Open Access Article
Open Access ArticleOne pot synthesis of higher order quasi-block copolymer libraries via sequential RAFT polymerization in an automated synthesizer†
Joris J.
Haven
ab,
Carlos
Guerrero-Sanchez
*acd,
Daniel J.
Keddie
ae,
Graeme
Moad
*a,
San H.
Thang
a and
Ulrich S.
Schubert
cd
aCSIRO, Materials Science and Engineering, Bag 10, Clayton South MDC, 3169 Victoria, Australia. E-mail: carlos.guerrero-sanchez@csiro.au; graeme.moad@csiro.au
bPolymer Reaction Design Group Institute for Materials Research (IMO-IMOMEC), Universiteit Hasselt, Agoralaan Building D, B-3590 Diepenbeek, Belgium
cLaboratory of Organic and Macromolecular Chemistry (IOMC), Friedrich Schiller University Jena, Humboldstrasse 10, 07743 Jena, Germany
dJena Center for Soft Matter (JCSM) and Polymer Libraries, Friedrich Schiller University Jena, Philosophenweg 7, 07743 Jena, Germany
eChemistry, School of Science and Technology, University of New England, Armidale, 2351 New South Wales, Australia
First published on 16th May 2014
Abstract
Recently developed sequential reversible addition–fragmentation chain transfer (RAFT) polymerization protocols allow the rapid, fully unattended preparation of quasi-block copolymer libraries that cover a wide range of copolymer compositions in an automated synthesizer. This contribution explores the scope and limitations of this sequential approach for the synthesis of higher order quasi-multiblock copolymers (including copolymer sequences of BAB, CBABC, ABC and ABCD). These syntheses illustrate the utility of this high-throughput approach for the one pot synthesis of functional polymers of increased complexity. Additionally, the use of this experimental technique for method development is highlighted.
Introduction
One of the major benefits to stem from reversible addition–fragmentation chain transfer (RAFT) polymerization1 is ready access to block copolymers.2 However, the protocol for polymer synthesis, requiring multiple isolation and purification steps, can be demanding. Thus the development of one-pot methods for the synthesis of block copolymers using RAFT polymerization3–5 and other reversible-deactivation radical polymerization (RDRP)6 methods, such as nitroxide mediated polymerization (NMP)7 and atom transfer radical polymerization (ATRP),8 is currently a research topic of substantial interest to industry and academia. The adoption of such protocol inevitably leads to lower production costs through avoidance of expensive and time-consuming intermediate purification steps.3–5,7,8During RAFT polymerization, every mole of initiator decomposed will produce between one and two moles of dead chains. Those formed during synthesis of a first block of an A–B block will constitute a homopolymer A impurity in the block copolymer. It will also produce between one and two moles of initiator-derived chains. Those formed during synthesis of the second block of an A–B block will constitute a homopolymer B impurity in the block copolymer. Thus to minimize impurity one should minimize the amount of initiator consumed and take the polymerization to form block A to <100% conversion.9
In the synthesis of multi-block copolymers from high kp monomers such as acrylates and acrylamides near quantitative monomer conversions can be achieved rapidly with very low initiator concentrations and thus minimal formation of dead chains and/or homopolymer impurities.5,10 The fraction of living chains at any stage of RAFT polymerization can be easily estimated with knowledge of the concentrations of RAFT agent and initiator and readily available kinetic parameters.2b
For lower kp monomers, which include methacrylates and styrenes, where high conversions require longer polymerization times and/or higher initiator concentrations, a different strategy is required.
Two main strategies have been utilized to achieve the synthesis of block-like copolymers using one pot techniques via RDRP: (1) exploitation of differing monomer reactivity (i.e., reactivity ratios) in limited comonomer systems3a,7 and (2) utilizing sequential monomer addition.3b,c,4,5,8 This latter approach yields quasi-block copolymers when <100% of the first monomer has been consumed prior to a second monomer being incorporated. This approach is necessary with the low kp monomers where relatively high initiator concentrations are required to obtain acceptable rates of polymerization.3,4
The term quasi-block was introduced to refer to block copolymers formed by sequential addition of monomers A, B, … where the second (and subsequent) blocks are, in the general case, some form of gradient copolymer poly(A)-block-poly(A-grad-B)… due to the incorporation of residual first block monomer(s).4 For the case of an all methacrylate quasi-block where reactivity ratios are close to unity the product will be a poly(A)-block-poly(A-ran-B); i.e., the ratio of A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B will remain essentially constant. The determination of composition is not trivial from an experimental point of view. Nevertheless, it can be estimated by simulation using the appropriate monomer reactivity ratios.11
B will remain essentially constant. The determination of composition is not trivial from an experimental point of view. Nevertheless, it can be estimated by simulation using the appropriate monomer reactivity ratios.11
In this work, we use high-throughput polymer synthesis12 and build upon our previously developed one pot high-throughput synthetic strategy to develop protocols for the rapid synthesis of quasi-multiblock copolymer libraries based on methacrylates.4,13 Furthermore, we utilize this experimental technique for the optimization of the reaction conditions of the investigated systems. This method allows the rapid and systematic preparation of higher order (multi) quasi-block copolymer libraries with “new” block combinations that expand over a comprehensive copolymer composition range. Rapid access to these new materials is particularly pertinent for rapid screening of structure–property relationships and development of novel applications. These are areas where quasi-block copolymers are ideally suited and are currently being applied.14,15
Experimental
Materials
