Open Access Article
Open Access ArticleSynthesis of donor–acceptor conjugated polymers based on benzo[1,2-b:4,5-b′]dithiophene and 2,1,3-benzothiadiazole via direct arylation polycondensation: towards efficient C–H activation in nonpolar solvents†
Xiaochen
Wang
and
Mingfeng
Wang
*
School of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459, Singapore. E-mail: mfwang@ntu.edu.sg; Fax: +65 6794 7553; Tel: +65 6316 8746
First published on 17th June 2014
Abstract
This article describes the synthesis of donor–acceptor (D–A) type copolymers based on benzo[1,2-b:4,5-b′]dithiophene and 2,1,3-benzothiadiazole via direct-arylation cross-coupling polycondensation. To achieve high performance polymerization, we have systematically investigated the reaction factors including catalysts, solvents, ligands, bases, additives, concentration of reactants and phase transfer agents. In particular, 1,2-dimethylbenzene (ODMB), as a nonpolar high boiling point solvent, is a superior medium to perform this direct-arylation polymerization. In this nonpolar aromatic solvent, Pd2dba3/(o-MeOPh)3P, accompanied with a base potassium carbonate and an additive pivalic acid, serves as an efficient catalyst system to obtain high-quality polymers. Our optimized condition gave the polymer with a weight-average molecular weight (Mw) as high as 60 kg mol−1 in nearly quantitative yield and excellent C–H selectivity.
Introduction
Conjugated polymers (CPs), due to their excellent optical and electronic properties, have attracted tremendous interest in the past decades from both academic and industrial fields. The so-called D–A conjugated polymers containing alternating electron-rich and electron-deficient units are particularly attractive due to the facile tunability of electronic structures in their conjugated backbones and consequently optoelectronic properties of polymers.1–6 To date, most of D–A conjugated polymers have been synthesized by classic Stille coupling polycondensation or Suzuki coupling polycondensation.7–9 But these reactions show disadvantages such as the necessity of prefunctionalizing monomers (arylstannanes or arylboron derivatives) using flammable and nonstable butyllithium. Other issues, particularly for Stille coupling, include the difficulty of purifying arylstannane monomers and the formation of toxic byproducts. To synthesize conjugated polymers via an economically efficient, safe and environment-friendly approach, special attention has been paid to carbon–hydrogen (C–H) direct arylation cross-coupling reaction.9–12The protocols of C–H direct arylation towards synthesis of conjugated polymers have usually been borrowed from what has been learned from the synthesis of small organic molecules for pharmaceutics.13,14 For example, Fagnou et al. have explored an effective synthetic protocol, which involves palladium acetate (Pd(OAc)2) as a catalyst, N,N-dimethylacetamide (DMAc) as a solvent, potassium carbonate (K2CO3) as a base and pivalic acid (PivOH) as an additive, for direct arylation of aromatic compounds.15,16 This synthetic protocol has been successfully applied to the synthesis of many conjugated polymers.17–29 The catalyst Pd(OAc)2 is particularly efficient in highly polar solvents such as DMAc, in which most low polar or nonpolar conjugated polymers show limited solubility. Therefore, highly polar solvents are not ideal reaction media for synthesis of conjugated polymers, particularly for those decorated with hydrophobic alkyl side chains.
Very recently, there has been some progress in exploring low polar or nonpolar solvents as the reaction media for synthesis of conjugated polymers. For instance, Ozawa et al. have used tetrahydrofuran (THF) instead of highly polar solvents to synthesize poly(3-hexylthiophene-2,5-diyl) (P3HT), to ensure the solubility of the resulting polymers during polymerization.30 Herrmann's catalyst (trans-Di-μ-acetatobis[2-[bis(2-methylphenyl)phosphine]benzyl]dipalladium), in the presence of an appropriate ligand, was proven to be an effective catalyst in this reaction system to afford high-molecular-weight P3HT with high regioregularity (98%), whereas the reaction catalyzed with Pd(OAc)2 in the same solvent was not reproducible and frequently provided low molecular weight products. Later, Leclerc et al. have utilized this reaction condition with modifications to synthesize a series of D–A type conjugated polymers.31–36 The reaction was typically performed with heating at 120 °C. The overheated solvent (THF) and the necessity of using a sealed and pressurized reaction container, however, may compromise the reproducibility of the polymerization and raise the cost as well as safety concerns for performing and scaling up the synthesis. In addition, some monomers cannot be polymerized in THF with Herrmann's catalyst.36 More recently, Ozawa et al. have reported another efficient catalytic system based on tris(dibenzylideneacetone)dipalladium(0)-chloroform adducts (Pd2(dba)3·CHCl3) for polycondensation of 2,7-dibromo-9,9-dioctylfluorene and 1,2,4,5-tetrafluorobenzene.37 This catalytic system was sufficiently reactive in both THF and in toluene to afford the polyphenylene derivative with high molecular weight and high yield.
Despite these recent advances, little study has been carried out in exploring high boiling point nonpolar solvents for efficient synthesis of conjugated polymers via direct arylation polycondensation. Even less has been understood with reaction factors that affect the direct arylation polycondensation in nonpolar solvents.
