Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hierarchically porous π-conjugated polyHIPE as a heterogeneous photoinitiator for free radical polymerization under visible light†
Zi Jun
Wang
,
Katharina
Landfester
and
Kai A. I.
Zhang
*
Max Planck Institute for Polymer Research, Ackermannweg 10, D-55128 Mainz, Germany. E-mail: kai.zhang@mpip-mainz.mpg.de
First published on 25th March 2014
Abstract
A hierarchically porous π-conjugated polyHIPE was used as a heterogeneous visible light photoinitiator for the radical polymerization of methyl methacrylate (MMA) under a household energy saving light bulb at room temperature. The heterogeneous nature of the porous polymer ensures easy separation and reusability.
Photopolymerization has demonstrated its advantages and played an important role in various industrial applications such as coatings, adhesives, optical waveguides, microelectronics, dental fillings, and other biomaterials for bone and tissue engineering.1–4 In the past, numerous efficient photoinitiators with different absorption ranges for free radical polymerization were developed. However, they mainly absorb in the UV range.5 Taking nature as a role model, researchers have put tremendous effort in developing new photocatalysts which absorb mainly in the visible range of light. Many of the photocatalysts have established notable prominence in applications such as water splitting, solar energy storage, and photovoltaics.6–8 Among the visible light photocatalysts, rare metal complexes, especially ruthenium complexes, have found prominent use due to their synthesis, stability and photoredox properties.
However, the high cost and toxicity of those rare metals, as well as their limited availability, present a huge challenge in their sustainability. Therefore, there has been growing interest in developing metal-free photocatalysts in the field of visible light photocatalysis. In recent years, a number of organic chromophores and dyes were employed successfully in photoredox catalysis.9–11 Examples of some well-studied photoinitiators that perform in the visible range include titanocene, camphorquinone, organic ketones containing germanium, iridium complexes, and organic dyes.12–19 Despite their high initiation efficiency, strong odor, toxicity, and high migration are often observed with these low-molar-mass photoinitiators and the homogeneous nature makes them difficult to remove. One method to tackle such problems is to use macromolecular photoinitiators.20–23 Recently, Kiskan et al. reported the use of a phenolphthalein-based microporous polymer network24 and mesoporous graphitic carbon nitride as metal-free heterogeneous visible light photoinitiators.25
High internal phase emulsion (HIPE) polymerization is a relatively new technique that has found a wide variety of applications in tissue engineering scaffolds, enzyme immobilization, gas storage and separation media.26–29 Very recently, π-conjugated polyHIPEs combine the hierarchically interconnected pore structure of polyHIPEs and the π-conjugated polymer backbone throughout the network, showing high efficiency as a heterogeneous photosensitizer for singlet oxygen generation under visible light.30 In our previous study, we reported the micropore engineering and photocatalytic activity of conjugated microporous polyHIPEs for highly selective oxidation of organic sulfides to sulfoxides under visible light.31
In this study, we report for the first time the use of a π-conjugated polyHIPE B–(Boc-CB)2–BO (Scheme 1) as a heterogeneous, visible light photoinitiator in the free radical polymerization of methyl methacrylate (MMA). Via high internal phase emulsion (HIPE) polymerization, the π-conjugated polymer B–(Boc-CB)2–BO was synthesized via palladium-catalyzed Suzuki–Miyaura cross-coupling reactions using tert-butylcarbonate funtionalized dibromocarbazole (Boc-CB) with 1,3,5-benzenetriboronic acid tris(pinacol) ester (B) as the cross-linker combined with a strong electron acceptor, dibromobenzoxadiazole (BO). The chemical structure, morphology, and optical properties of the porous polymer were characterized by Fourier transform-infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), diffusive reflectance (DR) UV/Vis spectroscopy, solid-state 13C magic angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy, and N2 gas sorption measurements.
The system formed stable emulsions that resulted in a monolithic polymer with an interconnected pore structure (Fig. 1a and b). The polymer was insoluble in all common organic solvents tested. The SEM images show that the conjugated polyHIPE has a hierarchical pore structure, consisting of micrometer scale cavities, submicron scale interconnected pores, as well as nanometer scale micropores measured by the N2 gas sorption experiment (Fig. S5 and S6†). The Brunauer–Emmett–Teller (BET) surface area, micropore size, and total pore volume were measured to be 82 m2 g−1, 1.5 nm and 0.128 cm3 g−1, respectively.
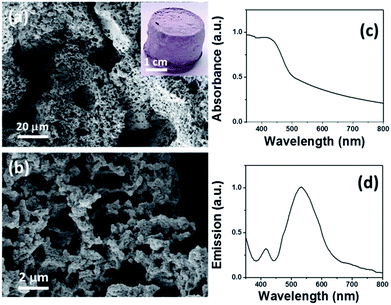 | ||
| Fig. 1 (a and b) SEM images of B–(Boc-CB)2–BO, the inset is a photograph of the monolithic polymer, (c) DRS UV/Vis, and (d) photoluminescence spectra. | ||
Solid state 13C/MAS NMR spectra show chemical shifts at δ = 30, 85 and 155 ppm, which are assigned to the –CH3, quart.-C and >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the Boc moiety. The signals between 110 and 150 ppm can be assigned to the aromatic rings in the polymer backbone (Fig. S7†). Thermal gravimetric analysis (TGA) measurements showed that the conjugated network remained intact up to 700 °C, the weight loss at around 200 °C can be attributed to the Boc functional group (Fig. S4†). Fig. 1c displays the very broad absorption band in the visible light range, an optical band gap of 2.16 eV can be derived from the absorption edge. The exhibited baseline increases with decreasing wavelength. This is an indication of light scattering inside the porous structure with sizes in the range of the visible wavelengths. B–(Boc-CB)2–BO exhibited weak emission with a maximum at about 540 nm (Fig. 1d), a large Stokes shift occurred, which indicates a better π–π* transition in the exited state of the polymer.
