A novel reusable and efficient nano-ZnS catalyst for green synthesis of xanthenes and its derivatives under solvent free conditions†
K. V. V.
Satyanarayana
a,
M.
Ravi Chandra
b,
P.
Atchuta Ramaiah
a,
Y. L. N.
Murty
a,
E. N.
Pandit
a and
S. V. N.
Pammi
*b
aDepartment of Organic Chemistry foods, drugs and water, College of science and technology, Andhra university, Visakhapatnam, 530003, India
bAdvanced Analytical Laboratory, DST-PURSE programme, Andhra University, Visakhapatnam, 530003, India. E-mail: sreepammi@gmail.com
First published on 24th January 2014
Abstract
A novel reusable and efficient nano-ZnS catalyst was prepared by a single pot wet chemical method and analyzed by SEM, XRD, FTIR and TEM techniques. We have studied the solvent effect, recyclability and reactions of various aromatic aldehydes with dimidone and β-naphthol for the synthesis of 1,8-dioxo-octahydroxanthenes and aryl-14H-dibenzo [a,j] xanthenes using ZnS nano particles as the catalyst under solvent free conditions without using any acidic catalyst or toxic materials. These results show that nano-ZnS is a potential catalyst for the development of green synthesis in the preparation of xanthenes and that the reaction proved to be economical which is of utmost importance for industrial applications.
Introduction
Xanthenes and their derivatives have been gaining much attention because of their biological and pharmacological activities, such as antiviral,1 antibacterial,2 and anti-inflammatory activities.3 In addition, these compounds can be employed as leuco-dyes,4 fluorescent materials for the visualization of biomolecules,5 and in laser technologies.6 Due to the unique biological profiles of xanthenes, there has been an upsurge interest in the development of many efficient synthetic protocols for the preparation of these molecules.7 The most widely used approach to synthesize xanthenes involves a one-pot thermal reaction of various aromatic aldehydes with β-naphthol in the presence of a number of catalysts.8–11 However, very few reports on the preparation of xanthenes and their derivatives via the condensation of dimidone with various aromatic aldehydes have been reported.7,12 Although these methods are effective, they suffer from some limitations, such as long reaction times, low yields, high catalyst loadings, and the use of toxic solvents or expensive catalysts. In order to overcome these disadvantages, attention has been paid to heterogeneous nano catalysts because of the unique properties of nano particles, thus, the usage of a new and efficient catalyst with a high catalytic activity, short reaction time, recyclability, ease in work-up capability and suitability in green chemistry for the synthesis of xanthenes and their derivatives would be highly desirable. From the literature, it was reported that ZnS is a semiconductor material, and can be used as a photo catalyst in the degradation of contaminated water containing halo benzene derivatives, organic dyes and toxic metal ions.13,14 Recently, the usage of a ZnS catalyst in organic synthesis i.e., the preparation of tetrazoles, has been reported.15 A nano-ZnS catalyst was prepared by a simple and efficient single pot wet chemical technique which provides the large scale production of nano-ZnS particles at a low cost and with a high purity. In this communication, we report for the first time, the preparation of xanthenes and their derivatives using various aromatic aldehydes with dimidone (Scheme 1) and β-naphthol (Scheme 2) in the presence of the nano-ZnS catalyst under solvent free conditions without using any co-catalyst, or toxic materials.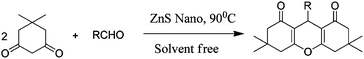 | ||
| Scheme 1 One pot synthesis of 1,8-dioxo-octahydroxanthenes using an aromatic aldehyde with dimidone catalyzed by nano-ZnS particles. | ||
 | ||
| Scheme 2 One pot synthesis of aryl-14H-dibenzo [a,j] xanthenes using an aromatic aldehyde with β-naphthol catalyzed by nano-ZnS particles. | ||
Nano-ZnS particles were prepared by a simple single pot wet chemical method with zinc sulfate (ZnSO4·7H2O), trisodium citrate, and NaOH solution by adding ethylene glycol containing PVP at near room temperature (∼35 °C). The product was separated from the reaction mixture by centrifugation, washed several times with ethanol and treated with 0.1 M HNO3. The detailed experimental method is given in ESI.† The morphological, structural and chemical composition of the ZnS nano particles was analyzed with SEM-EDS, XRD, and TEM techniques.