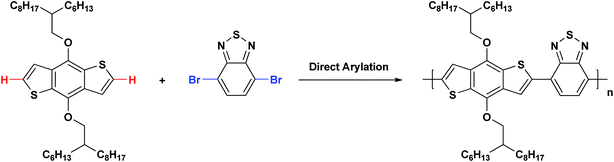
In this article, we report such a direct arylation polycondensation system. Our target polymer, denoted as PBDTBT, consists of alternating benzo[1,2-b:4,5-b′]dithiophene (BDT) as an electron donor (D) and 2,1,3-benzothiadiazole (BT) as the electron acceptor (A). Both BDT and BT have been among the most popular building blocks in a variety of D–A conjugated polymer semiconductors.1–6,38–47 Two long branched 2-hexyldecyloxy groups were introduced to the BDT segment to afford good solubility of PBDTBT in a variety of solvents.
To optimize the polymerization of BDT and BT under the scheme of direct arylation, we have systematically examined a broad range of factors, including a series of low polar or nonpolar solvents, catalysts, ligands, bases, additives, reactant concentrations and phase transfer agents. Our optimized condition for direct arylation gives high molecular weight PBDTBT in a nearly quantitative yield with good regioregularity.
Results and discussion
Scheme 1 shows the synthetic route to the polymer PBDTBTvia palladium-catalyzed direct arylation coupling reaction between monomers 4,8-di(2-hexyldecyloxy)benzo[1,2-b:4,5-b′]dithiophene (HDBDT) and 4,7-dibromobenzothiadiazole (BrBT). The following sections describe our systematic study of the factors that influence this direct arylation polymerization.Effect of catalysts
Palladium(0) (Pd(0)) usually serves as the major active species in most of palladium-catalyzed cross-coupling reactions. However, due to labile characteristics, the Pd(0) complexes usually need to be reduced in situ from palladium(II) (Pd(II)) complexes and manifolded with varying coordination shells to form reactive species.48,49 So the chemical characteristics of palladium catalysts, also known as catalytic precursors, are important to determine their activity in a cross-coupling reaction. We examined activities of different palladium complexes for polymerizing HDBDT and BrBT. As shown in Table 1, the polymerization using Herrmann's catalyst in THF resulted in PBDTBT with a low number-average molecular weight (Mn) of 4.1 kg mol−1, indicating that the activity of the Herrmann's catalyst is not sufficient to activate the C–H bonds at positions 2- and 6- of BDT to form high molecular weight polymers. As can be expected, PBDTBT prepared using Pd(OAc)2 in DMAc precipitated from the reaction system in the process of polymerization and formed metallic luster polymer pellets. The solubility limitation of the resulting polymer in DMAc blocked further growth of polymer chains, especially for the molecules surrounded by others in the precipitated particles. As a result, using DMAc as the reaction medium gave the polymer PBDTBT with a relatively high polydispersity index (PDI) of 2.9. Compared with Pd(OAc)2 in DMAc, the Pd2dba3 catalyst in THF led to similar weight-average molecular weight (Mw), but improved Mn with a relatively low PDI of 2.0.| Entry | Catalyst | Ligand | Solvent | Base | Yield (%) | M n (kg mol−1) | M w (kg mol−1) | PDI |
|---|---|---|---|---|---|---|---|---|
| a HDBDT (0.2 mmol) and BrBT (0.2 mmol) were polymerized in solvent (1 mL), in the presence of catalyst (5 mol%), ligand (10 mol%), base (0.6 mmol) and PivOH (0.06 mmol), at 100 °C for 24 h. | ||||||||
| 1 | Pd(OAc)2 | PCy3·HBF4 | DMAc | K2CO3 | 98 | 11.0 | 32.2 | 2.9 |
| 2 | Herrmann's catalyst | (o-MeOPh)3P | THF | Cs2CO3 | 88 | 4.1 | 9.8 | 2.4 |
| 3 | Pd2dba3 | (o-MeOPh)3P | THF | Cs2CO3 | 99 | 16.0 | 32.8 | 2.0 |
To further compare the polymers prepared using these different catalytic systems, absorption spectra of the polymers were collected (Fig. 1). As expected, PBDTBT synthesized using Herrmann's catalyst/THF, due to its much lower molecular weight, shows an absorption peak at a shorter wavelength (545 nm) than the other two polymers synthesized using Pd(OAc)2/DMAc (568 nm) and Pd2dba3/THF (638 nm), respectively. Surprisingly, the polymers synthesized using Pd(OAc)2/DMAc and Pd2dba3/THF, respectively, despite their similar Mw, show much different optical absorption properties. The latter shows an absorption peak and the onset at longer wavelengths, corresponding to a longer average conjugation length. The average conjugation length of CPs is determined by maximum effective conjugation length, degree of polymerization (molecular weight) and structural defects.4 Here, the difference of the conjugation length between PBDTBTs prepared with Pd(OAc)2/DMAc and Pd2dba3/THF, respectively, might be mainly attributed to the structural defects, which is further discussed in the final section (Optical properties). The possible structural defects in PBDTBTs prepared by direct arylation polymerizations are shown in Scheme 2.
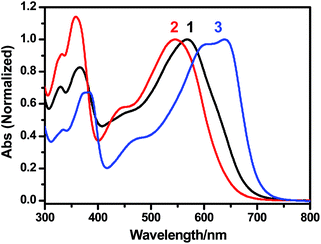 | ||
| Fig. 1 Absorption spectra of chloroform solutions of PBDTBTs synthesized with different catalysts: line 1, 2, and 3 correspond to the polymers synthesized under the condition shown in entry 1, 2, and 3, respectively, in Table 1. | ||
To obtain more information about the molecular structures of PBDTBTs synthesized using Pd(OAc)2/DMAc and Pd2dba3/THF, 1H-NMR spectra of these polymers were collected. As highlighted with green rectangles in Fig. 2, marked regio-irregular sequence peaks at around 7.9 and 8.8 ppm are observed in the spectrum (line a) of PBDTBT synthesized using Pd(OAc)2/DMAc. These two peaks may be assigned to the protons of 6-unsubstituted benzo[1,2-b:4,5-b′]dithiophene and protons at 5 and 6 positions of benzothiodiazole which linked on the 3 and/or 7 positions of BDT, respectively, as shown in Scheme 2. In contrast, only negligible peaks exist in those regions for the polymer synthesized using Pd2dba3/THF (line b).