n-Butyl methacrylate (BMA), methyl methacrylate (MMA), di(ethylene glycol) methyl ether methacrylate (DEGMA) and benzyl methacrylate (BzMA) monomers were purchased from Sigma-Aldrich and purified by stirring in the presence of inhibitor-remover for hydroquinone or hydroquinone monomethyl ether (Aldrich) for 30 minutes prior to use. RAFT agent: 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid was purchased from Strem Chemicals and utilized as received. Bis-RAFT agent: 4-cyano-4((dodecylthiocarbonothioylthio)pentanoyloxy)butyl 4-cyano-4-(dodecylthiocarbonothioylthio)pentanoate was prepared according to the reported literature procedure.14 1,1′-Azobis(cyclohexanecarbonitrile) (ACHN) initiator (DuPont VAZO-88) was used as received. N,N-Dimethylformamide (DMF) (AR grade) was purchased from Merck.Automated parallel synthesizer
The commercially available synthesizer utilized in this work was a Chemspeed Swing-SLT automated parallel synthesizer.13 The synthesizer was equipped with a glass reactor block consisting of 16 reaction vessels (13 mL) with thermal jackets connected in series through the reaction block to a heating/cooling system (Hüber, −90 °C to 140 °C). In addition, all reaction vessels were equipped with cold-finger reflux condensers (∼7 °C). Mixing was achieved by vortex agitation (up to 1400 rpm). Liquid transfers were handled by a 4-needle head (4-NH) capable of four simultaneous sample transfers. The 4-NH was connected to a reservoir bottle (degassed DMF solvent) for needle rinsing after each liquid transfer step. This DMF solvent reservoir was degassed by continuous sparging with nitrogen and was also utilized to prime the tubing lines of the 4-NH. When experiments were carried out, the synthesizer was maintained under an inert atmosphere by supplying a constant flow of nitrogen to the hood of the synthesizer. A nitrogen atmosphere was also applied to reactors and stock solutions at all times. Prior to the experiments, the reaction vessels were heated to 135 °C and subjected to 10 cycles of vacuum (2 min each) and filling with nitrogen (2 min each) to ensure the elimination of oxygen. After this pre-treatment, the RAFT polymerization experiment was carried out following similar procedures to those reported elsewhere.4,13 It is worth mentioning that the characteristics of the RAFT-synthesized polymers prepared in the automated parallel synthesizer are very similar to those obtained in conventional batch polymerization performed in sealed ampoules as demonstrated in a previous contribution.13aAutomated synthesis of high order (multi) quasi-block copolymer libraries
Monomers and solvent (BMA, MMA, DEGMA, BzMA and DMF), and stock solutions of ACHN (4.12 mg mL−1 in DMF), RAFT (136.24 mg mL−1 in DMF) and bis-RAFT (140 mg mL−1 in DMF) agents were prepared, degassed by sparging with nitrogen for 15 min, and placed inside the automated synthesizer. The sequential RAFT polymerization method utilized in this work was adapted from that previously reported4 and is described below. Schematic representations of the followed synthetic procedures are summarized in Scheme 1.The utilized characterization methods, i.e., proton nuclear magnetic resonance (1H-NMR) and size exclusion chromatography (SEC), are described in the ESI.† Fig. S1† in the ESI displays representative 1H-NMR spectra and their analysis to calculate conversions for the four different monomers investigated in this work.
SEC and NMR samples for analysis were prepared with the automated liquid handling system of the synthesizer at the end of each sampling sequence by adding the corresponding SEC and NMR solvents. Once the pre-established reaction time elapsed, the polymerization mixture was cooled to 20 °C.
Results and discussion
Within this investigation the simplest library synthesis corresponds to the BAB block copolymer case, which utilizes a similar procedure to that described in our previous contribution for quasi-diblock copolymer libraries.4b For this a series of one pot (two step) sequential polymerizations were performed in the automated synthesizer using a bis-macro-RAFT agent to yield a library of 24 PMMA-qb-PBMA-qb-PMMA quasi-triblock copolymers derived from three different bis-macro-RAFT agents. Table 1 summarizes the synthetic results and the reaction conditions utilized (see Table 1 footnote a) of this library and shows that all materials have Đ values below 1.27. Fig. 1 displays representative SEC traces demonstrating an efficient chain extension process for each specific case. The same data is presented with the SEC traces scaled against monomer conversion (MMA) during the chain extension reaction of bis-RAFT agent precursor 1 in Fig. 1D.| ID | Reaction time (h) | M n (g mol−1) | Đ | M n(theory) (g mol−1) | MMA conversion (%) |
|---|---|---|---|---|---|
a Number average molar mass (Mn) and dispersity (Đ = Mw/Mn) were estimated by SEC and are reported as PMMA equivalents. The monomer to polymer conversion was determined by 1H-NMR. Mn(theory) was estimated using the formula: Mn(theory) = ([MBMA]o × MBMA × % conversionBMA + [MMMA]o × MMMA × % conversionMMA)/[bis-RAFT]o + Mbis-RAFT.MBMA, MMMA and Mbis-RAFT are the molar masses of BMA, MMA and bis-RAFT agent, respectively. [MBMA]o, [MBMA]o and [bis-RAFT]o are the initial concentrations of BMA, MMA and bis-RAFT agent, respectively. For the synthesis of the A bis-macro-RAFT agents, [MBMA]o = 2.143 M, reaction temperature = 85 °C and reaction time = 12 h; [RAFT]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Initiator] [Initiator]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MBMA] ratios of 1 [MBMA] ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1 50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 and 1 75 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 were utilized for the cases of bis-macro-RAFT agents 1, 2 and 3, respectively. For the chain extension reaction of the respective bis-macro-RAFT agents, [MMMA]o = 2.318 M, reaction temperature = 105 °C and reaction time = 11 h. 100 were utilized for the cases of bis-macro-RAFT agents 1, 2 and 3, respectively. For the chain extension reaction of the respective bis-macro-RAFT agents, [MMMA]o = 2.318 M, reaction temperature = 105 °C and reaction time = 11 h.
