Open Access Article
Open Access ArticleSynthesis and chiral recognition ability of helical polyacetylenes bearing helicene pendants†
Emmanuel
Anger
ab,
Hiroki
Iida
a,
Tomoko
Yamaguchi
a,
Koutarou
Hayashi
a,
Daisuke
Kumano
a,
Jeanne
Crassous
*b,
Nicolas
Vanthuyne
c,
Christian
Roussel
c and
Eiji
Yashima
*a
aDepartment of Molecular Design and Engineering, Graduate School of Engineering, Nagoya University, Nagoya 464-8603, Japan. E-mail: yashima@apchem.nagoya-u.ac.jp
bSciences Chimiques de Rennes, UMR 6226, Campus de Beaulieu, CNRS-Université de Rennes 1, 35042 Rennes Cedex, France. E-mail: jeanne.crassous@univ-rennes1.fr
cAix Marseille Université, Centrale Marseille, CNRS, iSm2 UMR 7313, 13397, Marseille, France
First published on 9th June 2014
Abstract
Novel polyacetylenes bearing an optically active or racemic [6]helicene unit as the pendant groups directly bonded to the main-chain (poly-1s) were prepared by the polymerisation of the corresponding acetylenes (1-rac, 1-P and 1-M) using a rhodium catalyst. The optically active polyacetylenes (poly-1-P and poly-1-M) formed a preferred-handed helical conformation biased by the optically active helicene pendants, resulting in the induced circular dichroism (ICD) in their π-conjugated polymer backbone regions. The optically active helical polymers, when employed as an enantioselective adsorbent, showed a high chiral recognition ability towards racemates, such as the monomeric [6]helicene and 1,1′-binaphthyl analogues, and enantioselectively adsorbed one of the enantiomers.
Introduction
Helicenes are unique polycyclic aromatic compounds that are inherently chiral because of their nonplanar screw-shaped skeletons.1 Due to such intriguing features of the helicenes that involve helical chirality, conjugated π-systems and fluorescent and chiroptical properties,1,2 optically active helicene-containing polymers have the potential to be used in many fields of the materials science including optoelectric materials, asymmetric catalysts and chiral separation and sensing materials. To date, several groups have attempted to introduce helicene units into macromolecules,3 but the applications of optically active helicene-containing polymers as chiral materials are quite limited.1a,b,e In particular, the application of optically active helicene-containing polymers as enantioselective adsorbents and chiral stationary phases (CSPs) for high-performance liquid chromatography (HPLC) has not been previously reported, in spite of the recent significant advance in chromatographic enantioseparation that has become an essential technique for the development of chiral drugs in the pharmaceutical industry.4On the other hand, the development of artificial helical polymers with a controlled helical sense has significantly advanced over the past decades because of their possible applications to chiral materials.5 Among the synthetic helical polymers prepared so far, polyacetylenes are one of the most extensively studied helical polymers,5,6 and some of them have been successfully utilised as asymmetric catalysts,7 chiral adsorbents8 and CSPs,9,10 in which the preferred-handed helical structures appear to be important for their enantioselectivities and resolving abilities.
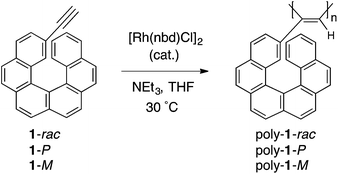
We anticipated that an optically active [6]helicene derivative might be a suitable chiral component as the pendant group to develop a novel optically active helical polyacetylene, since the helicene residues, once covalently introduced to the polyacetylene backbone, could induce a preferred-handed helical structure in the polymer accompanied by a one-handed helical array of the helicene pendants, thus showing an intriguing chiral recognition ability originating from the helical chiralities of the helicene itself and polymer main-chain as well. To this end, we have designed and synthesised a series of novel optically inactive (poly-1-rac) and active polyacetylenes (poly-1-P and poly-1-M) (Scheme 1) bearing a racemic or optically active helicene unit as the pendant groups directly connected to the polymer backbone, and also investigated the chiroptical properties of poly-1-P and poly-1-M and their chiral recognition abilities were evaluated on the basis of an enantioselective adsorption method toward several racemates.11
Results and discussion
The helicene monomer, 2-ethynyl-carbo[6]helicene (1-rac), was prepared and resolved into enantiomers by chiral HPLC separation according to a previously reported method2h (1-P and 1-M with right- and left-handed screw senses, respectively; enantiomeric excess (ee) >99%) (ESI).†Helically twisted helicene molecules are known to form self-associated supramolecular aggregates through noncovalent, face-to-face π–π interactions, which frequently trigger spontaneous resolution; P- and M-helicenes favorably form homochiral assemblies over heterochiral ones due to a greater π–π overlap between the helicenes with the same configuration.1a,b,e,12 If this is the case, we anticipated that the enantiomerically pure 1-P and 1-M could polymerise faster than their racemic mixture (1-rac), producing higher molecular weight (MW) homochiral polymers (poly-1-P and poly-1-M, respectively) or the racemic 1-rac could polymerise in a stereoselective manner (poly-1-rac) via a growing chain-end control mechanism, thus producing a mixture of polymers rich in either 1-P or 1-M.13
In order to investigate this possibility, we first polymerised 1s on a small scale under various conditions, namely, in different solvents (tetrahydrofuran (THF) and chloroform) in the presence of NEt3, different concentrations of 1s (0.02–0.2 M) and rhodium catalysts (1–6 mol%) (neutral [Rh(nbd)Cl]2 and cationic [Rh(nbd)2BF4] (nbd: norbornadiene), respectively) based on previously reported results.14 However, the polymers precipitated during the polymerisation reactions independent of the conditions, affording polymers with relatively low MWs in moderate yields. Therefore, the difference in the polymerisation reactivity between 1-rac and 1-P or 1-M was difficult to investigate.
