Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Scalable syntheses of well-defined pentadecablock bipolymer and quintopolymer†
Dean R.
Carroll
 a,
Anna P.
Constantinou
a,
Anna P.
Constantinou
 a,
Natalie
Stingelin
a,
Natalie
Stingelin
 ab and
Theoni K.
Georgiou
ab and
Theoni K.
Georgiou
 *a
*a
aDepartment of Materials, Exhibition Road, Royal School of Mines, Imperial College London, SW7 2AZ, UK. E-mail: t.georgiou@imperial.ac.uk
bSchool of Materials Science and Engineering, Georgia Institute of Technology, USA
First published on 7th May 2018
Abstract
The one-pot syntheses of two pentadeca-(15)-block methacrylate-based amphiphilic copolymers, specifically a bipolymer (AB)7A and a quintopolymer (ABCDE)3, are being reported using a fast and easy to scale up procedure that does not require any intermediate purification steps. Both syntheses were carried out using sequential group transfer polymerisation (GTP) and took under 3.5 h. Amino-containing (DMAEMA, DEAEMA), ether (THFMA, MEGMA) and alkyl (EtMA) methacrylates were used to produce the multiblock copolymers with a final Đ < 1.3.
In nature, monomer sequence control and regulation in vital molecules like proteins and DNA play a crucial role in biology and life such as complex self-assembly and self-replication. Thus, mimicking nature by trying to establish sequence control in polymer synthesis has gained much interest.1–5
Recent synthetic strategies have incorporated the precise control of the sequence of monomers, facilitating single monomer insertion. However, these approaches are not easy to scale up, so there is increasing interest in synthesizing multiblock copolymers through scalable methods.6 Several groups have successfully produced multiblock copolymers by using reversible-deactivation radical polymerisation (RDRP) methods. Specifically, Perrier et al. produced acrylamide polymers with hepta-block (7),7 octa-block (8),8 deca-block (10),9 dodeca-block (12)10 and icosa-block (20)11 copolymer architectures using reversible addition–fragmentation chain-transfer (RAFT) polymerisation. Haddleton et al. used acrylates and acrylamides with photo-living radical polymerisation (photoLRP) and atom-transfer radical polymerisation (ATRP), respectively, to produce copolymers with hepta (7)12 and henicosa (21)6 blocks. Where conversion is high enough (>99%), RDRPs are one-pot synthetic methods. However, in order to achieve high conversion, the polymerisation conditions will need to be optimised and studied thoroughly for each set of monomers used and this, depending on the monomers, can be a time consuming procedure. Furthermore, the actual polymerisation time varies from 30 minutes to 8 days for each block.
All of the above-mentioned studies polymerised acrylates or acrylamides, that polymerize much faster than methacrylates. Only one study reported methacrylate-based multiblocks with RAFT using a bifunctional initiator. A hepta-block copolymer was synthesised but poor monomer conversion required purification between each polymerisation.13 This is due to the “poor” kinetics of methacrylate monomers with RDRP methods which could not be overcome until a recent publication in Nature Chemistry.6 In this study, several methacrylate multiblock copolymers were synthesised using an innovative methodology: sulphur-free RAFT emulsion polymerisation.6 The henicosa-block (21 blocks) copolymer produced had a dispersity (Đ) of 1.25 and a total polymerisation time of 60 hours. In a follow-up study by the same authors, deca (10)- and hexa (6)-block multiblock copolymers with similar dispersities were also synthesised.14 The main disadvantage of this method is that it limits the chemistry of the monomers that can be used since it is an emulsion polymerisation method. Thus, only hydrophilic or only hydrophobic monomers can be polymerised under optimised conditions depending on the choice of the disperse phase limiting the possibility for amphiphilic copolymers with a multiblock architecture, unless combined with post-polymerisation modification(s).
Multiblock copolymers have also been reported with living polymerisation methods, typically having a narrower Đ. Using the conventional living anionic polymerisation, high molar mass copolymers of hexa (6),15 hepta (7),16 and octa (8)17 blocks have been reported with a Đ of 1.09, 1.5 and 1.25, respectively. Coupling reactions in combination with living anionic polymerisation have produced a hepta-block with a Đ of 1.07.18
Several studies have also been reported using group transfer polymerisation, GTP, which is an easy to scale up living anionic polymerisation that was developed by Du Pont in the 1980s.19,20 The mechanism of GTP has undergone much debate and is believed to depend on the nucleophilicity of the catalyst used. Monomers are added to the active chain by repetitive Mukaiyama aldol additions. However, two proposed mechanisms exist to describe the interaction of the catalyst with the active species: (1) a dissociative mechanism with enolate anions as the active species or (2) an associative mechanism with an intramolecular transfer of the silyl group through a concerted reaction. We believe that the initiator/catalyst we use follows an anionic, dissociative mechanism.