|
|||||
| 1. A bis-macro-RAFT agent (M n = 5400 g mol −1 , Đ = 1.14) | |||||
| 1A | 1 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.12 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632 632 |
29 |
| 1B | 2 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.14 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708 708 |
51 |
| 1C | 3 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.15 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 428 |
64 |
| 1D | 4 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.16 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
71 |
| 1E | 5 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.17 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 358 358 |
78 |
| 1F | 6 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.14 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
83 |
| 1G | 8 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.18 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 860 |
89 |
| 1H | 11 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.19 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 463 463 |
93 |
| 2. A bis-macro-RAFT agent (M n = 7800 g mol −1 , Đ = 1.13) | |||||
| 2A | 1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.14 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279 279 |
31 |
| 2B | 2 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.16 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 478 478 |
52 |
| 2C | 3 | 22 600 | 1.17 | 22 669 | 63 |
| 2D | 4 | 24 400 | 1.18 | 24 512 | 72 |
| 2E | 5 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.19 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 641 641 |
78 |
| 2F | 7 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.20 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971 971 |
84 |
| 2G | 9 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.21 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 918 918 |
89 |
| 2H | 11 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.20 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493 493 |
92 |
3. A bis-macro-RAFT agent (M
n
= 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1, Đ = 1.13) 600 g mol−1, Đ = 1.13) |
|||||
| 3A | 1 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.17 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 408 |
31 |
| 3B | 2 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.19 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 574 574 |
50 |
| 3C | 3 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.20 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926 926 |
59 |
| 3D | 4 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.21 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 588 588 |
65 |
| 3E | 5 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.23 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256 256 |
72 |
| 3F | 6 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.25 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 264 264 |
79 |
| 3G | 8 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.26 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558 558 |
84 |
| 3H | 11 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.26 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
87 |
 | ||
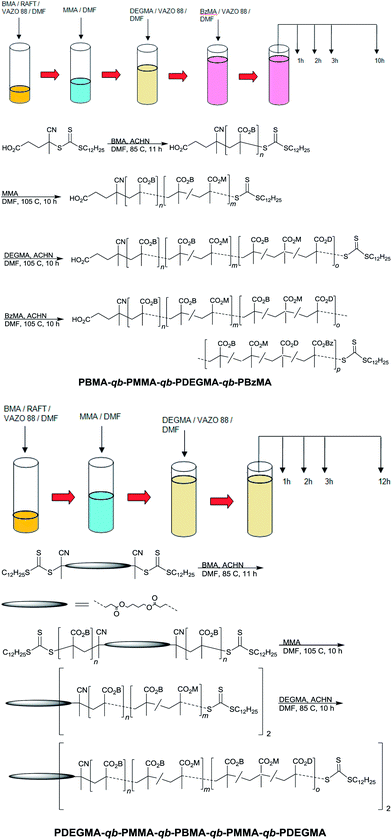
| Fig. 1 SEC traces of the chain extension bis-macro-RAFT polymerization (Table 1) of the synthesized PMMA-qb-PBMA-qb-PMMA quasi-triblock copolymer materials derived from bis-macro-RAFT (A) precursor agent 1, (B) precursor agent 2, (C) precursor agent 3 and (D) precursor agent 1 scaled against conversion of MMA. | ||
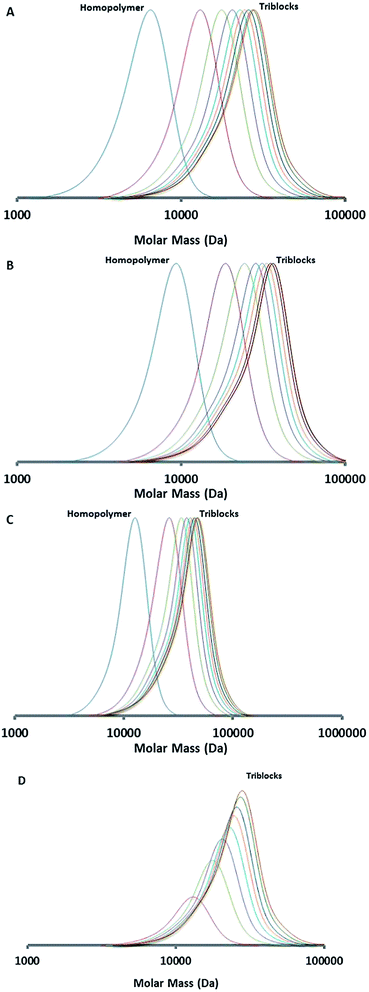
Fig. 2 displays kinetic plots of the chain extension reaction of one of the investigated cases (bis-macro-RAFT agent 2 in Table 1) where a linear relationship can be observed between the number average molar mass (Mn) vs. conversion (x). and the −ln(1 − x) vs. reaction time indicating good control over the consecutive polymerizations. Additional kinetic plots for the cases of the bis-macro-RAFT agents 1 and 3 (Table 1) can be found in Fig. S2† in the ESI. As full conversion was not achieved during the first polymerization the residual BMA is incorporated within the PMMA blocks of the PMMA-qb-PBMA-qb-PMMA quasi-triblock copolymers. The monomer conversions for the three different bis-macro-RAFT agents of Table 1 were 86, 91 and 89% for 1, 2 and 3, respectively. Based on these measurements and using a similar 1H-NMR analysis as reported elsewhere,4 the amount of BMA incorporated into the PMMA blocks during the second polymerization step can be estimated. In all cases of Table 1 was found that the PMMA-qb-PBMA-qb-PMMA quasi-triblock copolymers have BMA units within the PMMA blocks below or at a value of 4 mol%.
 | ||
| Fig. 2 Kinetic data of the synthesized PMMA-qb-PBMA-qb-PMMA quasi-triblock copolymer materials derived from bis-macro-RAFT agent 2 (Table 1). Mn and Đ = Mw / Mn as a function of the MMA conversion (top) and MMA conversion as a function of reaction time (bottom). | ||
Next, we consider the synthesis of a PDEGMA-qb-PMMA-qb-PBMA-qb-PMMA-qb-PDEGMA quasi-pentablock copolymer library (CBABC case). For this CBABC case, three similar bis-macro-RAFT agents to those reported in Table 1 were sequentially chain extended with MMA to obtain the respective BAB bis-macro-RAFT agents of the type PMMA-qb-PBMA-qb-PMMA quasi-triblock copolymers. Thereafter, a second chain extension polymerization step with DEGMA to the three BAB bis-macro-RAFT agents was sequentially undertaken to yield a library of 24 PDEGMA-qb-PMMA-qb-PBMA-qb-PMMA-qb-PDEGMA quasi-pentablock copolymers. Table 2 summarizes the synthetic results and the reaction conditions utilized (see Table 2 footnote a) of this library and shows that all the materials had Đ values below or at 1.37, whereas Fig. 3 displays representative SEC traces demonstrating an efficient second chain extension process for each specific case. Fig. 4 displays kinetic plots of the chain extension reaction of one of the investigated cases (BAB bis-macro-RAFT agent 4 in Table 2) where a linear relationship can be observed between Mnvs. conversion (x) and the −ln(1 − x) vs. reaction time indicating good control over the consecutive polymerization. Additional kinetic plots for the cases of the BAB bis-macro-RAFT agents 5 and 6 (Table 2) can be found in Fig. S3† in the ESI.