Typical polymerisation results in THF in the presence of NEt3 using [Rh(nbd)Cl]2 as a catalyst, yielding stereoregular (cis-transoidal) polyacetylenes,15 poly-1-rac, poly-1-P and poly-1-M (Scheme 1) are shown in Table 1 (entries 1–3, 4, 9, and 14). The number-average molecular weights (Mn) of poly-1-P and poly-1-M (Mn = 2.6 × 103) estimated by size exclusion chromatography (SEC) using polystyrene standards were higher than that of poly-1-rac (Mn = 1.6 × 103) (entries 1–3). However, this tendency was not reproducible (see entries 4, 9 and 14 in Table 1), because the polymers precipitated during the polymerisation as already mentioned. The SEC profiles of poly-1s showed multimodal peaks, thus we fractionated poly-1s (Table 1, entries 4, 9 and 14) into four fractions (indicated by a subscript notation using A–D) based on the MW by SEC (Fig. S4† and Table 1)17,18 and their chiroptical properties were investigated.
| Entry | Monomer | Polymer | |||||
|---|---|---|---|---|---|---|---|
| Code | Yieldd (%) | M n × 10−3 | M w/Mne | Δε1stf (M−1 cm−1) | Δε2ndf (M−1 cm−1) | ||
| a Polymerised under nitrogen; [monomer] = 0.2 M, [Rh]/[monomer] = 100. b [Rh]/[monomer] = 33. c [Rh]/[monomer] = 17. d Diethyl ether-insoluble part. e Estimated by SEC (polystyrene standards) with THF as the eluent. f The intensities of the first (Δε1st) and second Cotton effects (Δε2nd) at around 250 and 330 nm, respectively, measured in THF (0.02 mg mL−1) at 25 °C. g Not measured because the amounts of poly-1-PA and poly-1-MA obtained by SEC fractionation were too small. h For their MALDI-TOF MS spectra, see Fig. S5.† | |||||||
| 1 | 1-rac | poly-1-rac | 50 | 1.6 | 3.4 | — | — |
| 2b | 1-P | Poly-1-P | 25 | 2.6 | 5.3 | 0.98 | −0.88 |
| 3 | 1-M | Poly-1-M | 17 | 2.6 | 7.1 | −0.94 | 0.82 |
| 4c | 1-rac | poly-1-rac | 46 | 1.8 | 3.6 | — | — |
| 5 | poly-1-racA | — | 5.8 | 2.0 | — | — | |
| 6 | poly-1-racB | — | 11 | 1.3 | — | — | |
| 7 | poly-1-racC | — | 3.0 | 1.6 | — | — | |
| 8 | poly-1-racD | — | 0.74 | 1.1 | — | — | |
| 9c | 1-P | Poly-1-P | 52 | 1.7 | 2.7 | 1.22 | —1.13 |
| 10 | poly-1-PA | — | —g | —g | —g | —g | |
| 11 | poly-1-PB | — | 7.0 | 1.4 | 1.27 | −1.12 | |
| 12 | poly-1-PC | — | 2.7 | 1.3 | 1.29 | −1.14 | |
| 13 | poly-1-PD | — | 0.73 | 1.4 | 1.32 | −1.25 | |
| 14c | 1-M | Poly-1-M | 47 | 1.8 | 2.7 | −1.16 | 1.07 |
| 15 | poly-1-MA | — | —g | —g | —g | —g | |
| 16 | poly-1-MB | — | 5.2h | 1.3 | −1.24 | 1.07 | |
| 17 | poly-1-MC | — | 2.1h | 1.6 | −1.25 | 1.10 | |
| 18 | poly-1-MD | — | 0.79h | 1.4 | −1.31 | 1.23 | |
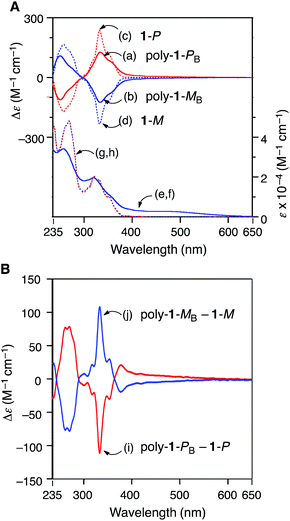
Fig. 1A shows the circular dichroism (CD) and absorption spectra of the optically active 1-P, 1-M, poly-1-PB and poly-1-MB measured in THF at 25 °C. The monomers 1-P and 1-M gave rise to the typical absorption signals centered at around 270 and 320 nm assigned to the helicene moiety (g and h in Fig. 1A) and characteristic intense Cotton effects in the regions (c and d).2h On the other hand, poly-1-PB and poly-1-MB showed an apparent CD in the π-conjugated main-chain region (380–600 nm) induced by the chiral helicene pendants as well as CDs in the helicene chromophore region (235–380 nm) (a and b), whose CD spectral patterns were different from those of the monomers 1-P and 1-M. Fig. 1B shows the differential CD spectra in which the contributions arising from the CD absorption due to the enantiomerically pure 1-P and 1-M themselves are subtracted from the observed CD spectra of poly-1-PB and poly-1-MB, respectively (i and j in Fig. 1B). The differential CD spectral patterns seem to be like those of helical polyacetylenes with optically active pendant groups,19 suggesting that poly-1-P and poly-1-M most likely possess an excess of a one-handed helical conformation induced by the optically active helicene pendants, which may further result in a preferred-handed helical array of the helicene pendants along the single-handed helical polymer backbone.