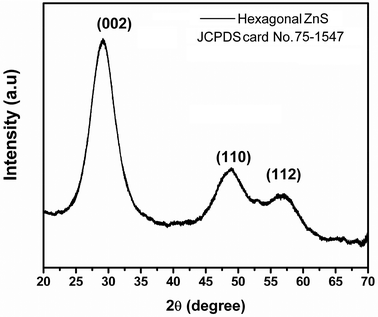
The XRD pattern (Fig. 1) of the nano-ZnS particles exhibits diffraction peaks which are significantly broad indicating a very small crystalline size. The nano-ZnS particles exhibit a hexagonal structure (JCPDS card No. 75-1547) with a preferred orientation (002) along the (110), and (112) directions. No metal or other phases were detected by XRD. We cannot exclude the existence of the cubic ZnS phase from XRD patterns due to large similarities in the structures between cubic and hexagonal ZnS. The interplanar spacing (d-spacing) obtained from XRD analysis is about 0.35 nm corresponding to the (002) lattice plane of hexagonal ZnS. The crystalline size of the nano-ZnS particles was calculated by using the Scherrer formula and was found to be about 2.5 nm indicating a nano crystalline nature. From the reported literature, the synthesized ZnS nano particles at ≤200 °C may consist of a physical mixture of the cubic and hexagonal phases.
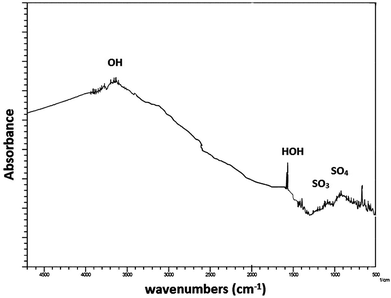
Fig. 2 shows the FTIR spectra of the ZnS nano particles which were synthesized by a single pot wet chemical method. From the FTIR data, bands around 3000–3600 cm−1 represent (OH) stretching vibrations and the band at 1620 cm−1 is due to (HOH) bending vibrations which seems to be in good agreement with the reported values.16 In addition, the group of peaks around 1000 cm−1 are indicative of sulfate groups, which have been previously reported for water on bulk ZnS.16 However, there is no evidence of a distinct ZnO phase from the diffraction data and the FTIR data.
The morphology and size of the ZnS nano particles were analyzed by scanning electron microscopy (SEM-EDS) and transmission electron microscopy (TEM) as shown in Fig. 3. The low magnification SEM image (Fig. 3(a)) shows that the nano-ZnS spheres have rough surfaces composed of nano particles with an average diameter of about 70 nm which indicates the nano crystalline nature of the ZnS nano particles. The presence of some larger particles is attributed to aggregation or overlapping of smaller particles. On further investigation, the size of the ZnS nano particles was analyzed by TEM which shows (Fig. 3(b) & (c)) that the nano particles had diameters of 2–5 nm. From the HRTEM image, the interplanar spacing is about 0.35 nm (Fig. 3(d)) and it represents the (002) lattice plane of the hexagonal wurtzite structure of the ZnS nano particles.
We analyzed the ZnS nano catalyst with energy dispersive spectroscopy (EDS) before and after treatment with 0.1 M HNO3 (given in ESI†) which showed that the catalyst contains only zinc and sulfur elements, indicating that the synthesized nano particles contain pure ZnS. It is noteworthy that there is an increase in atomic % of Zn due to a decrease in the atomic % of sulfur which indicates the formation of H2S gas during treatment, thus leading to the retaining and creation of sulfur vacancies.