| ID | Reaction time (h) | M n (g mol−1) | Đ | M n(theory) (g mol−1) | DEGMA conversion (%) |
|---|---|---|---|---|---|
a Number average molar mass (Mn) and dispersity (Đ = Mw/Mn) were estimated by SEC and are reported as PMMA equivalents. The monomer to polymer conversion was determined by 1H-NMR. Mn(theory) was estimated using the formula: Mn(theory) = ([MBMA]o × MBMA × % conversionBMA + [MMMA]o × MMMA × % conversionMMA + [MDEGMA]o × MDEGMA × % conversionDEGMA)/[bis-RAFT]o + Mbis-RAFT.MBMA, MMMA, MDEGMA and Mbis-RAFT are the molar masses of BMA, MMA, DEGMA and bis-RAFT agent, respectively. [MBMA]o, [MBMA]o, [MDEGMA]o and [bis-RAFT]o are the initial concentrations of BMA, MMA, DEGMA and bis-RAFT agent, respectively. For the synthesis of the A bis-macro-RAFT agents, [MBMA]o = 2.143 M, reaction temperature = 85 °C and reaction time = 12 h; [RAFT]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Initiator] [Initiator]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MBMA] ratios of 1 [MBMA] ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1 50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 and 1 75 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 were utilized for the cases of bis-macro-RAFT agents 4, 5 and 6, respectively. For the synthesis of the BAB bis-macro-RAFT agents, [MMMA]o = 2.318 M, reaction temperature = 105 °C and reaction time = 10 h. For the chain extension reaction of the respective BAB bis-macro-RAFT agents with DEGMA, [MDEGMA]o = 0.853 M, additional [Initiator]o = 6.638 × 10−4 M, reaction temperature = 85 °C and reaction time = 12 h. 100 were utilized for the cases of bis-macro-RAFT agents 4, 5 and 6, respectively. For the synthesis of the BAB bis-macro-RAFT agents, [MMMA]o = 2.318 M, reaction temperature = 105 °C and reaction time = 10 h. For the chain extension reaction of the respective BAB bis-macro-RAFT agents with DEGMA, [MDEGMA]o = 0.853 M, additional [Initiator]o = 6.638 × 10−4 M, reaction temperature = 85 °C and reaction time = 12 h.
|
|||||
4. BAB bis-macro-RAFT agent (M
n
= 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1, Đ = 1.15) 900 g mol−1, Đ = 1.15) |
|||||
| 4A | 1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.18 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 594 |
25 |
| 4B | 2 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.19 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 471 471 |
37 |
| 4C | 3 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.20 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
48 |
| 4D | 4 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.22 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 554 554 |
55 |
| 4E | 6 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.25 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
64 |
| 4F | 8 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.25 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 869 |
68 |
| 4G | 10 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.26 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
74 |
| 4H | 12 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.27 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538 538 |
78 |
5. BAB bis-macro-RAFT agent (M
n
= 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, Đ = 1.18) 800 g mol−1, Đ = 1.18) |
|||||
| 5A | 1 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.20 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 428 |
25 |
| 5B | 2 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.24 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357 357 |
37 |
| 5C | 3 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.24 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536 536 |
47 |
| 5D | 4 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.26 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 033 033 |
53 |
| 5E | 6 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.28 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676 676 |
63 |
| 5F | 8 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.29 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 126 |
69 |
| 5G | 10 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.30 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421 421 |
75 |
| 5H | 12 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.30 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569 569 |
80 |
6. BAB bis-macro-RAFT agent (M
n
= 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1, Đ = 1.22) 600 g mol−1, Đ = 1.22) |
|||||
| 6A | 1 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.27 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 392 392 |
25 |
| 6B | 2 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.30 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
34 |
| 6C | 3 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.31 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 151 151 |
42 |
| 6D | 4 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.34 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138 |
48 |
| 6E | 6 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.34 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626 626 |
58 |
| 6F | 8 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.33 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 084 084 |
63 |
| 6G | 10 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.34 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 |
69 |
| 6H | 12 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.37 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 427 |
73 |
 | ||
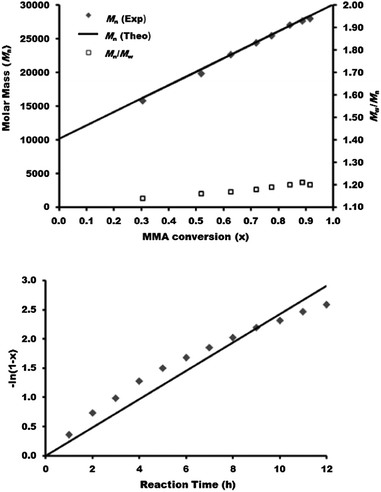
| Fig. 3 SEC traces of the chain extension bis-macro-RAFT polymerization (Table 2) of the synthesized PDEGMA-qb-PMMA-qb-PBMA-qb-PMMA-qb-PDEGMA quasi-pentablock copolymer materials derived from bis-macro-RAFT (A) precursor agent 4 (B) precursor agent 5 and (C) precursor agent 6. | ||
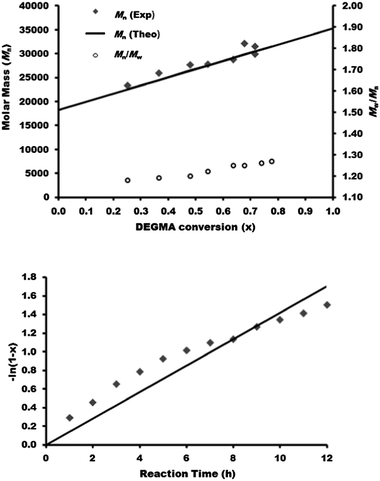 | ||
| Fig. 4 Kinetic data of the synthesized PDEGMA-qb-PMMA-qb-PBMA-qb-PMMA-qb-PDEGMA quasi-pentablock copolymer materials derived from BAB bis-macro-RAFT agent 4 (Table 2). Mn and Đ = Mw / Mn of as a function of the DEGMA conversion (top) and DEGMA conversion as a function of reaction time (bottom). | ||
Similar to the previous analysis, full conversion was also not reached during the second polymerization step. Thus, the residual MMA was incorporated within the PDEGMA blocks of the PDEGMA-qb-PMMA-qb-PBMA-qb-PMMA-qb-PDEGMA quasi-pentablock copolymers. The MMA monomer conversions for the three different BAB bis-macro-RAFT agents of Table 2 were 82, 86 and 82% for 4, 5 and 6, respectively. Based on these measurements and using the 1H-NMR analysis described in the experimental section, the amount of MMA incorporated into the PDEGMA blocks during the third polymerization step can be estimated. It was found that the PDEGMA-qb-PMMA-qb-PBMA-qb-PMMA-qb-PDEGMA quasi-pentablock copolymers have MMA units within the PDEGMA blocks in the range of 18 to 25 mol%. The relative high impurity found in the PDEGMA block can be ascribed to the lower MMA monomer conversion obtained during the second polymerization as well as to the low concentration of DEGMA monomer utilized for the third polymerization step. It was found that, within the investigated reaction conditions, higher conversions during the second polymerization, higher concentration of third monomer values in the materials or dead polymer in the third sequential polymerization led to higher dispersity chains. This aspect is analysed in more detail below for the ABC quasi-triblock copolymer library case. Nevertheless, further optimization to reduce these defects or impurities in the sequentially formed blocks could be easily achieved.