The CD and absorption spectra of the fractionated poly-1-PC,D and poly-1-MC,D were also recorded in THF at 25 °C (Fig. S6A, C and D†), which, together with the differential CD spectra (Fig. S6B†) indicated that the CD intensities more or less depended on the MW of the polymers, but their difference was not significant, probably because poly-1-P and poly-1-M likely possess a rather stable helical conformation with an excess one-handedness stabilised by the attractive intramolecular π–π interaction between the helically arranged helicene pendants with the same configuration. This speculation was supported by the facts that the CD spectra of poly-1-PB,C and poly-1-MB,C hardly changed over the temperature range of −10 to 55 °C in THF and remained unchanged after heating at 55 °C for 2 h (Fig. S7†). Such a thermal stability of the present helical poly-1-P and poly-1-M is noticeable, since most of the reported helical polyacetylenes are dynamic in nature and highly sensitive to temperature, which frequently exhibits an inversion of the macromolecular helicity with temperature.5d,6a,c,f
Fig. 2A and B display a possible right-handed helical structure of poly-1-M (20 mer) based on molecular mechanics calculations with a 23 unit per 10 turn (23/10) helical poly(phenylacetylene) whose helical structure was determined by X-ray diffraction measurements (see ESI†).20 The calculated structure revealed that the helicene pendant units are closely packed and the neighboring helicene pendant units are overlapped via π–π interactions, resulting in the formation of the one-handed helical array along the helical polymer backbone.21
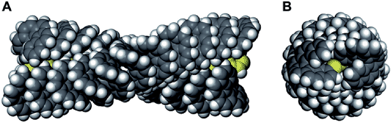 | ||
| Fig. 2 (A) Side view and (B) top view of a possible right-handed helical structure of poly-1-M (20 mer). The structure is shown using the space-filling model; the main-chain atoms are shown in yellow for clarity. The pendant helicene residues are arranged in a left-handed helical array along the right-handed helical poly-1-M backbone. The helix-sense of the main-chain is assumed from the Cotton effect sign in the main-chain region (>380 nm) according to the literature.19 | ||
We next investigated if the racemic 1-rac could polymerise in a stereoselective fashion to produce a mixture of poly-1s rich in either 1-P or 1-M by an enantioselective adsorption technique11 using optically active (−)-poly(triphenylmethyl methacrylate) ((−)-PTrMA) with a one-handed helical conformation.5a–c,24 The optically active helical PTrMA is a practically useful chiral packing material for HPLC and has been used to resolve a variety of aromatic racemic compounds including [6]helicene as well as racemic helical polymers such as (±)-PTrMA.5a–c,25 In fact, (−)-PTrMA selectively adsorbed poly-1-M from an equimolar mixture of poly-1-P and poly-1-M in hexane–THF (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v), and its supernatant solution exhibited a CD whose spectral pattern and intensity were identical to those of poly-1-P (ee = 36.5%) (for more detailed experimental procedures, see ESI, Table S1 and Fig. S8†). In the same way, an enantioselective adsorption experiment was performed for poly-1-rac with (−)-PTrMA. However, the supernatant solution showed almost no CD due to poly-1-P or poly-1-M (Table S1 and Fig. S8†), suggesting that 1-rac polymerised in an almost random way, or a highly stereoselective polymerisation (racemate-forming enantiomer-differentiating polymerisation) may not occur during the present polymerisation of 1-rac.