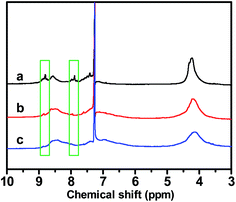 | ||
| Fig. 2 1H-NMR spectra of PBDTBTs synthesized under different conditions: line a and b correspond to the polymers synthesized under the condition shown in entry 1 and 3 in Table 1; line c corresponds to the polymer synthesized under the optimized condition in ODMB (entry 1, Table 5). | ||
All the results described above indicate that Pd2dba3 is a superior catalyst in THF, compared to Herrmann's catalyst in the same solvent and Pd(OAc)2 in DMAc for direct arylation polymerization of HDBDT and BrBT. Therefore, in the following sections, we focus on Pd2dba3 catalyst and discuss how other factors influence the direct arylation polymerization.
Effect of solvents
For most reactions involving C–H direct arylation coupling, transition-metal based catalysts, largely Pd(OAc)2, show relatively higher reactivity in polar solvents than in nonpolar ones. Nevertheless, the influence of solvents on direct arylation still remains complex, as other factors also affect the polymerization result. To explore solvents appropriate for the Pd2dba3-catalyzed direct arylation polymerization, we systematically studied this polymerization in a variety of solvents, including THF, 1,4-dioxane (DIO), anisole (methoxybenzene, MOB), toluene (methylbenzene, MB), xylene (dimethylbenzene, DMB), mesitylene (1,3,5-trimethylbenzene, TMB), ODMB and tetrahydronaphthalene (THN). The chemical structures of the solvents used in the direct arylation polymerizations are summarized in Scheme 3. As shown in Table 2, the common low polar or nonpolar ether and arene solvents can serve as the reaction media for efficient Pd2dba3-catalyzed direct arylation. Interestingly, all the tested solvents give the polymers with a rather high yield (≥95%) but quite different molecular weights. Although no clear relationship between polarity of the reaction solvent and molecular weight of the resulting polymers can be concluded from these experiments, the ether solvents with higher polarity than the arene solvents tend to give polymers with higher molecular weights. In addition, it appears that some extremely high boiling point solvents (e.g. TMB and THN) are unfavorable for the Pd2dba3-catalyzed polymerization. | ||
| Scheme 3 Chemical structures and abbreviations of solvents used in the direct arylation polymerization. | ||
| Entry | Solvent | Boiling point (°C) | Dielectric constant | Dipole moment (10−10 C m) | Yield (%) | M n (kg mol−1) | M w (kg mol−1) | PDI |
|---|---|---|---|---|---|---|---|---|
| a The physical properties of the solvents were from ref. 50. b HDBDT (0.2 mmol) and BrBT (0.2 mmol) were polymerized in a solvent (1 mL), in the presence of Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), Cs2CO3 (0.6 mmol) and PivOH (0.06 mmol), at 100 °C for 24 h. | ||||||||
| 1 | THF | 66 | 7.58 | 5.67 | 99 | 16.0 | 32.8 | 2.0 |
| 2 | DIO | 101.3 | 2.209 | 1.50 | 97 | 14.1 | 31.0 | 2.2 |
| 3 | MOB | 153.7 | 4.33 | 4.00 | 96 | 13.2 | 23.7 | 1.8 |
| 4 | MB | 110.6 | 2.24 | 1.23 | 99 | 13.6 | 24.3 | 1.8 |
| 5 | DMB | 137–140 | — | — | 96 | 11.3 | 22.5 | 2.0 |
| 6 | TMB | 164.7 | 2.279 | 0.23 | 95 | 6.8 | 11.9 | 1.7 |
| 7 | ODMB | 144.4 | 2.266 | 1.47 | 98 | 14.2 | 31.1 | 2.2 |
| 8 | THN | 207.6 | 2.733 | 1.33 | 95 | 5.2 | 8.2 | 1.6 |
It is worthwhile to note that, in ODMB, the polymerization gives a comparable result to that in THF, indicating that ODMB is a promising reaction medium for Pd2dba3-catalyzed coupling polymerization. As mentioned in Introduction, most of direct arylation polymerizations for the synthesis of conjugated polymers have been carried out in high-boiling-point, highly polar solvents such as DMAc, in which high-molecular-weight polymer products often show limited solubility. In contrast, ODMB described above is a nonpolar aromatic solvent with a boiling point of 144.4 °C. Based on the “like dissolves like” principle, ODMB should possess good solubility for aromatic compounds, particularly for nonpolar and low polar molecules. Therefore, ODMB should be favorable for synthesis of a broad range of conjugated polymers. In addition, the significantly higher boiling point of ODMB than that of THF allows polymerization to be readily performed under ambient pressure.