For the PBMA-qb-PMMA-qb-PDEGMA ABC case, two macro-RAFT agents were sequentially chain extended with MMA monomer utilizing the reaction conditions described in the Experimental section to obtain the respective AB macro-RAFT agents of the type PBMA-qb-PMMA quasi-diblock copolymers. Thereafter, a second chain extension with DEGMA monomer to the two AB macro-RAFT agents was sequentially undertaken to yield a library of 16 PBMA-qb-PMMA-qb-PDEGMA quasi-triblock copolymers. Table 3 summarizes the synthetic results and the utilized reaction conditions (see Table 3 footnote a) of this library and shows that all the materials have Đ values below or at 1.34, whereas Fig. 5 displays representative SEC traces demonstrating an efficient second chain extension process for each specific case. Fig. 6 displays kinetic plots of the chain extension reaction of one of the investigated cases (AB macro RAFT agent 7 in Table 3) where a linear relationship can be observed between the Mnvs. conversion (x) and the −ln(1 − x) vs. reaction time indicating all in all a good control over the consecutive polymerization. Kinetic plots for the additional case of the AB macro-RAFT agent 8 (Table 3) can be found in Fig. S4† in the ESI. Similar to the previous analysis, full conversion was also not reached during the second polymerization step (synthesis of the AB macro-RAFT agents). Thus, the residual MMA was incorporated within the PDEGMA blocks of the PBMA-qb-PMMA-qb-PDEGMA quasi-triblock copolymers. The MMA monomer conversions for the two different AB macro-RAFT agents of Table 3 were 80% for both cases (7 and 8). Attempts for bringing to higher conversions (>80%) these polymerization reactions led to the appearance of a considerable amount of dead polymer chains as revealed by SEC traces (appearance of shoulders and tails in the low molar mass range during the second chain extension reaction; see Fig. S5† in the ESI for an example where a reaction time of 12 h was utilized instead of 11 h). Similar to our optimization of the reaction time, to provide a reasonable balance between high monomer conversion and minimal dead polymer chains, optimization experiments could be performed using our high-throughput methodology to establish the minimum amount of initiator required to reach a desired conversion and level of end group fidelity.5,10 This optimal initiator level might also be estimated by simulations11,16 for systems where kinetic parameters are available.2b As a direct consequence of the relatively low conversion in the first chain extension reaction (polymerization of MMA), higher amounts of MMA monomer will be incorporated into the PDEGMA block as “impurity” during the second chain extension reaction. Based on this and using the 1H-NMR analysis, the amount of MMA incorporated into the PDEGMA blocks during the third polymerization step was estimated to be in the range of 18 to 27 mol%. Similar to the previous discussed case, the relatively high impurity found in the PDEGMA block can be ascribed to the low MMA monomer conversion during the second polymerization and the relatively low concentration of DEGMA monomer utilized for the third polymerization step.
| ID | Reaction time (h) | M n (g mol−1) | Đ | M n(theory) (g mol−1) | DEGMA conversion (%) |
|---|---|---|---|---|---|
a Number average molar mass (Mn) and dispersity (Đ = Mw/Mn) were estimated by SEC and are reported as PMMA equivalents. The monomer to polymer conversion was determined by 1H-NMR. Mn(theory) was estimated using the formula: Mn(theory) = ([MBMA]o × MBMA × % conversionBMA + [MMMA]o × MMMA × % conversionMMA + [MDEGMA]o × MDEGMA × % conversionDEGMA)/[RAFT]o + MRAFT.MBMA, MMMA, MDEGMA and MRAFT are the molar masses of BMA, MMA, DEGMA and RAFT agent, respectively. [MBMA]o, [MBMA]o, [MDEGMA]o and [RAFT]o are the initial concentrations of BMA, MMA, DEGMA and RAFT agent, respectively. For the synthesis of the A macro RAFT agents, [MBMA]o = 2.143 M, reaction temperature = 85 °C and reaction time = 11 h; [RAFT]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Initiator] [Initiator]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MBMA] ratios of 1 [MBMA] ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 and 1 75 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 were utilized for the cases of macro RAFT agents 7 and 8, respectively. For the synthesis of the AB macro RAFT agents, [MMMA]o = 2.318 M, reaction temperature = 105 °C and reaction time = 10 h. For the chain extension reaction of the respective AB macro RAFT agents with DEGMA, [MDEGMA]o = 0.853 M, additional [Initiator]o = 6.638 × 10−4 M, reaction temperature = 85 °C and reaction time = 12 h. 100 were utilized for the cases of macro RAFT agents 7 and 8, respectively. For the synthesis of the AB macro RAFT agents, [MMMA]o = 2.318 M, reaction temperature = 105 °C and reaction time = 10 h. For the chain extension reaction of the respective AB macro RAFT agents with DEGMA, [MDEGMA]o = 0.853 M, additional [Initiator]o = 6.638 × 10−4 M, reaction temperature = 85 °C and reaction time = 12 h.