GTP, unlike the conventional living anionic polymerisation, can be conducted at temperatures up to 100 °C but is frequently conducted at ambient temperatures; thus there is no need to cool down to temperatures below 0 °C. However, to mitigate fluctuations in the polymerisation kinetics of GTP, at industrial scales, a reflux condenser is sometimes used to control the reaction exotherm. Furthermore, it should be noted that the recent studies on living anionic polymerisation have been performed at room temperature.21,22 However these were conducted using flow tubular or microreactor devices, so scalability is a concern.
Patrickios’ group has reported the synthesis of methacrylate-based copolymers by GTP with penta (5)23 and hepta (7)24,25 blocks using a bifunctional initiator as well as a hexablock18 using a monofunctional initiator. More recently, Kakuchi's group reported the synthesis of an acrylate icosablock copolymer using GTP with novel catalysts.26 The latter was done in small scales (<1 g) using a glove box.
Here we report the synthesis of two novel methacrylate pentadecablock (15) copolymers using GTP. The synthesis was performed in one pot using sequential polymerisation and the overall polymerisation time was less than 3.5 hours. At 15 minutes per block, this is faster than any previous studies with a similar number of blocks. Unlike traditional anionic polymerisation methods, GTP can be conducted at ambient temperatures and at higher concentrations. Furthermore, due to exceptionally high conversion (>99%), no intermediate purification steps are needed and this methodology can be easily scaled up. For these reasons, this methodology is cost-effective when compared to RDRP and the conventional anionic polymerisation. Additionally, GTP has been routinely used in industry for the synthesis of block copolymers such as emulsifiers and narrow dispersity poly(methyl methacrylate) GPC calibration standards. The organic catalyst remains in the polymer solution at very low concentrations (typically 0.05% w/w catalyst/polymer). In applications where the presence of the catalyst is undesirable, the end product can be easily purified by precipitation to remove the non-sulphurous, non-metallic and colourless catalyst.
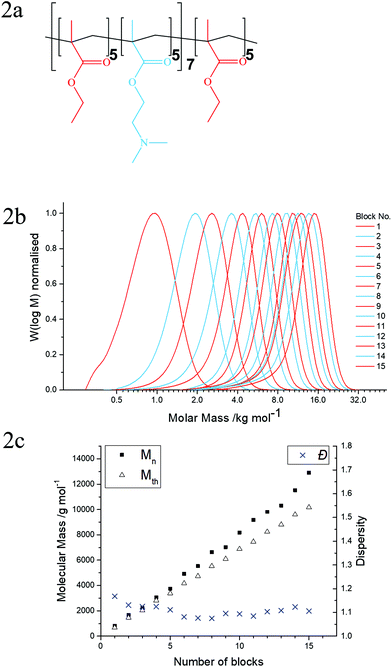
In this study, the first multiblock copolymer synthesised was a pentadecablock bipolymer based on ethyl methacrylate (EtMA) and 2-(dimethylamino)ethyl methacrylate (DMAEMA). These monomers were chosen for the initial multiblock study because of their different properties (specifically hydrophilicity), in order to produce amphiphilic, pH-responsive copolymers, and both monomers are readily available from commercial sources. Furthermore, the blocks were alternated between DMAEMA and EtMA for a subsequent investigation into the self-assembly of these block copolymers in the solution.
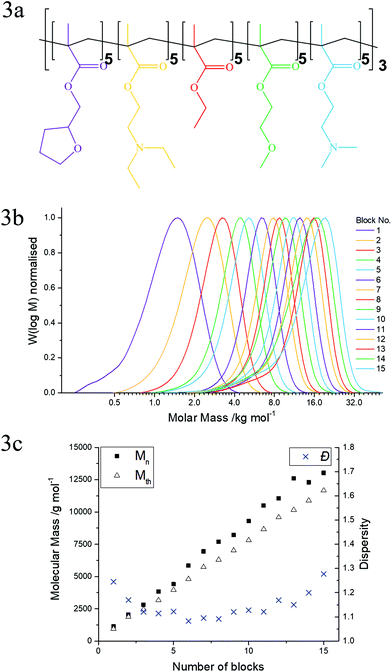
After confirming that the polymerisation of the bipolymer was successful, a second multiblock copolymer was synthesised based on 5 monomers in order to highlight the diversity of functional groups which can be polymerised with GTP. The chemical structures of the five commercially available monomers chosen to synthesise the pentadecablock copolymer are shown in Fig. 1. These monomers are EtMA, DMAEMA, 2-(diethylamino)ethyl methacrylate (DEAEMA), tetrahydrofurfuryl methacrylate (THFMA) and ethylene glycol methyl ether methacrylate (MEGMA). The chemical structures of the resulting polymers are shown in Fig. 2a and 3a. DEAEMA, similarly to DMAEMA, is a pH-responsive monomer that is hydrophobic under normal physiological conditions instead of being hydrophilic. THFMA and MEGMA are cyclic and linear esters, respectively, and both are polar.