Effect of ligands
Tris(2-methoxyphenyl)phosphine ((o-MeOPh)3P) served as an exceptionally effective ligand for Pd2dba3-catalyzed coupling copolymerization of HDBDT and BrBT in ODMB. There was no polymerization, however, when (o-MeOPh)3P was absent or replaced with other ligands, such as triphenylphosphane (Ph3P), tris(2-methylphenyl)phosphine ((o-Tol)3P), and tri-t-butyl phosphine ((t-Bu)3P). The coordinating ability of ortho-methoxy groups may play a particularly important role in the high efficiency of (o-MeOPh)3P in the polymerization.37Effect of bases
The presence of a base is necessary in a transition metal catalyzed coupling reaction to promote the efficiency of catalysts and increase the yield of products. The role of a base is not only to abstract protons and neutralize acids introduced and/or produced in the reaction system, but also to activate catalysts and facilitate regeneration of the reactive species. The performance of bases in reactions is determined by many factors, including the intrinsic factors such as basicity, nucleophilicity, and steric hindrance, as well as the extrinsic factors such as solubility, ionization ability, aggregation state, and coordination ability.The results of copolymerization of HDBDT and BrBT in the presence of different bases are summarized in Table 3. Carbonates were examined firstly, due to their wide use in previous direct arylation polymerizations. Among the tested carbonates, potassium carbonate (K2CO3) gave optimal results; cesium carbonate (Cs2CO3) showed an acceptable polymerization result; sodium carbonate (Na2CO3) was ineffective, only giving trace oligomer/polymer; calcium carbonate (CaCO3) and barium carbonate (BaCO3) did not give any oligomer/polymer at all after a brief purification procedure.
| Entry | Base | Yield (%) | M n (kg mol−1) | M w (kg mol−1) | PDI |
|---|---|---|---|---|---|
| a HDBDT (0.2 mmol) and BrBT (0.2 mmol) were polymerized in ODMB (1 mL), in the presence of Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), base and PivOH (0.06 mmol), at 100 °C for 24 h. | |||||
| 1 | Cs2CO3 (3 eq.) | 98 | 14.2 | 31.1 | 2.2 |
| 2 | K2CO3 (3 eq.) | 98 | 19.5 | 46.5 | 2.4 |
| 3 | Na2CO3 (3 eq.) | Trace | — | — | — |
| 4 | CaCO3 (3 eq.) | 0 | — | — | — |
| 5 | BaCO3 (3 eq.) | 0 | — | — | — |
| 6 | K3PO4 (3 eq.) | 96 | 18.0 | 39.7 | 2.2 |
| 7 | KOAc (3 eq.) | 85 | 5.4 | 8.5 | 1.6 |
| 8 | t-BuOK (3 eq.) | 0 | — | — | — |
| 9 | TEA (3 eq.) | 0 | — | — | — |
| 10 | DIPEA (3 eq.) | 0 | — | — | — |
| 11 | DBU (3 eq.) | 0 | — | — | — |
| 12 | TEDA (3 eq.) | 0 | — | — | — |
| 13 | K2CO3 (2 eq.) | 97 | 18.6 | 42.8 | 2.3 |
| 14 | K2CO3 (5 eq.) | 98 | 22.0 | 54.4 | 2.5 |
| 15 | K2CO3 (10 eq.) | 97 | 20.2 | 47.3 | 2.3 |
The performance of carbonates should be related to their basicity and solubility. Solubility of the carbonates, both in typical solvents51,52 and water, follows an order of Cs2CO3 > K2CO3 > Na2CO3 > CaCO3 ≈ BaCO3. In a Pd-catalyzed cross-coupling reaction, it is important to maintain a reasonable concentration of basic anions in the reaction system.53 We speculate that the concentration of carbonate from K2CO3 in ODMB at the polymerization temperature (i.e. 100 °C) should be at a favorable level, compared to other carbonates, for the direct arylation polymerization of HDBDT and BrBT.
As K2CO3 showed better performance than other metal carbonates in the direct arylation polymerization, we further tested potassium bases with other anions. The polymerization results are shown in Table 3. Potassium phosphate (K3PO4) is also an effective base, outranked only by K2CO3. Compared to K3PO4, potassium acetate (KOAc) gives polymer products with a lower reaction yield and a lower Mn, which may be caused by its weak alkaline and less effective coordination with reactive catalytic centers. When potassium tert-butoxide (t-BuOK) was added to the polymerization system, the reaction mixture turned to dark brown while being heated in an oil bath. This phenomenon suggested that some reagents were decomposed or some side reactions occurred in the presence of such a strong base.
Finally, organic bases such as triethylamine (TEA), diisopropylethylamine (DIPEA), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and triethylenediamine (TEDA) were tested in the copolymerization of HDBDT and BrBT. But none of them resulted in the formation of polymer products. The presence of organic bases may lead to debromination of dibromobenzothiadiazole as the main reaction54 and consequently interferes with the polymerization.
As K2CO3 showed the best performance among all the bases tested above, we further optimized the equivalence of K2CO3 (entries 2, 13, 14 and 15, Table 3) in the reaction mixtures. With the increase of K2CO3 from 2 to 5 equivalents, the molecular weight of the resulting polymers increased gradually. Further increase of the amount of K2CO3 to 10 equivalents, the molecular weight of the resulting polymers cannot be further improved.
