Sustainable polymers: replacing polymers derived from fossil fuels
Stephen A.
Miller
*
The George and Josephine Butler Laboratory for Polymer Research, Department of Chemistry, University of Florida, Gainesville, Florida 32611-7200, USA. E-mail: miller@chem.ufl.edu; Fax: +1-352-392-9741; Tel: +1-352-392-7773
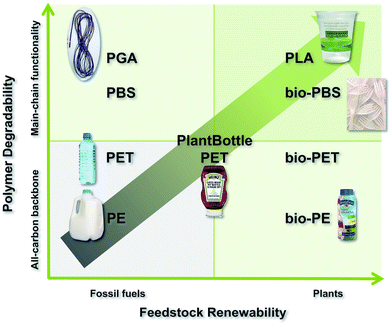
Fortunately, an increasing number of polymer chemists—both academic and industrial—are considering the sustainability of their creations. The two overarching goals are to employ renewable resources—instead of fossil fuels—and to engineer degradation pathways that can operate under reasonable time frames—decades instead of millennia. Fig. 1 allows one to visualize this renewability/degradability matrix in two dimensions. Over recent decades polymer chemists have departed the lower left quadrant of this matrix and targeted polymers in the upper right. Thus, traditional, fossil fuel-based polymers with slow degradation behaviours are eschewed for biogenic polymers that degrade readily and benignly in the environment. The aim of this special issue is to concentrate the most recent efforts in the field of sustainable polymers that have been designed to replace classic polymers derived from fossil fuel feedstocks. Topics include: the development and utilization of abundant and/or inexpensive biorenewable monomers to create thermoplastics; the mimicry of thermal properties inherent to successful commercial plastics; and the creation of bio-based polymers that are functional equivalents of fossil fuel-based polymers with respect to their properties, yet are capable of benign degradation over much shorter timescales.
A parallel thematic issue is available from Green Chemistry. Guest edited by Michael Meier (Karlsruhe Institute of Technology, Germany), that special issue also showcases sustainable polymers, but in the context of reduced environmental impact, renewable raw materials, and innovative catalytic approaches for monomers and polymers alike.
This Polymer Chemistry special issue contains fourteen contributions, covering a range of sustainable polymers with varying approaches and goals. Headlining this list is a rather comprehensive review on sustainable polyesters by Vilela et al. titled The quest for sustainable polyesters – insights into the future (DOI: 10.1039/c3py01213a). Given that nature is generous with carbon, hydrogen, and oxygen, polyesters are among the most promising classes of sustainable polymers. This review fills a void in the literature with thorough discussions about renewable monomers, aliphatic polyesters, aromatic polyesters, and synthetic approaches to all of the above.
A second, more targeted review is available from Caillol, Wadgaonkar, et al. titled Functionalization of cardanol: towards biobased polymers and additives (DOI: 10.1039/c3py01194a). Available from cashew nut shell liquid, cardanol is readily valorized into a variety of resins and polymer additives, including curing agents, plasticizers, surfactants, and anti-oxidants.
Among the twelve papers of this special issue, three are focused on novel polyesters. For example, Muñoz-Guerra et al. create polybutylene terephthalate (PBT) copolymers with diols and diacids derived from glucose (DOI: 10.1039/c3py01425h). Thus, the properties of PBT can be modified and its hydrolytic degradation accelerated. Thermoplastic polyesters and copolyesters of monomers derived from vegetable oil are reported by Narine et al. (DOI: 10.1039/c3py01261a). This paper reveals optimal polymerization parameters for the melt polycondensation of ω-hydroxyacids. Miller and Garcia describe the formation of polyester relatives via oxalate metathesis polymerization, thereby creating new polyoxalates with controllable properties and surprisingly rapid degradation behaviours (DOI: 10.1039/c3py01185b).
Sustainable elastomers are the focus of another three papers of this special issue. Clark and Hoong create polyol macromonomers from tetrahydrofuran (THF) and epoxidized vegetable oil and enlist them for making elastomeric polyurethanes (DOI: 10.1039/c3py01527k). The incorporated THF affords a substantial improvement in the mechanical properties. Hillmyer, Macosko, et al. polymerize bio-based δ-decalactone for the soft segment in thermoplastic block polyurethanes (DOI: 10.1039/c3py01120h). The elastomeric properties correlate with the block structures achieved. Tang, Chu, et al. synthesize novel thermoplastic elastomeric polyesters from renewable cellulose, rosin, and fatty acids (DOI: 10.1039/c3py01260c). By controlling the graft microstructure, both thermal and mechanical properties can be tuned.
Two papers focus on bio-based terpenes. Johansson et al. employ D-limonene in a free-radical thiol–ene macromonomer preparation (DOI: 10.1039/c3py01302b). The macromonomers are then successfully transformed into thermoset coatings with a useful range of thermo-mechanical properties. Kamigaito, Satoh, et al. subject β-pinene to living cationic polymerization and post-polymerization hydrogenation, acquiring polycycloolefins with a variety of promising properties—especially directed toward optoelectronic materials (DOI: 10.1039/c3py01320k).
Advances in reversible addition–fragmentation chain transfer (RAFT) polymerization are found in two contributed papers. Du Prez et al. apply a “grafting from” RAFT strategy to a fatty acid-based hydroxyl functionalized polyester (DOI: 10.1039/c3py01288c). Thus, this renewable macroRAFT agent was decorated with polyacrylate chains and served as the backbone for a novel amphiphilic graft polymer. Kamigaito, Satoh, et al. prepare thermally robust styrenic copolymers from renewable β-methyl styrenes, which are normally reluctant to copolymerize (DOI: 10.1039/c3py01066j). By using RAFT and specialized solvents, the renewable content of the polymers reached a comparatively high value of 50 mol%.
A paper contribution by Cavallo and Chen reports a novel organocatalytic polymerization by a phosphazene superbase (DOI: 10.1039/c3py01579c). Thus, the rapid and direct vinyl-type polymerization of a biorenewable methylmethylene-buyrolactone (MMBL) is achieved.
Finally, a paper contribution by Miller et al. describes the first cyclic and spirocyclic polyacetals derived from lignin-based aromatics (DOI: 10.1039/c4py00178h). The congested nature of these polymers affords high glass transition temperatures and the acetal functionality allows for facile water-degradability.
Indeed, there are many challenges set before the sustainable polymer community. As testified by the aforementioned contributions, tangible steps are continuously being taken that guide us from the old quadrant to the future quadrant. That is to say, we are progressing away from indestructible polymers built with fossil fuels and toward polymers that originate from biomass and degrade harmlessly back into the environment (Fig. 1).
| This journal is © The Royal Society of Chemistry 2014 |