|
|||||
7. AB macro-RAFT agent (M
n
= 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1, Đ = 1.16) 400 g mol−1, Đ = 1.16) |
|||||
| 7A | 1 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.19 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 338 338 |
17 |
| 7B | 2 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.22 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 601 |
31 |
| 7C | 3 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.23 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932 932 |
41 |
| 7D | 4 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.24 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709 709 |
48 |
| 7E | 6 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.26 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 071 071 |
58 |
| 7F | 8 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.27 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694 694 |
65 |
| 7G | 10 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.29 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 161 161 |
71 |
| 7H | 12 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.30 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 341 |
76 |
8. AB macro-RAFT agent (M
n
= 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, Đ = 1.21) 800 g mol−1, Đ = 1.21) |
|||||
| 8A | 1 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.22 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 921 921 |
17 |
| 8B | 2 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.25 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 265 265 |
32 |
| 8C | 3 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.27 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688 688 |
39 |
| 8D | 4 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.27 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938 938 |
46 |
| 8E | 5 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.25 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 583 583 |
52 |
| 8F | 7 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.31 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
60 |
| 8G | 10 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.33 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 458 458 |
70 |
| 8H | 12 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.34 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 052 052 |
75 |
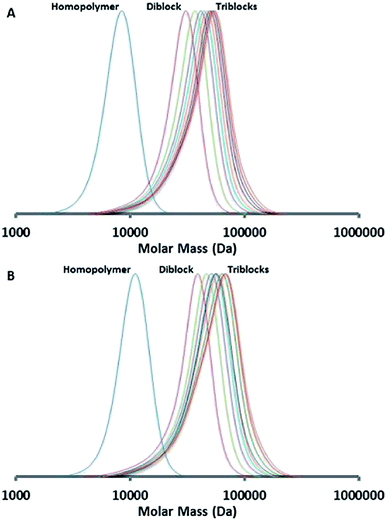 | ||
| Fig. 5 SEC traces of the chain extension RAFT polymerization (Table 3) of the synthesized PBMA-qb-PMMA-qb-PDEGMA quasi-triblock copolymer materials derived from macro-RAFT (A) precursor agent 7 and (B) precursor agent 8. | ||
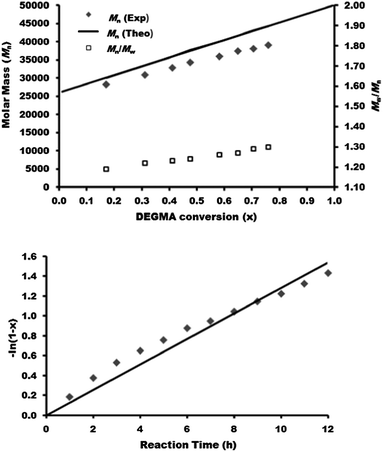 | ||
| Fig. 6 Kinetic data of the synthesized PBMA-qb-PMMA-qb-PDEGMA quasi-triblock copolymer materials derived from AB macro RAFT agent 7 (Table 3). Mn and Đ = Mw / Mn of as a function of the DEGMA conversion (top) and DEGMA conversion as a function of reaction time (bottom). | ||
For the PBMA-qb-PMMA-qb-PDEGMA-qb-PBzMA ABCD case, the ABC macro-RAFT agent was sequentially chain extended with BzMA monomer in a third polymerization step. This resulted in 8 PBMA-qb-PMMA-qb-PDEGMA-qb-PBzMA quasi-tetrablock copolymers. Table 4 summarizes the synthetic results and the reaction conditions utilized (see Table 4 footnote a) of this library and shows that all the materials had Đ values below or at 1.46, whereas Fig. 7 displays representative SEC traces demonstrating an efficient third chain extension process for this case. Fig. 8 displays kinetic plots of the chain extension reaction of these cases (ABC macro-RAFT agent 9 in Table 4) where a linear relationship can be observed between the Mnvs. conversion (x) and the −ln(1 − x) vs. reaction time indicating good control over the consecutive polymerizations. However, after three consecutive polymerization reactions the Đ values of the materials become higher with tailing to low molar mass evident in the SEC traces due to the unavoidable contribution of initiator-derived dead chains from the necessary use of relatively high initiator concentrations.