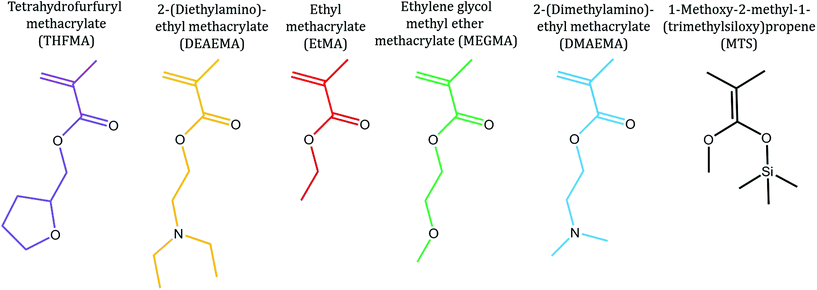 | ||
| Fig. 1 Chemical structures of the monomers and initiator used for the synthesis of multiblock copolymers with GTP. | ||
For this one-pot synthesis, the monomers were distilled from calcium hydride and purged with argon. The polymerisation catalyst (tetrabutylammonium bibenzoic acid, TBBAB), the solvent (tetrahydrofuran) and the initiator (1-methoxy-1(trimethylsiloxy)-2-methyl propene, MTS) were added into a dry flask containing argon. Subsequently, the first monomer (THFMA) was syringed in the flask, drop-wise. After the reaction exotherm attenuated, samples for gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) spectroscopy were obtained and the polymerisation was terminated with methanol. Sequential additions of THFMA, DEAEMA, EtMA, MEGMA and DMAEMA were executed in a similar manner. This sequence of additions was repeated 3 times to produce a pentadecablock copolymer. GPC samples were analysed to determine the molecular mass of the polymer after each addition and NMR samples confirmed the composition of the polymers and the full conversion of the monomers to the polymer. The bipolymer was synthesised in a similar manner, where only EtMA and DMAEMA were polymerised in an alternating sequence. The concentration of the monomers in the solution was kept constant at 25 wt% for both reactions.
The final Mn's were 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 12
000 g mol−1 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 for the quintopolymer and bipolymer, respectively. More than 19 g of the polymer, specifically 19.7 g (bipolymer) and 26.2 g (quintopolymer), was produced. The reactions could have been easily scaled up to produce more polymer if required; in fact, GTP works better at larger scales by minimising the deactivation caused by trace impurities or humidity. The total polymerisation time was <3 h for the bipolymer and <3.5 h for the quintopolymer. Each addition/block polymerised between 10 and 15 minutes. The temperature of the reaction ranged between 23 and 28 °C for both syntheses, with a typical temperature increase of 0.5 to 1 °C as each monomer was added. It should be pointed out that no cooling was required unlike the conventional anionic polymerisation. If a higher degree of polymerisation (DP) for each block was desired, then a water bath may be required to avoid the exotherm from compromising the Đ. With this technique, the DP of each block could be increased, as required; however, the rate of addition should be reduced for larger volumes of the monomer and the final molecular mass of the copolymer should be reduced to retain control over the Đ.
200 g mol−1 for the quintopolymer and bipolymer, respectively. More than 19 g of the polymer, specifically 19.7 g (bipolymer) and 26.2 g (quintopolymer), was produced. The reactions could have been easily scaled up to produce more polymer if required; in fact, GTP works better at larger scales by minimising the deactivation caused by trace impurities or humidity. The total polymerisation time was <3 h for the bipolymer and <3.5 h for the quintopolymer. Each addition/block polymerised between 10 and 15 minutes. The temperature of the reaction ranged between 23 and 28 °C for both syntheses, with a typical temperature increase of 0.5 to 1 °C as each monomer was added. It should be pointed out that no cooling was required unlike the conventional anionic polymerisation. If a higher degree of polymerisation (DP) for each block was desired, then a water bath may be required to avoid the exotherm from compromising the Đ. With this technique, the DP of each block could be increased, as required; however, the rate of addition should be reduced for larger volumes of the monomer and the final molecular mass of the copolymer should be reduced to retain control over the Đ.