A study was initiated to check the feasibility of nano-ZnS as a catalyst for the synthesis of xanthenes and their derivatives, we examined various bulk and nano particle (synthesized in our lab) catalysts under our experimental conditions using ethanol as the solvent. Zn based commercial salts, i.e. Zn acetate, ZnBr2, ZnCl2 and ZnSO4·7H2O, as catalysts (Table 1, entries 1–4) exhibit moderate yields with longer reaction times. It was observed that commercial bulk ZnO and ZnS gave poor yields (entries 5, 6) whereas nano-ZnO and nano-ZnS catalysts (prepared in our lab, entries 7–10) show better yields in short reaction times. The increased catalytic activity of the synthesized nano particles (ZnS or ZnO) over commercial bulk catalysts may be attributed to the higher surface areas, thus resulting in higher surface concentrations of the reactive sites. Interestingly, the experimental results reveal that the acid treated (0.1 M HNO3) catalyst (nano-ZnS – entry 10) shows superior catalytic properties in terms of reaction time (16 min) and yield (88%) when compared to other Zn based commercial and/or nano catalysts. This is evident from the colors of the commercial and as prepared ZnS nano catalysts. Usually, when commercial and nano-ZnS (white in color) are exposed to air, commercial ZnS turns to a light yellow color due to surface oxidation resulting in degradation of the catalytic activity, but in our study, we observed that the as prepared nano-ZnS remains white in color due to the usage of PVP in its preparation, as it protects the ZnS from oxidation and thereby increases the catalytic activity of the ZnS nano particles. This can also be affirmed from the reaction of commercial ZnS and the as prepared nano-ZnS with HNO3 to produce H2S, by which some S vacancies may be created and or retained which makes ZnII unsaturated, resulting in exposure to reactants, which leads to an improvement in catalytic activity.15 But in the case of commercial ZnS, H2S in solution or SII in solid ZnS in air easily oxidize to form a sulfur solution or nanoparticles in air, which get deposited on the surface of ZnS to cover the active sites, thus degrading the catalytic activity of the treated commercial ZnS. Interestingly this character is different with acid treated nano-ZnS, as PVP is used in the preparation of nano-ZnS by the one pot wet chemical method. PVP played a crucial role in abolishing the deposition of sulfur sol particles on the surface of ZnS in solution and in air, PVP protects ZnS from oxidation. Thus, the results of catalytic activity indicate that the treated nano-ZnS is much better than the as prepared (untreated) nano-ZnS and acid treated commercial ZnS.
| Entry | Catalyst | Time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: dimidone (2 mmol), benzaldehyde (1 mmol), catalyst (10 mol%), solvent – ethanol, temperature 90 °C. b Based on isolated yields. | |||
| 1 | Zn acetate | 25 | 63 |
| 2 | Zn Br2 | 25 | 66 |
| 3 | ZnCl2 | 25 | 64 |
| 4 | ZnSO4·7H2O | 25 | 68 |
| 5 | Bulk ZnO commercial | 30 | 60 |
| 6 | Bulk ZnS commercial | 30 | 65 |
| 7 | Nano-ZnO | 20 | 76 |
| 9 | Nano-ZnS untreated | 20 | 82 |
| 10 | Nano-ZnS treated | 16 | 91 |
In order to investigate the performance of the nano-ZnS catalyst, we studied the effect of solvents (Table 2) and recyclability (Fig. 4). Surprisingly, the reaction without a solvent exhibits superior catalytic activity in terms of yield and reaction time than that with a solvent. It was noticed that ethanol and water show better yields (Table 2 entries 3, 5), when compared to other solvents (entries 1, 2 and 4). At the end of the reaction, the catalyst was filtered, washed with diethyl ether, dried at 130 °C for 1 h, and reused in another reaction. We found that the acid treated nano-ZnS particles show a high catalytic activity in a very short reaction time. Moreover, they can be recovered and reused several times (runs from 1 to 9) without any significant loss of activity, the yields ranged from 94% to 89%. These results prove that the recyclability and non toxicity of the nano-ZnS catalyst help in the development of green synthesis in the preparation of xanthenes, and the reaction is economical and potentially valuable for industrial applications.