| ID | Reaction time (h) | M n (g mol−1) | Đ | M n(theory) (g mol−1) | BzMA conversion (%) |
|---|---|---|---|---|---|
| a Number average molar mass (Mn) and dispersity (Đ = Mw/Mn) were estimated by SEC and are reported as PMMA equivalents. The monomer to polymer conversion was determined by 1H-NMR. Mn(theory) was estimated using the formula: Mn(theory) = ([MBMA]o × MBMA × % conversionBMA + [MMMA]o × MMMA × % conversionMMA + [MDEGMA]o × MDEGMA × % conversionDEGMA + [MBzMA]o × MBzMA × % conversionBzMA)/[RAFT]o + MRAFT.MBMA, MMMA, MDEGMA, MBzMA and MRAFT are the molar masses of BMA, MMA, DEGMA, BzMA and RAFT agent, respectively. [MBMA]o, [MBMA]o, [MDEGMA]o, [MBzMA]o and [RAFT]o are the initial concentrations of BMA, MMA, DEGMA, BzMA and RAFT agent, respectively. The synthesis of the ABC macro RAFT agent was an extension of 7 in Table 3, [MDEGMA]o = 0.853 M, additional [Initiator]o = 6.638 × 10−4 M, reaction temperature = 85 °C and reaction time = 12 h. For the chain extension reaction of this ABC macro RAFT agent with BzMA, [MBzMA]o = 0.682 M, additional [Initiator]o = 5.848 × 10−4 M, reaction temperature = 85 °C and reaction time = 10 h. | |||||
9. ABC macro-RAFT agent (M
n
= 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1, Đ = 1.28) 600 g mol−1, Đ = 1.28) |
|||||
| 9A | 1 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.34 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
15 |
| 9B | 2 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.36 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 767 767 |
29 |
| 9C | 3 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.37 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 615 |
36 |
| 9D | 4 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.39 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 413 413 |
43 |
| 9E | 6 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.43 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183 183 |
54 |
| 9F | 8 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.46 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 876 876 |
67 |
| 9G | 10 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.45 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 955 955 |
70 |
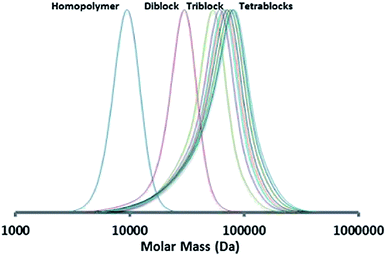 | ||
| Fig. 7 SEC trace of the chain extension RAFT polymerization (Table 4) of the synthesized PBMA-qb-PMMA-qb-PDEGMA-qb-PBzMA quasi-tetrablock copolymer materials derived from macro-RAFT precursor agent 9. | ||
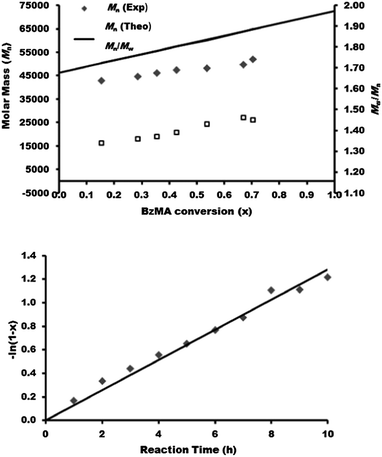 | ||
| Fig. 8 Kinetic data of the synthesized PBMA-qb-PMMA-qb-PDEGMA-qb-BzMA quasi-tetrablock copolymer materials derived from ABC macro-RAFT agent 9 (Table 4). Mn and Đ = Mw / Mn of as a function of the BzMA conversion (top) and BzMA conversion as a function of reaction time (bottom). | ||
Furthermore, similar to the previous analysis, full conversion was also not reached during the third polymerization step (synthesis of the ABC macro-RAFT agent). Thus, the residual DEGMA was incorporated within the PBzMA blocks of the PBMA-qb-PMMA-qb-PDEGMA-qb-PBzMA quasi-tetrablock copolymers. The DEGMA monomer conversion for ABC macro-RAFT agent of Table 4 was 70%. Attempts to obtain higher conversion (>70%) in these polymerization reactions led to the appearance of a considerable amount of dead chains evident through SEC traces (see Fig. S6† in the ESI for an example where a lower molar mass ABC macro-RAFT agent was obtained at the level of 74% conversion). As a direct consequence of the relatively low conversion in the second chain extension reaction (polymerization of DEGMA), higher amounts of DEGMA monomer will be incorporated to the PBzMA block as “impurity” during the third chain extension reaction. Based on these findings and using the 1H-NMR analysis described in the experimental section, the amount of DEGMA incorporated into the PBzMA blocks during the fourth polymerization step was estimated. It was found that the PBMA-qb-PMMA-qb-PDEGMA-qb-PBzMA quasi-tetrablock copolymers have DEGMA units within the PBzMA blocks in the range of 22 to 35 mol%. Similar to the previous discussed case, the relatively high impurity found in the PBzMA block can be ascribed to the relative lower DEGMA monomer conversion obtained during the third polymerization as well as to the relative low concentration of BzMA monomer utilized for the fourth polymerization step.
Hadjiantoniou et al. synthesized a series of methacrylate based “pure” block copolymers in consecutive polymerization steps utilizing the RAFT technique.10e In specific, they synthesized di-, tri, tetra- and pentablock copolymers and obtained Đ values of 1.32, 1.48, 1.58 and 1.83, respectively. The results reported in this contribution clearly demonstrate that properly optimized one pot RAFT synthetic approaches can yield multi-block copolymers with lower Đ values as compared to other more demanding methods where intermediate purification steps are applied for the synthesis of each block.
Conclusion
In this work, we have developed a convenient high-throughput approach for the one pot synthesis of quasi-block copolymer libraries of methacrylic monomers by RAFT polymerization. Utilizing this powerful technique, we could optimize reaction conditions and synthesize up to 71 different quasi-block copolymers, including diblocks, triblocks, tetrablocks and pentablocks, demonstrating that highly comprehensive and systematic polymer libraries can be obtained in a short period of time. Future efforts in this direction will include the synthesis of materials that utilize monomers of varying reactivity to further expand the range of materials that are easily accessible through process automation.Acknowledgements
J.J.H. acknowledges CSIRO-CMSE for financial support.Notes and references
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559 CrossRef CAS.
- (a) Y. K. Chong, T. P. T. Le, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1999, 32, 2071 CrossRef CAS; (b) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379 CrossRef CAS; (c) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2006, 59, 669 CrossRef CAS; (d) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2009, 62, 1402 CrossRef CAS; (e) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2012, 65, 985 CrossRef CAS; (f) D. J. Keddie, Chem. Soc. Rev., 2014, 43, 496 RSC.
- (a) S. Harrisson, F. Ercole and B. W. Muir, Polym. Chem., 2010, 1, 326 RSC; (b) I. Chaduc, W. Zhang, J. Rieger, M. Lansalot, F. D'Agosto and B. Charleux, Macromol. Rapid Commun., 2011, 32, 1270 CrossRef CAS PubMed; (c) J. Jennings, M. Beija, A. P. Richez, S. D. Cooper, P. E. Mignot, K. J. Thurecht, K. S. Jack and S. M. Howdle, J. Am. Chem. Soc., 2012, 134, 4772 CrossRef CAS PubMed; (d) A. Anastasaki, C. Waldron, P. Wilson, C. Boyer, P. B. Zetterlund, M. R. Whittaker and D. Haddleton, ACS Macro Lett., 2013, 2, 896–900 CrossRef CAS.
- (a) C. Guerrero-Sanchez, L. O'Brien, C. Brackley, D. J. Keddie, S. Saubern and J. Chiefari, Polym. Chem., 2013, 4, 1857 RSC; (b) J. J. Haven, C. Guerrero-Sanchez, D. J. Keddie and G. Moad, Macromol. Rapid Commun., 2014, 35, 492 CrossRef CAS PubMed.