20, v/v), and its supernatant solution exhibited a CD whose spectral pattern and intensity were identical to those of poly-1-P (ee = 36.5%) (for more detailed experimental procedures, see ESI, Table S1 and Fig. S8†). In the same way, an enantioselective adsorption experiment was performed for poly-1-rac with (−)-PTrMA. However, the supernatant solution showed almost no CD due to poly-1-P or poly-1-M (Table S1 and Fig. S8†), suggesting that 1-rac polymerised in an almost random way, or a highly stereoselective polymerisation (racemate-forming enantiomer-differentiating polymerisation) may not occur during the present polymerisation of 1-rac.
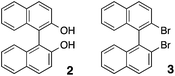
The chiral recognition ability of the optically active poly-1-P and poly-1-M was then investigated for the aromatic racemic compounds (1–3) including 1-rac by enantioselective adsorption experiments (Table 2 and ESI†).11,26,27
| Entry | Analyte | Polymer | Yield of adsorbed analyteb (%) | ee of adsorbed analyteb (%) | Separation factorc (α) |
|---|---|---|---|---|---|
| a Experimental conditions for entries 1 and 2: poly-1-P or poly-1-M, 2.0 mg; analyte, 0.04 mg (1 mL portion from a 0.04 mg mL−1 solution in methanol). Experimental conditions for entries 3–6: poly-1-P, 2.0 mg or poly-1-M, 3.5 mg; analyte, 0.4 mg for poly-1-P or 0.7 mg for poly-1-M (1 mL or 1.75 mL portion from a 0.4 mg mL−1 solution in methanol). b Determined by chiral HPLC analysis of analytes adsorbed on poly-1-P or poly-1-M using a UV-visible detector. c Calculated according to the equation α = (Fmajor (%)/Fminor (%))/(Amajor (%)/Aminor (%)), where Fmajor and Fminor are the percentages of major and minor enantiomers of the free analyte in the supernatant solutions, respectively, and Amajor and Aminor are those of major and minor enantiomers of the adsorbed analyte, respectively. d Average values of two runs. | |||||
| 1 | 1 | poly-1-P | 5.0 | 3.6 (P) | 1.08 |
| 2d | poly-1-M | 5.0 | 3.7 (M) | 1.08 | |
| 3d | 2 | poly-1-P | 0.64 | 37 (S) | 2.18 |
| 4d | poly-1-M | 0.66 | 35 (R) | 2.09 | |
| 5d | 3 | poly-1-P | 2.1 | 26 (S) | 1.71 |
| 6d | poly-1-M | 2.3 | 29 (R) | 1.84 | |
poly-1-P and poly-1-M selectively adsorbed one of the enantiomers of 1–3 in methanol. The adsorbed analytes on the polymers were almost completely recovered by desorption in methanol and subsequently in a methanol–THF (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) mixture (ESI†), and their amounts and ee values were estimated by chiral HPLC analysis (Table 2).
50, v/v) mixture (ESI†), and their amounts and ee values were estimated by chiral HPLC analysis (Table 2).
As anticipated,1a,b,e,12 poly-1-P and poly-1-M preferentially adsorbed 1-P and 1-M with the same configuration of the helicene residues, furnishing 3.6 and 3.7% ee, respectively (entries 1 and 2). Interestingly, poly-1-P and poly-1-M showed a much better and more remarkable chiral recognition toward the racemic 1,1′-binaphthyl analogues (2 and 3), and selectively adsorbed the (S)- and (R)-enantiomers, respectively, with up to 37% ee (entries 3–6). Based on the amounts and ee values of the analytes (1–3) adsorbed on the polymers, the separation factor (α), which is a useful measure in chiral HPLC to evaluate the chiral resolving ability of optically active hosts toward racemic analytes,11 can be calculated (Table 2).4 The calculated α values (1.71–2.18) are high enough for the complete separation of enantiomers when used as a CSP for HPLC,4,5a,b indicating that the helicene-bound helical polyacetylenes possess a practically useful chiral recognition power for aromatic racemic compounds like a commercially available (+)-PTrMA column.