Effect of additives
Carboxylate additives play an important role in transition metal catalyzed direct arylation reactions. It has been proposed that C–H bond transformations assisted by carboxylates proceed via a mechanism in which metalation takes place via a concerted base-assisted deprotonation.55 Fagnou et al. have proved that the pivalate anion is a key component in cleaving of a C–H bond by lowering the cleavage energy of the C–H bond and acting as a catalytic proton shuttle from an unactivated substrate to the stoichiometric carbonate base.15 In addition, DIPEA as an alternative to carboxylates shows improved selectivity in some cases.56The effect of additives on the Pd2dba3-catalyzed copolymerization of HDBDT and BrBT in ODMB is summarized in Table 4. Surprisingly, the Pd2dba3-catalyzed polymerization was quenched upon addition of DIPEA as an additive. The presence of DIPEA may result in debromination of bromide monomers and thus quenches the coupling reaction.54 In contrast to DIPEA, the addition of PivOH to the reaction mixture promoted the coupling copolymerization. For example, the addition of 0.5 equivalent (vs. the monomer) PivOH resulted in a more than 6-fold increase of molecular weight of the formed polymer, compared to that of the polymer synthesized without PivOH. In addition, this increase of molecular weight was accompanied by an increase of the reaction yield from 68% to 98%. A further increase of the amount of PivOH up to 1 equivalent (vs. the monomer) did not lead to significant improvement of the polymerization (entry 5, Table 4).
| Entry | Additive | Yield (%) | M n (kg mol−1) | M w (kg mol−1) | PDI |
|---|---|---|---|---|---|
| a HDBDT (0.2 mmol) and BrBT (0.2 mmol) were polymerized in ODMB (1 mL), in the presence of Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (0.6 mmol) and additive, at 100 °C for 24 h. | |||||
| 1 | None | 68 | 3.4 | 7.7 | 2.3 |
| 2 | PivOH (0.3 eq.) | 98 | 19.5 | 46.5 | 2.4 |
| 3 | DIPEA (0.3 eq.) | 0 | — | — | — |
| 4 | PivOH (0.5 eq.) | 98 | 21.9 | 53.6 | 2.4 |
| 5 | PivOH (1 eq.) | 97 | 21.1 | 51.0 | 2.4 |
Effect of reactant concentration
Under the optimized reaction condition (entry 1 in Table 5), after several hours of polymerization, the resulting polymers precipitated from the reaction mixture occasionally onto the inner surface of the reaction vessel, producing a metallic luster “conjugated polymer mirror” on the inner vessel surface. One possible reason for the early precipitation of polymer products during the reaction can be the very high concentration (>160 mg mL−1) of the formed polymers in the reaction medium. The precipitation of polymers from the concentrated reaction medium may limit the formation of high molecular weight polymers. To resolve the issue of precipitation, we added an extra solvent, ODMB, to dilute the reaction mixture. The polymerization results at different concentrations are summarized in Table 5. Unfortunately, the dilution of the reaction mixture slowed down the polymerization significantly. Although the yield of the reaction was little affected upon dilution of the reaction mixture, the average molecular weight of the polymer products fell gradually with the decrease of the reactant concentration from 0.2 to 0.05 M.| Entry | Concentration | Yield (%) | M n (kg mol−1) | M w (kg mol−1) | PDI |
|---|---|---|---|---|---|
| a HDBDT (0.2 mmol) and BrBT (0.2 mmol) were polymerized in ODMB (1, 2, or 4 mL), in the presence of Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (1 mmol) and PivOH (0.1 mmol), at 100 °C for 24 h. | |||||
| 1 | 0.2 M | 98 | 24.5 | 60.1 | 2.4 |
| 2 | 0.1 M | 99 | 19.5 | 45.0 | 2.3 |
| 3 | 0.05 M | 97 | 13.8 | 26.6 | 1.9 |
Effect of phase transfer agents
As discussed above, pivalic acid has been proven to be one of the most effective additives to enhance the reactivity of C–H bonds. Thompson et al. have proposed that in situ generated carboxylate anions acted as a soluble organic base to promote the reaction.9 From this point of view, it appears that increasing the solubility of an inorganic base (e.g. K2CO3) in a reaction system might benefit direct arylation cross-coupling. Phase transfer agents (PTAs) have been widely used in heterogeneous reactions to facilitate the migration of an inorganic base from an aqueous phase or a solid state into an organic phase where the reaction occurs. Here, we studied the effect of a series of PTAs, including 1,4,7,10,13,16-hexaoxacyclooctadecane (18-Crown-6), aliquat336, tetra-n-butylammoniumbromide (TBAB), tetra-n-butylammoniumfluoride (TBAF), and tetra-n-butylammonium hexafluorophosphate (TBAPF6) on the direct arylation polymerization in ODMB. The results (Table 6) appeared to be too scattered to reach a clear conclusion. While the addition of 18-Crown-6, TBAPF6 or distilled water gave polymers with relatively low molecular weights; quaternary ammonium salts associated with simple anions (i.e. halide ions, including chloride ion in Aliquat 336, bromide ion in TBAB and fluoride ion in TBAF, respectively) nearly quenched the polymerization. This effect might have been caused by these extra anions from PTAs (if any) and the inorganic base solubilized by PTAs, that may block the free coordination sites of low-ligated Pd(0) complexes, which is necessary for the turnover of the catalyst.53 As a result, the reactions were slowed down or even quenched.| Entry | PTA | Yield (%) | M n (kg mol−1) | M w (kg mol−1) | PDI |