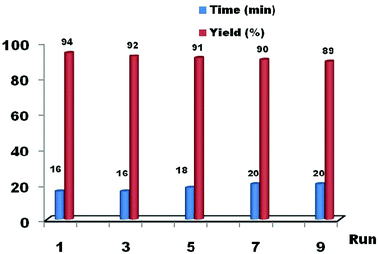 | ||
| Fig. 4 The reusability of the catalyst (nano-ZnS, 15 mol%) in the synthesis of 1,8-dioxo-octahydroxanthenes using benzaldehyde and dimidone under solvent free conditions. | ||
| Entry | Solvent | Time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: dimidone (2 mmol), benzaldehyde (1 mmol), catalyst (10 mol%), reflux (entries 1–5), temperature 90 °C. b Based on isolated yields. | |||
| 1 | Acetonitrile | 20 | 67 |
| 2 | Methanol | 20 | 75 |
| 3 | Ethanol | 16 | 88 |
| 4 | Ethyl acetate | 20 | 79 |
| 5 | H2O | 16 | 84 |
| 6 | Neat-ZnS NPs (10%) | 16 | 91 |
| 7 | Neat-ZnS NPs (15%) | 16 | 94 |
| 8 | Neat-ZnS NPs (20%) | 16 | 92 |
| 9 | Neat-ZnS NPs (25%) | 16 | 90 |
In order to ascertain the scope and limitation of the ZnS-nano particle catalyst for the synthesis of xanthenes, we extended the use of this catalyst to the reaction of various aromatic aldehydes with dimidone (Table 3) and various aromatic aldehydes with β-naphthol (Table 4) for the synthesis of 1,8-dioxo-octahydroxanthenes and aryl-14H-dibenzo [a,j] xanthenes. The aldehyde functional group is a more reactive centre for the nucleophilic dimidone. The para and ortho substituted –NO2 groups increase the electrophilic nature of the aldehyde because the –NO2 group acts as a strong electron withdrawing group compared to the remaining substituents. So, entries 6 and 7 (Tables 3 & 4) exhibit higher yields and take less time to complete the reaction. Entries 11 and 12 (Tables 3 & 4) show lower yields because all substituents are electron donating groups thereby decreasing the electrophilic nature of the aldehyde group and will take more time to complete the reaction.
| Entry | R | Time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: dimidone (2 mmol), aromatic aldehydes (1 mmol), catalyst (10 mol%), temperature 90 °C. b Based on isolated yields. | |||
| 1 | C6H5 | 16 | 91 |
| 2 | o-ClC6H4 | 10 | 89 |
| 3 | m-ClC6H4 | 15 | 87 |
| 4 | p-ClC6H4 | 12 | 95 |
| 5 | p-CH3C6H4 | 15 | 85 |
| 6 | p-O2NC6H4 | 5 | 97 |
| 7 | o-O2NC6H4 | 6 | 95 |
| 8 | p-HOC6H4 | 15 | 88 |
| 9 | p-MeOC6H4 | 20 | 87 |
| 10 | m-O2NC6H4 | 11 | 90 |
| 11 | m,p-(MeO)2C6H3 | 30 | 84 |
| 12 | p-N(CH3)2C6H3 | 30 | 75 |
| 13 | p-Br-C6H4 | 8 | 91 |
| 14 | 3-MeO,4-OHC6H3 | 10 | 84 |
| Entry | R | Time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: β-naphthol (2 mmol), aromatic aldehydes (1 mmol), catalyst (10 mol%), temperature 90 °C. b Based on isolated yields. | |||
| 1 | C6H5 | 18 | 90 |
| 2 | o-ClC6H4 | 10 | 86 |
| 3 | m-ClC6H4 | 15 | 85 |
| 4 | p-ClC6H4 | 12 | 93 |
| 5 | p-CH3C6H4 | 16 | 82 |
| 6 | p-O2NC6H4 | 6 | 96 |
| 7 | o-O2NC6H4 | 8 | 94 |
| 8 | p-HOC6H4 | 18 | 88 |
| 9 | p-MeO C6H4 | 22 | 87 |
| 10 | m-O2NC6H4 | 14 | 89 |
| 11 | m,p-(MeO)2 C6H3 | 30 | 84 |
| 12 | p-N(CH3)2C6H3 | 35 | 72 |
| 13 | p-Br-C6H4 | 10 | 92 |
In conclusion, we have synthesized nano-ZnS particles as a new heterogeneous catalyst with superior catalytic activity for the synthesis of xanthenes and their derivatives in short reaction times and without using any acidic catalyst, solvent or toxic materials. In addition, nano-ZnS offers the competitive advantage of easy separation from the reaction mixture and therefore can be reused, the recyclability of the catalyst showed no significant degradation in catalytic activity. These results proved that the nano-ZnS catalyst used in the preparation of xanthenes provides a development in green synthesis and the reaction is economical and potentially valuable for industrial applications.