The enantioselective adsorption results on the poly-1-P and poly-1-M (Table 2) revealed insight into the chiral recognition mechanism, in other words, the stereochemistry of the poly-1–analyte interaction; helically arranged helicene-pendant residues along the poly-1 helical backbone (Fig. 2) appear to be responsible for the observed efficient separation of aromatic analytes (1–3). The two naphthyl rings of the more retained enantiomers of (R)-2 and (R)-3 on poly-1-M may be favorably twisted into a left-handed helix so as to interact with the adjacent M-helicene groups arranged in a left-handed helical array through the aromatic interactions, whereas due to a considerable π–π overlap between the helicene pendants, may interfere with the favorable close helicene–helicene interactions between poly-1 and 1-rac, resulting in a relatively lower separation factor than we anticipated.
Conclusions
In summary, we have, for the first time, prepared stereoregular helical polyacetylenes bearing optically active and racemic [6]helicene groups as the pendants. Although the molecular weights of the polymers were rather low because of the low solubility of the helicenes, the optically active helicene-bound polymers exhibited an apparent CD in the polymer backbone chromophore region due to a preferred-handed helix formation biased by the chiral helicene residues. Moreover, we found that the optically active helicene-bound polyacetylenes showed a high chiral recognition ability toward racemic 1,1′-binaphthyl derivatives largely arising from a one-handed helical array of the pendant helicene residues.The present results suggest that a more practically useful chiral material for the separation of enantiomers may be developed based on analogous helicene- and organometallic helicene2f-bound helical polyacetylenes by introducing a long alkyl chain on the helicene residue, which will overcome the solubility problem of polymers, thereby providing a unique chiral stationary phase with a high enantioselectivity composed of one-handed helical polyacetylenes with screw-shaped helicene and organometallic helicene pendants, and further studies along this line are now in progress.
Acknowledgements
This work was supported in part by Grant-in-Aid for Scientific Research (S) from the Japan Society for the Promotion of Science (JSPS) and Research Grants in the Natural Sciences from the Mitsubishi Foundation. E.A. expresses his thanks for the JSPS Summer Program.Notes and references
- For recent reviews, see: (a) R. Amemiya and M. Yamaguchi, Org. Biomol. Chem., 2008, 6, 26–35 RSC; (b) Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed; (c) M. Gingras, Chem. Soc. Rev., 2013, 42, 968–1006 RSC; (d) M. Gingras, G. Felix and R. Peresutti, Chem. Soc. Rev., 2013, 42, 1007–1050 RSC; (e) M. Gingras, Chem. Soc. Rev., 2013, 42, 1051–1095 RSC.
- For recent examples of [n]helicenes, see: (a) J. Guin, C. Besnard and J. Lacour, Org. Lett., 2010, 12, 1748–1751 CrossRef CAS PubMed; (b) J. K. Zak, M. Miyasaka, S. Rajca, M. Lapkowski and A. Rajca, J. Am. Chem. Soc., 2010, 132, 3246–3247 CrossRef CAS PubMed; (c) J.-D. Chen, H.-Y. Lu and C.-F. Chen, Chem.–Eur. J., 2010, 16, 11843–11846 CrossRef CAS PubMed; (d) L. Norel, M. Rudolph, N. Vanthuyne, J. A. G. Williams, C. Lescop, C. Roussel, J. Autschbach, J. Crassous and R. Reau, Angew. Chem., Int. Ed., 2010, 49, 99–102 CrossRef CAS PubMed; (e) O. Songis, J. Misek, M. B. Schmid, A. Kollarovie, I. G. Stara, D. Saman, I. Cisarova and I. Stary, J. Org. Chem., 2010, 75, 6889–6899 CrossRef CAS PubMed; (f) E. Anger, M. Rudolph, L. Norel, S. Zrig, C. Shen, N. Vanthuyne, L. Toupet, J. A. G. Williams, C. Roussel, J. Autschbach, J. Crassous and R. Reau, Chem.–Eur. J., 2011, 17, 14178–14198 CrossRef CAS PubMed; (g) Y. Sawada, S. Furumi, A. Takai, M. Takeuchi, K. Noguchi and K. Tanaka, J. Am. Chem. Soc., 2012, 134, 4080–4083 CrossRef CAS PubMed; (h) E. Anger, M. Srebro, N. Vanthuyne, L. Toupet, S. Rigaut, C. Roussel, J. Autschbach, J. Crassous and R. Réau, J. Am. Chem. Soc., 2012, 134, 15628–15631 CrossRef CAS PubMed and references therein.