|---|---|---|---|---|---|
| a HDBDT (0.2 mmol) and BrBT (0.2 mmol) were polymerized in ODMB (2 mL), in the presence of Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (1 mmol), PivOH (0.1 mmol) and PTA (0.06 mmol), at 100 °C for 24 h. | |||||
| 1 | None | 99 | 19.5 | 45.0 | 2.3 |
| 2 | 18-Crown-6 | 96 | 10.2 | 16.7 | 1.6 |
| 3 | Aliquat336 | Trace | — | — | — |
| 4 | TBAB | Trace | — | — | — |
| 5 | TBAF | Trace | — | — | — |
| 6 | TBAPF6 | 99 | 16.8 | 35.9 | 2.1 |
| 7 | Water (0.4 mL) | 99 | 9.2 | 15.7 | 1.7 |
Optical properties
We further studied the optical properties of the polymers synthesized under different conditions of direct arylation polymerizations described above. The absorption spectra of some representative polymers with different molecular weights synthesized in ODMB are presented in Fig. 3. The PBDTBT synthesized using Pd(OAc)2/DMAc is also included for comparison. Table 7 summarizes the optical data, including absorption peak wavelengths (λabs), onset-absorption wavelengths (λonset), and full width at half maximum absorption (FWHM) of the polymers.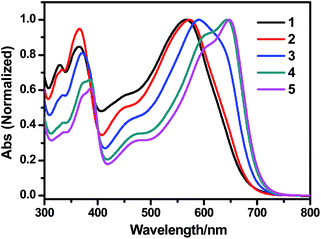 | ||
| Fig. 3 Absorption spectra of chloroform solutions of PBDTBTs synthesized under different conditions: lines 1–5 correspond to the polymers entry 1–5 listed in Table 7 with the same sequence. | ||
| Entry | M n/Mw (kg mol−1) | Polymerization condition | λ abs in CHCl3 (nm) | λ onset in CHCl3 (nm) | FWHM (nm) |
|---|---|---|---|---|---|
| a Details of the polymerizations: DMAc (1 mL), Pd(OAc)2 (5 mol%), PCy3·HBF4 (10 mol%), K2CO3 (0.6 mmol), and PivOH (0.06 mmol). b ODMB (1 mL), Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), KOAc (0.6 mmol) and PivOH (0.06 mmol). c ODMB (2 mL), Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (1 mmol), PivOH (0.1 mmol) and water (0.4 mL). d ODMB (2 mL), Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (1 mmol), PivOH (0.1 mmol) and TBAPF6 (0.06 mmol). e ODMB (1 mL), Pd2dba3 (5 mol%), (o-MeOPh)3P (10 mol%), K2CO3 (1 mmol) and PivOH (0.1 mmol). All these polymerizations were carried out with HDBDT (0.2 mmol) and BrBT (0.2 mmol) at 100 °C for 24 h. | |||||
| 1 | 11.0/32.2 | Pd(OAc)2/DMAca | 329, 365, 567 | 678 | 210 |
| 2 | 5.4/8.5 | Pd2(dba)3/ODMBb | 333, 366, 572 | 685 | 185 |
| 3 | 9.2/15.7 | Pd2(dba)3/ODMBc | 334, 370, 590 | 691 | 163 |
| 4 | 16.8/35.9 | Pd2(dba)3/ODMBd | 336, 385, 643 | 699 | 139 |
| 5 | 24.5/60.1 | Pd2(dba)3/ODMBe | 337, 387, 648 | 702 | 128 |
As expected, for the polymers synthesized in ODMB, gradual bathochromic shifts of both the absorption peak and the onset of absorption were observed with the increase of the molecular weight, accompanied with a decrease of the FWHM. The PBDTBT prepared using Pd(OAc)2/DMAc (line 1) showed a slight hypsochromic shift in its absorption spectrum, compared with the PBDTBT synthesized using Pd2dba3/ODMB and with a much lower molecular weight (line 2). In other words, the conjugation length of PBDTBT prepared using Pd(OAc)2/DMAc with an Mn/Mw of 11.0/32.2 kg mol−1, was even inferior to that of PBDTBT polymerized in ODMB with an Mn/Mw of 5.4/8.5 kg mol−1. These results imply that some structural defects, presumably caused by the relatively poor regioselectivity in the polymerization, may exist in the polymer prepared using Pd(OAc)2/DMAc.9,11,22,23,27
To further probe the structure of the polymers synthesized in ODMB, 1H-NMR spectrum of a representative PBDTBT prepared under the optimized reaction condition (entry 1 in Table 5) was collected and the result is presented in Fig. 2 for comparison. A close inspection of the NMR spectrum reveals only slight distortion without any distinguishable peaks in the regions around 8.8 and 7.9 ppm. This result further suggests that the relatively poor C–H regioselectivity observed in Pd(OAc)2-catalyzed polymerization in DMAc was suppressed in the Pd2dba3-catalyzed polymerization in ODMB.
In addition, the PBDTBTs that we synthesized here via direct arylation polymerization in ODMB (line 3 and 4) shows similar or even red-shifted absorption compared with the same type of polymer with a similar Mn but synthesized via Stille coupling by other research groups.40,41 Again, these results suggest the good regioregularity of the polymers synthesized via Pd2dba3-catalyzed direct arylation polymerization in ODMB.