Acknowledgements
We are thankful to the DST-PURSE Programme, Analytical Laboratory, Andhra University for their support in carrying out this research work regarding the SEM-EDS and XRD analysis.References
- T. Hideo and J. Teruomi, (Sankyo Co.) Jpn. Patent, 56005480, 1981 Search PubMed.
- J. P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Marcisse, G. Uchida-Ernouf and R. Lacroix, Eur. J. Med. Chem., 1978, 13, 67–71 CAS.
- K. Chibale, M. Visser, D. V. Schalkwyk, P. J. Smith, A. Saravanamuthu and A. H. Fairlamb, Tetrahedron, 2003, 59, 2289–2296 CrossRef CAS.
- R. W. Lambert, J. A. Martin, J. H. Merrett, K. E. B. Parkes and G. J. Thomas, PCT Int. Appl., WO9706178, 1997 Search PubMed.
- A. Banerjee and A. K. Mukherjee, Stain. Technol., 1981, 56, 83–85 CAS.
- C. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683–689 CAS.
- B. Karami, S. J. Hoseini, K. Eskandari, A. Ghasemib and H. Nasrabadia, Catal. Sci. Technol., 2012, 2, 331–338 CAS (reference there in).
- A. K. Bhattacharya, K. C. Rana, M. Mujahid, I. Sehar and A. K. Saxena, Bioorg. Med. Chem. Lett., 2009, 19, 5590 CrossRef CAS PubMed.
- D. Prasad and M. Nath, Catal. Sci. Technol., 2012, 2, 93–96 CAS (references there in).
- M. A. Zolfigol, A. R. Moosavi-Zare, P. A. Hadi, A. Zare, V. Khakyzadeh and G. Darvishi, RSC Adv., 2012, 2, 3618–3620 RSC (references there in).
- R. Kumar, G. C. Nandi, R. K. Verma and M. S. Singh, Tetrahedron Lett., 2010, 51, 442 CrossRef CAS PubMed (references there in).
- N. G. Khaligh, Catal. Sci. Technol., 2012, 2, 2211–2215 Search PubMed (references there in).
- J. S. Hu, L. L. Ren, Y. G. Guo, H. P. Liang, A. M. Cao, L. J. Wan and C. L. Bai, Angew. Chem., Int. Ed., 2005, 44, 1269–1273 CrossRef CAS PubMed.
- J. H. Bang, R. J. Helmich and K. S. Suslick, Adv. Mater., 2008, 20, 2599–2603 CrossRef CAS.
- L. Lang, B. Li, W. Liu, L. Jiang, Z. Xu and G. Yin, Chem. Commun., 2010, 46, 448–450 RSC.
- R. Sharma, S. J. Dhoble, D. P. Bisen, N. Brahme and B. P. Chandra, Int. J. Nanopart., 2011, 4, 64 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3qi00016h |
| This journal is © the Partner Organisations 2014 |