In Fig. 3b and 2b, the GPC chromatograms of the quintopolymer (THFMA5-b-DEAEMA5-b-EtMA5-b-MEGMA5-b-DMAEMA5)3 and the bipolymer (EtMA5-b-DMAEMA5)7-b-EtMA5 are shown, respectively. The corresponding number average molar mass (Mn), theoretical molar mass (Mth) and dispersity (Đ) are plotted against the number of blocks for the bipolymer (Fig. 2c) and the quintopolymer (Fig. 3c). Also, provided in the ESI† are two tables which summarise these data and the theoretical and experimentally determined polymer compositions. The NMR spectra of both the final polymers are also provided in the ESI.† Noteworthy is the fact that the NMR spectra for the precursors and polymers did not reveal any monomer traces, confirming >99% conversion of the monomer to the polymer. This is typical for GTP synthesis under these conditions, demonstrating the advantage of GTP for the synthesis of block copolymers by sequential polymerisation.
Furthermore, as can be seen in Fig. 2b and 3b, there was slight deactivation between each block addition. As the polymerisation proceeds to above 12 blocks, the rubber septum sealing the polymerisation flask has been pierced more than 20 times for monomer addition and sample acquisition. These polymers were synthesised in a normal fume hood with no control over humidity, resulting in some unavoidable deactivation during the polymerisation. This factor and initial deactivation of the initiator, which is typical for GTP synthesis,6,27–36 cause the Mn to increase more than the Mth. The Đ remained low (<1.3) at all times during the polymerisation for both polymers. However, the final polymers had a slightly higher Đ than for a typical GTP synthesis. This can be attributed to chain transfer reactions that occur at longer polymerisation times. The quintopolymer had a higher Đ than the bipolymer possibly due to the slightly better kinetic rate for EtMA and DMAEMA with GTP; the polymerisation is more controlled in comparison with THFMA and MEGMA. At this point it should be noted that the aimed average degree of polymerisation per block was 5, knowing that normally there is some initial deactivation that will result in at least an average 6 units per block. This ensures that at least one unit was attached until the addition of the next monomer according to a recent theoretical study.37
In conclusion, the successful sequential GTP syntheses of two pentadecablock copolymers are reported. The short polymerisation times, large scale, no need of heating/cooling and the relatively high concentration (when compared to the conventional/traditional living anionic polymerisation) makes GTP a versatile technique to manufacture multiblock copolymers with tailorable number of blocks, composition and hydrophilicity/hydrophobicity (amphiphilicity).
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The Department of Materials and EPSRC are acknowledged for funding DRC and APC.References
- J.-F. Lutz, Nat. Chem., 2010, 2(2), 84–85 CrossRef PubMed
.
- H. Colquhoun and J.-F. Lutz, Nat. Chem., 2014, 6(6), 455–456 CrossRef PubMed
.
- J. F. Lutz, Acc. Chem. Res., 2013, 46(11), 2696–2705 CrossRef PubMed
.
- J. Lutz, M. Ouchi, D. R. Liu and M. Sawamoto, Science, 2013, 341(6146), 1238149 CrossRef PubMed
.
- H. Mutlu and J. F. Lutz, Angew. Chem., Int. Ed., 2014, 53(48), 13010–13019 CrossRef PubMed
.
- N. G. Engelis, A. Anastasaki, G. Nurumbetov, N. P. Truong, V. Nikolaou, A. Shegiwal, M. R. Whittaker, T. P. Davis and D. M. Haddleton, Nat. Chem., 2016, 9(2), 171–178 CrossRef PubMed
.
- G. Gody, R. Barbey, M. Danial and S. Perrier, Polym. Chem., 2015, 6(9), 1502–1511 RSC
.
- L. Martin, G. Gody and S. Perrier, Polym. Chem., 2015, 6(27), 4875–4886 RSC
.
- G. Gody, T. Maschmeyer, P. B. Zetterlund and S. Perrier, Macromolecules, 2014, 47(2), 639–649 CrossRef
.
- G. Gody, T. Maschmeyer, P. B. Zetterlund and S. Perrier, Macromolecules, 2014, 47(10), 3451–3460 CrossRef
.
- G. Gody, T. Maschmeyer, P. B. Zetterlund and S. Perrier, Nat. Commun., 2013, 4, 2505 CrossRef PubMed
.