- G. Gody, T. Maschmeyer, P. B. Zetterlund and S. Perrier, Nat. Commun., 2013, 4, 2505 Search PubMed.
- A. D. Jenkins, R. I. Jones and G. Moad, Pure Appl. Chem., 2010, 82, 483 CAS.
- D. Chan-Seng, M. Zamfir and J. F. Lutz, Angew. Chem., 2012, 124, 12420 CrossRef.
- (a) J. Shu, C. Cheng, Y. Zheng, L. Shen, Y. Qiao and C. Fu, Polym. Bull., 2011, 67, 1185 CrossRef CAS PubMed; (b) H. Wei, S. Perrier, S. Dehn, R. Ravarian and F. Dehghani, Soft Matter, 2012, 8, 9526 RSC; (c) J. Vandenbergh, G. Reekmans, P. Adriaensens and T. Junkers, Chem. Commun., 2013, 49, 10358 RSC.
- (a) Y. K. Chong, T. P. T. Le, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1999, 32, 2071–2074 CrossRef CAS; (b) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379 CrossRef CAS.
- (a) C. Boyer, A. H. Soeriyadi, P. B. Zetterlund and M. R. Whittaker, Macromolecules, 2011, 44, 8028 CrossRef CAS; (b) A. H. Soeriyadi, C. Boyer, F. Nyström, P. B. Zetterlund and M. R. Whittaker, J. Am. Chem. Soc., 2011, 133, 11128 CrossRef CAS PubMed; (c) J. Vandenbergh, T. De Moraes Ogawa and T. Junkers, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2366 CrossRef CAS; (d) P. B. Zetterlund, G. Gody and S. Perrier, Macromol. Theory Simul., 2014 DOI:10.1002/mats.201300165; (e) N. A. Hadjiantoniou, T. Krasia-Christoforou, E. Loizou, L. Porcar and C. S. Patrickios, Macromolecules, 2010, 43, 2713 CrossRef CAS.
- (a) C. Toloza Porras, D. R. D'Hooge, P. H. M. van Steenberge, M. F. Reyniers and G. B. Marin, Macromol. React. Eng., 2013, 7, 311 CrossRef CAS; (b) P. H. M. van Steenberge, D. R. D'Hooge, Y. Wang, M. Zhong, M. F. Reyniers, D. Konkolewicz, K. Matyjaszewski and G. B. Marin, Macromolecules, 2012, 45, 8519 CrossRef CAS.
- (a) D. J. Siegwart, M. Leiendecker, R. Langer and D. G. Anderson, Macromolecules, 2012, 45, 1254 CrossRef CAS PubMed; (b) P. Chapon, C. Mignaud, G. Lizarraga and M. Destarac, Macromol. Rapid Commun., 2003, 24, 87 CrossRef CAS; (c) A. W. Bosman, A. Heumann, G. Klaerner, D. Benoit, J. M. J. Frechet and C. J. Hawker, J. Am. Chem. Soc., 2001, 123, 6461 CrossRef CAS; (d) R. Rojas, N. K. Harris, K. Piotrowska and J. Kohn, J. Polym. Sci., Part A: Polym. Chem., 2008, 47, 49 CrossRef; (e) A. Ekin and D. C. Webster, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 4880 CrossRef CAS; (f) J. Bonilla-Cruz, C. Guerrero-Sanchez, U. S. Schubert and E. Saldivar-Guerra, Eur. Polym. J., 2010, 46, 298 CrossRef CAS PubMed; (g) C. Guerrero-Sanchez, B. G. G. Lohmeijer, M. A. R. Meier and U. S. Schubert, Macromolecules, 2005, 38, 10388 CrossRef CAS; (h) A. A. A. Smith, B. M. Wohl, M. B. L. Kryger, N. Hedemann, C. Guerrero-Sanchez, A. Postma and A. N. Zelikin, Adv. Healthcare Mater., 2014, 3 DOI:10.1002/adhm.201300637; (i) K. Zuwala, A. A. A. Smith, A. Postma, C. Guerrero-Sanchez, P. Ruiz-Sanchis, J. Melchjorsen, M. Tolstrup and A. N. Zelikin, Adv. Healthcare Mater., 2014, 3 DOI:10.1002/adhm.201400148.
- (a) C. Guerrero-Sanchez, D. J. Keddie, S. Saubern and J. Chiefari, ACS Comb. Sci., 2012, 14, 389 CrossRef CAS PubMed; (b) D. J. Keddie, C. Guerrero-Sanchez and G. Moad, Polym. Chem., 2013, 4, 3591 RSC; (c) D. J. Keddie, C. Guerrero-Sanchez, G. Moad, R. J. Mulder, E. Rizzardo and S. H. Thang, Macromolecules, 2012, 45, 4205 CrossRef CAS; (d) C. Guerrero-Sanchez, S. Harrisson and D. J. Keddie, Macromol. Symp., 2013, 325–326, 38 CrossRef CAS; (e) D. J. Keddie, C. Guerrero-Sanchez, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 2011, 44, 6738 CrossRef CAS.
- T. M. Hinton, C. Guerrero-Sanchez, J. E. Graham, T. Le, B. W. Muir, S. Shi, M. L. V. Tizard, P. A. Gunatillake, K. M. McLean and S. H. Thang, Biomaterials, 2012, 33, 7631 CrossRef CAS PubMed.
- C. Pietsch, U. Mansfeld, C. Guerrero-Sanchez, S. Hoeppener, A. Vollrath, M. Wagner, R. Hoogenboom, S. Saubern, S. H. Thang, C. R. Becer, J. Chiefari and U. S. Schubert, Macromolecules, 2012, 45, 9292 CrossRef CAS.
- E. Saldívar, O. Araujo, R. Giudici and C. Guerrero-Sanchez, J. Appl. Polym. Sci., 2002, 84, 1320 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional 1H-NMR spectra, integration methods, SEC traces and kinetic plots of the discussed experiments. See DOI: 10.1039/c4py00496e |
| This journal is © The Royal Society of Chemistry 2014 |