Conclusions
D–A type conjugated polymers based on BDT and BT have been synthesized by direct-arylation polycondensation. We have systematically investigated the reaction parameters, including catalysts, ligands, solvents, bases, additives and concentrations, and studied how each factor influences the direct arylation polycondensation. ODMB as a nonpolar and high-boiling-point solvent is a promising reaction medium to perform the direct-arylation polymerization. An optimized condition for reacting 0.2 M of HDBDT and stoichiometric BrBT with 5 mol% of Pd2dba3, 10 mol% of (o-MeOPh)3P, 5 equivalents of K2CO3, 0.5 equivalents of PivOH in ODMB gives PBDTBT with a weight-average molecular weight of 60 kg mol−1 in almost quantitative yields and good regioselectivity. We expect that the knowledge that we gain from this system of direct arylation polymerization will be useful for economically efficient and environmentally-green synthesis of a broad scope of conjugated polymers for applications in optoelectronic devices, sensing and bioimaging.Experimental section
Materials
HDBDT was synthesized according to the procedure described in a previous report.44BrBT and other reagents, solvents were of commercial grade and used as received without further purification. All reactions were performed under a nitrogen atmosphere.Measurements and characterization
The number-average molecular weights (Mn), weight-average molecular weights (Mw) and polydispersity index (PDI, Mw/Mn) of the polymers were measured by gel permeation chromatography (GPC) using an Agilent 1260 Infinity system at 30 °C, with polystyrenes as reference standard and THF as an eluent. All new compounds were characterized by nuclear magnetic resonance spectroscopy (NMR). The NMR spectra were recorded on a Bruker AV 300 spectrometer at room temperature. UV-vis absorption spectra were recorded on a Shimadzu spectrometer model UV-2450. Absorption spectra measurements of the polymer solutions were carried out in chloroform at room temperature.General procedures of polymerization
In a glove box, HDBDT (0.2 mmol), BrBT (0.2 mmol), catalyst (10 μmol), ligand (20 μmol), base, additive(s) and solvent were added in a reaction vial with a magnetic stirring bar. The vial was sealed with a rubber cap and then removed from the glove box. The vial was heated in a 100 °C oil bath for 24 hours. After being cooled to room temperature, the reaction mixture was diluted with 30 mL of chloroform and then filtered to remove the insoluble species. The filtrate was concentrated and added dropwise to 100 mL of ethanol, filtered through a Soxhlet thimble, and then subjected to Soxhlet extraction with methanol and chloroform sequentially. The chloroform fraction was concentrated and precipitated in 100 mL of ethanol. The precipitates were collected by filtration and dried under vacuum for one day to yield the target polymerWhen purifying the reaction mixtures from different batches of polymerizations, we tried to remove unreacted starting materials and low molecular weight organic and inorganic impurities and collect all the resulting polymers and oligomers present in final products in order to completely compare the polymerizations under different reaction conditions.
Acknowledgements
M.W. is grateful to the funding support by a start-up grant from Nanyang Technological University and AcRF Tier 1 (M4011061.120, RG49/12) from the Ministry of Education, Singapore.References
- X. Guo, M. Baumgarten and K. Müllen, Prog. Polym. Sci., 2013, 38, 1832 CrossRef CAS PubMed.
- B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef CAS PubMed.
- J. W. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709 CrossRef CAS PubMed.
- C. L. Chochos and S. A. Choulis, Prog. Polym. Sci., 2011, 36, 1326 CrossRef CAS PubMed.
- Y. F. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed.
- C. L. Wang, H. L. Dong, W. P. Hu, Y. Q. Liu and D. B. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed.
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed.
- B. Carsten, F. He, H. J. Son, T. Xu and L. P. Yu, Chem. Rev., 2011, 111, 1493 CrossRef CAS PubMed.
- P. P. Khlyabich, B. Burkhart, A. E. Rudenko and B. C. Thompson, Polymer, 2013, 54, 5267 CrossRef CAS PubMed.
- K. Okamoto, J. X. Zhang, J. B. Housekeeper, S. R. Marder and C. K. Luscombe, Macromolecules, 2013, 46, 8059 CrossRef CAS.
- L. G. Mercier and M. Leclerc, Acc. Chem. Res., 2013, 46, 1597 CrossRef CAS PubMed.
- K. Wang and M. F. Wang, Curr. Org. Chem., 2013, 17, 999 CrossRef CAS.
- D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed.
- L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed.
- M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496 CrossRef CAS PubMed.
- B. Liégault, D. Lapointe, L. Caron, A. Vlassova and K. Fagnou, J. Org. Chem., 2009, 74, 1826 CrossRef PubMed.
- W. Lu, J. Kuwabara and T. Kanbara, Macromolecules, 2011, 44, 1252 CrossRef CAS.
- W. Lu, J. Kuwabara, T. Iijima, H. Higashimura, H. Hayashi and T. Kanbara, Macromolecules, 2012, 45, 4128 CrossRef CAS.
- Y. Fujinami, J. Kuwabara, W. Lu, H. Hayashi and T. Kanbara, ACS Macro Lett., 2012, 1, 67 CrossRef CAS.
- W. Lu, J. Kuwabara and T. Kanbara, Polym. Chem., 2012, 3, 3217 RSC.
- J. Kuwabara, Y. Nohara, S. J. Choi, Y. Fujinami, W. Lu, K. Yoshimura, J. Oguma, K. Suenobu and T. Kanbara, Polym. Chem., 2013, 4, 947 RSC.
- A. E. Rudenko, C. A. Wiley, S. M. Stone, J. F. Tannaci and B. C. Thompson, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3691 CrossRef CAS.
- A. E. Rudenko, C. A. Wiley, J. F. Tannaci and B. C. Thompson, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2660 CrossRef CAS.
- N. I. Abdo, A. A. El-Shehawy, A. A. El-Barbary and J.-S. Lee, Eur. J. Org. Chem., 2012, 5540 CrossRef CAS.
- S.-W. Chang, H. Waters, J. Kettle, Z.-R. Kuo, C.-H. Li, C.-Y. Yu and M. Horie, Macromol. Rapid Commun., 2012, 33, 1927 CrossRef CAS PubMed.