- A. Simula, V. Nikolaou, A. Anastasaki, F. Alsubaie, G. Nurumbetov, P. Wilson, K. Kempe and D. M. Haddleton, Polym. Chem., 2015, 6(12), 2226–2233 RSC
.
- K. S. Pafiti, C. S. Patrickios, C. Abetz and V. Abetz, J. Polym. Sci., Part A: Polym. Chem., 2013, 51(23), 4957–4965 CrossRef
.
- N. G. Engelis, A. Anastasaki, R. Whitfield, G. R. Jones, E. Liarou, V. Nikolaou, G. Nurumbetov and D. M. Haddleton, Macromolecules, 2018, 51, 336–342 CrossRef
.
- G. Fleury and F. S. Bates, Macromolecules, 2009, 42(5), 1691–1694 CrossRef
.
- N. Hadjichristidis, M. Pitsikalis, S. Pispas and H. Iatrou, Chem. Rev., 2001, 101(12), 3747–3792 CrossRef PubMed
.
- M. Faber, V. S. D. Voet, G. ten Brinke and K. Loos, Soft Matter, 2012, 8(16), 4479 RSC
.
- N. A. Hadjiantoniou, A. I. Triftaridou, D. Kafouris, M. Gradzielski and C. S. Patrickios, Macromolecules, 2009, 42(15), 5492–5498 CrossRef
.
- O. W. Webster, W. R. Hertler, D. Y. Sogah, W. B. Farnham and T. V. Rajanbabu, J. Am. Chem. Soc., 1983, 105(17), 5706–5708 CrossRef
.
-
O. W. Webster, in New Synthetic Methods, Springer Berlin Heidelberg, Berlin, Heidelberg, 2004, pp. 1–34 Search PubMed
.
- A. Nagaki, A. Miyazaki and J.-I. Yoshida, Macromolecules, 2010, 43(20), 8424–8429 CrossRef
.
- A. Nagaki, Y. Takahashi, K. Akahori and J.-I. Yoshida, Macromol. React. Eng., 2012, 6(11), 467–472 CrossRef
.
- A. I. Triftaridou, M. Vamvakaki and C. S. Patrickios, Biomacromolecules, 2007, 8(5), 1615–1623 CrossRef PubMed
.
- M. T. Popescu, I. Athanasoulias, C. Tsitsilianis, N. A. Hadjiantoniou and C. S. Patrickios, Soft Matter, 2010, 6(21), 5417–5424 RSC
.
- M. T. Popescu, C. Tsitsilianis, C. M. Papadakis, J. Adelsberger, S. Balog, P. Busch, N. A. Hadjiantoniou and C. S. Patrickios, Macromolecules, 2012, 45(8), 3523–3530 CrossRef
.
- O.-T. Eric, Y. Chen, K. Takada, S. Sato, T. Satoh and T. Kakuchi, Polymers, 2015, 6(45), 7841–7850 Search PubMed
.
- N. H. Raduan, T. S. Horozov and T. K. Georgiou, Soft Matter, 2010, 6(10), 2321 RSC
.
- M. A. Ward and T. K. Georgiou, Soft Matter, 2012, 8(9), 2737 RSC
.
- M. A. Ward and T. K. Georgiou, J. Polym. Sci., Part A: Polym. Chem., 2010, 48(4), 775–783 CrossRef
.
- M. A. Ward and T. K. Georgiou, J. Polym. Sci., Part A: Polym. Chem., 2013, 51(13), 2850–2859 CrossRef
.
- M. A. Ward and T. K. Georgiou, Polym. Chem., 2013, 4(6), 1893 RSC
.
- J. J. Chung, J. R. Jones and T. K. Georgiou, Macromol. Rapid Commun., 2015, 36(20), 1773–1773 CrossRef
.
- A. P. Constantinou and T. K. Georgiou, Polym. Chem., 2016, 7(11), 2045–2056 RSC
.
- A. P. Constantinou, H. Zhao, C. McGilvery, A. Porter and T. Georgiou, Polymers, 2017, 9(1), 31 CrossRef
.
- N. Ghasdian, M. A. Ward and T. K. Georgiou, Chem. Commun., 2014, 50, 7114 RSC
.
- P. G. Falireas and M. Vamvakaki, Polym. Chem., 2017, 130, 50–60 Search PubMed
.
- G. Gody, P. B. Zetterlund, S. Perrier and S. Harrisson, Nat. Commun., 2016, 7, 10514 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, the synthetic procedure and GPC and NMR analysis. See DOI: 10.1039/c8py00565f |
| This journal is © The Royal Society of Chemistry 2018 |