- (a) A. Sudhakar, T. J. Katz and B. W. Yang, J. Am. Chem. Soc., 1986, 108, 2790–2791 CrossRef CAS; (b) Y. J. Dai, T. J. Katz and D. A. Nichols, Angew. Chem., Int. Ed. Engl., 1996, 35, 2109–2111 CrossRef CAS; (c) Y. J. Dai and T. J. Katz, J. Org. Chem., 1997, 62, 1274–1285 CrossRef CAS; (d) Z. Y. Wang, Y. Qi, T. P. Bender and J. P. Gao, Macromolecules, 1997, 30, 764–769 CrossRef CAS; (e) J. P. Chen, J. P. Gao and Z. Y. Wang, Polym. Int., 1997, 44, 83–87 CrossRef CAS; (f) Z. Y. Wang, T. P. Bender, H. B. Zheng and L. Z. Chen, Polym. Adv. Technol., 2000, 11, 652–657 CrossRef CAS; (g) J. M. Fox, D. Lin, Y. Itagaki and T. Fujita, J. Org. Chem., 1998, 63, 2031–2038 CrossRef CAS; (h) H. Sugiura, Y. Nigorikawa, Y. Saiki, K. Nakamura and M. Yamaguchi, J. Am. Chem. Soc., 2004, 126, 14858–14864 CrossRef CAS PubMed; (i) T. Iwasaki, Y. Kohinata and H. Nishide, Org. Lett., 2005, 7, 755–758 CrossRef CAS PubMed; (j) R. Amemiya, N. Saito and M. Yamaguchi, J. Org. Chem., 2008, 73, 7137–7144 CrossRef CAS PubMed.
- (a) Y. Okamoto and E. Yashima, Angew. Chem., Int. Ed., 1998, 37, 1020–1043 CrossRef; (b) E. Yashima, C. Yamamoto and Y. Okamoto, Synlett, 1998, 344–360 CrossRef CAS; (c) T. Ikai and Y. Okamoto, Chem. Rev., 2009, 109, 6077–6101 CrossRef CAS PubMed.
- (a) Y. Okamoto and T. Nakano, Chem. Rev., 1994, 94, 349–372 CrossRef CAS; (b) T. Nakano, J. Chromatogr. A, 2001, 906, 205–225 CrossRef CAS PubMed; (c) T. Nakano and Y. Okamoto, Chem. Rev., 2001, 101, 4013–4038 CrossRef CAS PubMed; (d) E. Yashima, K. Maeda, H. Iida, Y. Furusho and K. Nagai, Chem. Rev., 2009, 109, 6102–6211 CrossRef CAS PubMed.
- (a) T. Aoki, T. Kaneko and M. Teraguchi, Polymer, 2006, 47, 4867–4892 CrossRef CAS; (b) T. Masuda, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 165–180 CrossRef CAS; (c) E. Yashima and K. Maeda, Macromolecules, 2008, 41, 3–12 CrossRef CAS; (d) E. Yashima, K. Maeda and Y. Furusho, Acc. Chem. Res., 2008, 41, 1166–1180 CrossRef CAS PubMed; (e) K. Akagi, Chem. Rev., 2009, 109, 5354–5401 CrossRef CAS PubMed; (f) J. Liu, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799–5867 CrossRef CAS PubMed; (g) C. Song, X. Liu, D. Liu, C. Ren, W. Yang and J. Deng, Macromol. Rapid Commun., 2013, 34, 1426–1445 CrossRef CAS PubMed.
- (a) E. Yashima, Y. Maeda and Y. Okamoto, Polym. J., 1999, 31, 1033–1036 CrossRef CAS; (b) F. Sanda, H. Araki and T. Masuda, Chem. Lett., 2005, 34, 1642–1643 CrossRef CAS; (c) K. Maeda, K. Tanaka, K. Morino and E. Yashima, Macromolecules, 2007, 40, 6783–6785 CrossRef CAS; (d) Z. Tang, H. Iida, H.-Y. Hu and E. Yashima, ACS Macro Lett., 2012, 1, 261–265 CrossRef CAS; (e) D. Zhang, C. Ren, W. Yang and J. Deng, Macromol. Rapid Commun., 2012, 33, 652–657 CrossRef CAS PubMed; (f) H. Iida, Z. Tang and E. Yashima, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2869–2879 CrossRef CAS.