- H. Zhao, C.-Y. Liu, S.-C. Luo, B. Zhu, T.-H. Wang, H.-F. Hsu and H.-H. Yu, Macromolecules, 2012, 45, 7783 CrossRef CAS.
- S. Kowalski, S. Allard and U. Scherf, ACS Macro Lett., 2012, 1, 465 CrossRef CAS.
- L. A. Estrada, J. J. Deininger, G. D. Kamenov and J. R. Reynolds, ACS Macro Lett., 2013, 2, 869 CrossRef CAS.
- N. I. Abdo, J. Ku, A. A. El-Shehawy, H.-S. Shim, J.-K. Min, A. A. El-Barbary, Y. H. Jang and J.-S. Lee, J. Mater. Chem. A, 2013, 1, 10306 CAS.
- Q. Wang, R. Takita, Y. Kikuzaki and F. Ozawa, J. Am. Chem. Soc., 2010, 132, 11420 CrossRef CAS PubMed.
- P. Berrouard, S. Dufresne, A. Pron, J. Veilleux and M. Leclerc, J. Org. Chem., 2012, 77, 8167 CrossRef CAS PubMed.
- N. Allard, A. Najari, J.-R. Pouliot, A. Pron, F. Grenier and M. Leclerc, Polym. Chem., 2012, 3, 2875 RSC.
- F. Grenier, P. Berrouard, J.-R. Pouliot, H.-R. Tseng, A. J. Heeger and M. Leclerc, Polym. Chem., 2013, 4, 1836 RSC.
- L. G. Mercier, B. R. Aïch, A. Najari, S. Beaupré, P. Berrouard, A. Pron, A. Robitaille, Y. Tao and M. Leclerc, Polym. Chem., 2013, 4, 5252 RSC.
- M. P. Ouattara, S. Lenfant, D. Vuillaume, M. Pézolet, J.-F. Rioux-Dubé, J. Brisson and M. Leclerc, Macromolecules, 2013, 46, 6408 CrossRef CAS.
- J.-R. Pouliot, L. G. Mercier, S. Caron and M. Leclerc, Macromol. Chem. Phys., 2013, 214, 453 CrossRef CAS.
- M. Wakioka, Y. Kitano and F. Ozawa, Macromolecules, 2013, 46, 370 CrossRef CAS.
- L. J. Huo and J. H. Hou, Polym. Chem., 2011, 2, 2453 RSC.
- L. J. Huo, J. H. Hou, S. Q. Zhang, H.-Y. Chen and Y. Yang, Angew. Chem., 2010, 122, 1542 CrossRef.
- J. H. Hou, M.-H. Park, S. Q. Zhang, Y. Yao, L.-M. Chen, J.-H. Li and Y. Yang, Macromolecules, 2008, 41, 6012 CrossRef CAS.
- Y. W. Li, B. Xu, H. Li, W. D. Cheng, L. L. Xue, F. P. Chen, H. G. Lu and W. J. Tian, J. Phys. Chem. C, 2011, 115, 2386 CAS.
- A. C. Stuart, J. R. Tumbleston, H. X. Zhou, W. T. Li, S. B. Liu, H. Ade and W. You, J. Am. Chem. Soc., 2013, 135, 1806 CrossRef CAS PubMed.
- X. C. Wang, S. Chen, Y. P. Sun, M. J. Zhang, Y. F. Li, X. Y. Li and H. Q. Wang, Polym. Chem., 2011, 2, 2872 RSC.
- X. C. Wang, Y. P. Sun, S. Chen, X. Guo, M. J. Zhang, X. Y. Li, Y. F. Li and H. Q. Wang, Macromolecules, 2012, 45, 1208 CrossRef CAS.
- X. C. Wang, P. Jiang, Y. Chen, H. Luo, Z. G. Zhang, H. Q. Wang, X. Y. Li, G. Yu and Y. F. Li, Macromolecules, 2013, 46, 4805 CrossRef CAS.
- X. C. Wang, Z.-G. Zhang, H. Luo, S. Chen, S. Q. Yu, H. Q. Wang, X. Y. Li, G. Yu and Y. F. Li, Polym. Chem., 2014, 5, 502 RSC.
- Y. Liu, H. F. Wang, H. L. Dong, J. H. Tan, W. P. Hu and X. W. Zhan, Macromolecules, 2012, 45, 1296 CrossRef CAS.
- C. Amatore and A. Jutand, Acc. Chem. Res., 2000, 33, 314 CrossRef CAS PubMed.
- I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed.
- N.-L. Cheng, in Handbook of Solvent, Chemical Industry Press, Beijing, 2nd edn, 1994 Search PubMed.
- J. A. Cella and S. W. Bacon, J. Org. Chem., 1984, 49, 1122 CrossRef CAS.
- C.-T. Yang, Y. Fu, Y.-B. Huang, J. Yi, Q.-X. Guo and L. Liu, Angew. Chem., Int. Ed., 2009, 48, 7398 CrossRef CAS PubMed.
- A. Zapf and M. Beller, Chem.–Eur. J., 2001, 7, 2908 CrossRef CAS.
- L.-C. Campeau, M. Parisien, A. Jean and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 581 CrossRef CAS PubMed.
- L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed.
- M. Wakioka, Y. Nakamura, Q. F. Wang and F. Ozawa, Organometallics, 2012, 31, 4810 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4py00565a |
| This journal is © The Royal Society of Chemistry 2014 |