayashi and J. Jiang, Inorg. Chem., 2011, 50, 2562–2567 CrossRef CAS PubMed; (f) X. Zhang, A. Muranaka, W. Lv, Y. Zhang, Y. Bian, J. Jiang and N. Kobayashi, Chem.–Eur. J., 2008, 14, 4667–4674 CrossRef CAS PubMed.
- (a) N. Ishikawa, M. Sugita, T. Ishikawa, S. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694–8695 CrossRef CAS PubMed; (b) N. Ishikawa, Y. Mizuno, S. Takamatsu, T. Ishikawa and S. Koshihara, Inorg. Chem., 2008, 47, 10217–10219 CrossRef CAS PubMed.
- (a) R. Robles, N. Lorente, H. Isshiki, J. Liu, K. Katoh, B. K. Breedlove, M. Yamashita and T. Komeda, Nano Lett., 2012, 12, 3609–3612 CrossRef CAS PubMed; (b) T. Komed, H. Isshiki, J. Liu, Y.-F. Zhang, N. Lorente, K. Katoh, B. K. Breedlove and M. Yamashita, Nat. Commun., 2011, 2, 1–7 Search PubMed; (c) M. Gonidec, E. S. Davies, J. McMaster, D. B. Amabilino and J. Veciana, J. Am. Chem. Soc., 2010, 132, 1756–1757 CrossRef CAS PubMed; (d) A. Candini, S. Klyatskaya, M. Ruben, W. Wernsdorfer and M. Affronte, Nano Lett., 2011, 11, 2634–2639 CrossRef CAS PubMed.
- (a) H. Wang, W. Cao, T. Liu, C. Duan and J. Jiang, Chem.–Eur. J., 2013, 19, 2266–2270 CrossRef CAS PubMed; (b) H. Wang, K. Wang, J. Tao and J. Jiang, Chem. Commun., 2012, 48, 2973–2975 RSC; (c) H. Wang, T. Liu, K. Wang, C. Duan and J. Jiang, Chem.–Eur. J., 2012, 18, 7691–7694 CrossRef CAS PubMed; (d) H. Wang, K. Qian, K. Wang, Y. Bian, J. Jiang and S. Gao, Chem. Commun., 2011, 47, 9624–9626 RSC; (e) K. Wang, D. Qi, H. Wang, W. Cao, W. Li, T. Liu, C. Duan and J. Jiang, Chem.–Eur. J., 2013, 19, 11162–11166 CrossRef CAS PubMed.
- (a) J. Jiang and D. K. P. Ng, Acc. Chem. Res., 2009, 42, 79–88 CrossRef CAS PubMed; (b) J. Jiang, M. Bao, L. Rintoul and D. P. Arnold, Coord. Chem. Rev., 2006, 250, 424–448 CrossRef CAS PubMed; (c) J. Jiang, K. Kasuga and D. P. Arnold, in Supramolecular Photosensitive and Electroactive Materials, ed. H. S. Nalwa, Academic Press, New York, 2001, ch. 2, pp. 113–210 Search PubMed; (d) D. K. P. Ng and J. Jiang, Chem. Soc. Rev., 1997, 26, 433–442 RSC.
- (a) I. S. Kirin, P. N. Moskalev and Y. A. Makashev, Russ. J. Inorg. Chem., 1965, 10, 1065–1066 Search PubMed; (b) J. W. Buchler and D. K. P. Ng, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith, R. Guilard, Academic Press, San Diego, CA, 2000, vol. 3, pp. 245–294 Search PubMed; (c) X. Sun, X. Cui, D. P. Arnold, M. T. M. Choi, D. K. P. Ng and J. Jiang, Eur. J. Inorg. Chem., 2003, 8, 1555–1561 CrossRef; (d) X. Sun, R. Li, D. Wang, J. Dou, P. Zhu, F. Lu, C. Ma, Ch.-F. Choi, D. Y. Y. Cheng, D. K. P. Ng, N. Kobayashi and J. Jiang, Eur. J. Inorg. Chem., 2004, 19, 3806–3813 CrossRef.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751–767 CrossRef.
- (a) K. Wang, D. Qi, H. Wang, W. Cao, W. Li and J. Jiang, Chem.–Eur. J., 2012, 18, 15948–15952 CrossRef CAS PubMed; (b) K. Wang, H. Wang, J. Mack, W. Li, N. Kobayashi and J. Jiang, Acta Chim. Sin., 2012, 70, 1791–1797 CAS; (c) N. Kobayashi, R. Higashi, B. C. Titeca, F. Lamote and A. Ceulemans, J. Am. Chem. Soc., 1999, 121, 12018–12028 CrossRef CAS.
- (a) B. H. Koo, K. S. Lim, D. W. Ryu, W. R. Lee, E. K. Koh and C. S. Hong, Chem. Commun., 2012, 48, 2519–2521 RSC; (b) J. Kan, H. Wang, W. Sun, W. Cao, J. Tao and J. Jiang, Inorg. Chem., 2013, 52, 8505–8510 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation, MALDI-TOF mass spectrum, electronic absorption spectra, and electrochemical properties of compounds 1 and 2. Molecular structure, 1H NMR data, and crystal data for compound 1. The temperature dependence of χmT, M vs. H/T, and ac susceptibility under zero applied dc field for compound 2. CCDC 950171–950174. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3qi00097d |
| This journal is © the Partner Organisations 2014 |