- (a) R. Y. Liu, F. Sanda and T. Masuda, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4175–4182 CrossRef CAS; (b) F. Sanda, T. Fujii, J. Tabei, M. Shiotsuki and T. Masuda, Macromol. Chem. Phys., 2008, 209, 112–118 CrossRef CAS; (c) X. Du, J. Liu, J. Deng and W. Yang, Polym. Chem., 2010, 1, 1030–1038 RSC; (d) L. Li, X. Du, J. Deng and W. Yang, React. Funct. Polym., 2011, 71, 972–979 CrossRef CAS.
- (a) E. Yashima, S. Huang and Y. Okamoto, J. Chem. Soc., Chem. Commun., 1994, 1811–1812 RSC; (b) E. Yashima, T. Matsushima, T. Nimura and Y. Okamoto, Korea Polym. J., 1996, 4, 139–146 CAS; (c) Y. Naito, Z. Tang, H. Iida, T. Miyabe and E. Yashima, Chem. Lett., 2012, 41, 809–811 CrossRef CAS; (d) C. Zhang, F. Liu, Y. Li, X. Shen, X. Xu, R. Sakai, T. Satoh, T. Kakuchi and Y. Okamoto, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2271–2278 CrossRef CAS.
- Solid membranes for enantioselective permeations have also been demonstrated, see: (a) M. Teraguchi, J. Suzuki, T. Kaneko, T. Aoki and T. Masuda, Macromolecules, 2003, 36, 9694–9697 CrossRef CAS; (b) T. Aoki, T. Fukuda, K. I. Shinohara, T. Kaneko, M. Teraguchi and M. Yagi, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 4502–4517 CrossRef CAS; (c) S. Hadano, M. Teraguchi, T. Kaneko and T. Aoki, Chem. Lett., 2007, 36, 220–221 CrossRef CAS.
- (a) E. Yashima, J. Noguchi and Y. Okamoto, Macromolecules, 1995, 28, 8368–8374 CrossRef CAS; (b) T. Nakano, Y. Satoh and Y. Okamoto, Polym. J., 1998, 30, 635–640 CrossRef CAS; (c) T. Miyabe, H. Iida, M. Banno, T. Yamaguchi and E. Yashima, Macromolecules, 2011, 44, 8687–8692 CrossRef CAS.
- (a) C. Nuckolls, T. J. Katz and L. Castellanos, J. Am. Chem. Soc., 1996, 118, 3767–3768 CrossRef CAS; (b) C. Nuckolls, T. J. Katz, G. Katz, P. J. Collings and L. Castellanos, J. Am. Chem. Soc., 1999, 121, 79–88 CrossRef CAS; (c) K. Nakamura, H. Okubo and M. Yamaguchi, Org. Lett., 2001, 3, 1097–1099 CrossRef CAS PubMed; (d) H. Okubo, D. Nakano, S. Anzai and M. Yamaguchi, J. Org. Chem., 2001, 66, 557–563 CrossRef CAS PubMed.
- T. Tsuruta, J. Polym. Sci., Part D: Macromol. Rev., 1972, 6, 179–250 CrossRef CAS.
- (a) E. Yashima, S. Huang, T. Matsushima and Y. Okamoto, Macromolecules, 1995, 28, 4184–4193 CrossRef CAS; (b) M. A. Saito, K. Maeda, H. Onouchi and E. Yashima, Macromolecules, 2000, 33, 4616–4618 CrossRef CAS; (c) K. Morino, K. Maeda, Y. Okamoto, E. Yashima and T. Sato, Chem.–Eur. J., 2002, 8, 5112–5120 CrossRef CAS PubMed.
- The stereoregularity of the polymers was investigated by laser Raman spectroscopy, which revealed that poly-1s is rich in cis-transoid (Fig. S2†).16.
- (a) H. Shirakawa, T. Ito and S. Ikeda, Polym. J., 1973, 4, 460–462 CrossRef CAS; (b) M. Tabata, Y. Tanaka, Y. Sadahiro, T. Sone, K. Yokota and I. Miura, Macromolecules, 1997, 30, 5200–5204 CrossRef CAS; (c) M. Tabata, T. Sone, Y. Mawatari, D. Yonemoto, A. Miyasaka, T. Fukushima and Y. Sadahiro, Macromol. Symp., 2003, 192, 75–97 CrossRef.
- The highest-MW part of poly-1-rac (poly-1-racA) (Table 1, entry 4) may be composed of aggregates of poly-1-rac, since the Mn of poly-1-racA became lower than that before fractionation (Fig. S4d†).
- The accurate molecular weights of poly-1s could not be determined by SEC using polystyrene standards, and we recorded the matrix-assisted laser desorption/ionisation time-of-flight mass (MALDI-TOF-MS) spectra of poly-1-MB, poly-1-MC and poly-1-MD (Fig. S5†), which suggested that the MWs of poly-1s may be relatively higher than those estimated by SEC. A similar tendency was reported by Kumazawa et al.; the MWs of poly(phenylacetylene)s calculated by MALDI-TOF MS were 1.5 times larger than those estimated by SEC. See, S. Kumazawa, J. Rodriguez Castanon, N. Onishi, K. Kuwata, M. Shiotsuki and F. Sanda, Organometallics, 2012, 31, 6834–6842 CrossRef CAS.
- (a) S.-i. Sakurai, K. Okoshi, J. Kumaki and E. Yashima, Angew. Chem., Int. Ed., 2006, 45, 1245–1248 CrossRef CAS PubMed; (b) S.-i. Sakurai, K. Okoshi, J. Kumaki and E. Yashima, J. Am. Chem. Soc., 2006, 128, 5650–5651 CrossRef CAS PubMed.
- K. Nagai, K. Sakajiri, K. Maeda, K. Okoshi, T. Sato and E. Yashima, Macromolecules, 2006, 39, 5371–5380 CrossRef CAS.
- We attempted to observe the helical structures of poly-1s by high-resolution atomic force microscopy (AFM),6d,22 but it was difficult because the polymers were too short in length to form two-dimensional crystals on a substrate that was essential to observe the helical structures of the helical polyacetylenes, polyisocyanides and foldamers.19,23.
- J. Kumaki, S.-i. Sakurai and E. Yashima, Chem. Soc. Rev., 2009, 38, 737–746 RSC.
- (a) T. Kajitani, K. Okoshi, S. I. Sakurai, J. Kumaki and E. Yashima, J. Am. Chem. Soc., 2006, 128, 708–709 CrossRef CAS PubMed; (b) M. Banno, T. Yamaguchi, K. Nagai, C. Kaiser, S. Hecht and E. Yashima, J. Am. Chem. Soc., 2012, 134, 8718–8728 CrossRef CAS PubMed.
- (a) Y. Okamoto, K. Suzuki, K. Ohta, K. Hatada and H. Yuki, J. Am. Chem. Soc., 1979, 101, 4763–4765 CrossRef CAS; (b) Y. Okamoto, H. Shohi and H. Yuki, J. Polym. Sci., Part C: Polym. Lett., 1983, 21, 601–607 CrossRef CAS.
- H. Yuki, Y. Okamoto and I. Okamoto, J. Am. Chem. Soc., 1980, 102, 6356–6358 CrossRef CAS.
- For representative examples of chiral recognition by helicene derivatives, see ref. 12d and: (a) M. Nakazaki, K. Yamamoto, T. Ikeda, T. Kitsuki and Y. Okamoto, J. Chem. Soc., Chem. Commun., 1983, 787–788 RSC; (b) K. Deshayes, R. D. Broene, I. Chao, C. B. Knobler and F. Diederich, J. Org. Chem., 1991, 56, 6787–6795 CrossRef CAS; (c) D. J. Weix, S. D. Dreher and T. J. Katz, J. Am. Chem. Soc., 2000, 122, 10027–10032 CrossRef CAS; (d) E. Murguly, R. McDonald and N. R. Branda, Org. Lett., 2000, 2, 3169–3172 CrossRef CAS PubMed; (e) M. T. Reetz and S. Sostmann, Tetrahedron, 2001, 57, 2515–2520 CrossRef CAS; (f) D. Z. G. Wang and T. J. Katz, J. Org. Chem., 2005, 70, 8497–8502 CrossRef CAS PubMed; (g) W. Ichinose, M. Miyagawa, Z. An and M. Yamaguchi, Org. Lett., 2012, 14, 3123–3125 CrossRef CAS PubMed.
- A series of [4]helicene-based oligomers able to form self-aggregates in which the chirality of the helicene units significantly affects the self-assembly has also been reported.1a,3j,12c.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis and characterisation of poly-1s, the enantioselective adsorption on poly-1-M and poly-1-P and the molecular modeling and calculations of poly-1-M. See DOI: 10.1039/c4py00692e |
| This journal is © The Royal Society of Chemistry 2014 |