O groups of the Boc moiety. The signals between 110 and 150 ppm can be assigned to the aromatic rings in the polymer backbone (Fig. S7†). Thermal gravimetric analysis (TGA) measurements showed that the conjugated network remained intact up to 700 °C, the weight loss at around 200 °C can be attributed to the Boc functional group (Fig. S4†). Fig. 1c displays the very broad absorption band in the visible light range, an optical band gap of 2.16 eV can be derived from the absorption edge. The exhibited baseline increases with decreasing wavelength. This is an indication of light scattering inside the porous structure with sizes in the range of the visible wavelengths. B–(Boc-CB)2–BO exhibited weak emission with a maximum at about 540 nm (Fig. 1d), a large Stokes shift occurred, which indicates a better π–π* transition in the exited state of the polymer.
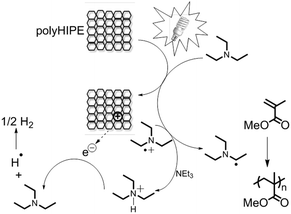
To demonstrate the photocatalytic activity under visible light, B–(BOC-CB)2–BO was employed to initiate the free radical photopolymerization of methyl methacrylate (MMA) in 50 wt% THF solution using a 23 W household energy saving light bulb at room temperature. Et3N was used as a co-initiator. The set-up is shown in Fig. S8 of the ESI.† A kinetic plot of the radical polymerization of methyl methacrylate (MMA) initiated by B–(Boc-CB)2–BO under visible light at room temperature is shown in Fig. 2.
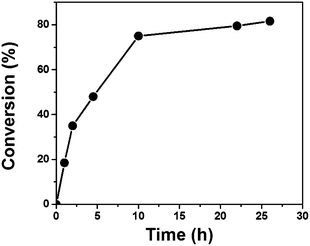 | ||
| Fig. 2 Photopolymerization of methyl methacrylate using B–(Boc-CB)2–BO as a heterogeneous photoinitiator at room temperature under visible light. | ||
It shows that the polymerization rate slowed down after reaching ca. 80% conversion, exhibiting the character of an exponential development. In this case a second kinetic order might have occurred. Table 1 presents the characterization data of the PMMA polymers obtained at different conversions including control experiments. Both the initiating components, namely, polyHIPE and Et3N, are indispensable, no polymerization was observed when either component was absent. An acceleration of the conversion, the so-called Trommsdorff effect, was not observed. The reason could be that the polymerization was conducted in 50 wt% THF solution with vigorous stirring, both of which alleviate the system with a sudden localized viscosity increase.
| Sample | hν | Conv. (%) | M n (g mol−1) | M w/Mn |
|---|---|---|---|---|
| a No light with polyHIPE and coinitiator. b No coinitiator with polyHIPE and light. c No polyHIPE with light and coinitiator. Conditions: MMA (1 mL, 9.39 mmol), B–(Boc-CB)2–BO (25 mg) and Et3N (30 mg) in 50 wt% THF. | ||||
| Blanka | — | — | — | — |
| Blankb | + | — | — | — |
| Blankc | + | — | — | — |
| B–(Boc-CB)2–BO | + | 82 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.67 |
| B–(Boc-CB)2–BO | + | 35 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.18 |
| B–(Boc-CB)2–BO | + | 35 | 8300 | 1.92 |
Control experiments conducted without light irradiation resulted in no polymer formation. In Fig. 3, we suggest an initiation mechanism similar to that in the literature.25 Under visible light irradiation, charge separation inside the conjugated polyHIPE occurred,32 which likely could be stabilized within the nanometer-sized pores. Et3N was oxidized by the hole inside the conjugated polyHIPE into the corresponding radical cation. Another Et3N molecule formed consequently a free radical after giving away one proton to a radical cation. The radical polymerization of MAA was initiated. Followed by the photoinitiation an electron could react with the amine cation, forming a neutral amine and a hydrogen radical.
Furthermore, to investigate the reusability of B–(Boc-CB)2–BO as a heterogeneous photoinitiator, three additional repeating experiments of photopolymerization were performed under the same reaction conditions in a large time scale of 24 h (Fig. S12†). PMMA was obtained without a significant loss of conversion, demonstrating the stability and reusability of the polyHIPE. The SEM image of B–(Boc-CB)2–BO after the third run still showed a similar porous structure, indicating promising stability of the conjugated polyHIPE (Fig. S9†). However, the efficiency of photoinitiation of this new-class conjugated porous polymer is still unknown; new photophysical studies are being conducted in order to gain more understanding and optimization of the system.
Conclusions
In summary, photopolymerization of MMA using π-conjugated porous polyHIPE as a heterogeneous photoinitiator could be efficiently achieved under visible light. A household energy saving light bulb was used as a light source, which provides an economically low-cost solution. The hierarchical porosity of the conjugated polyHIPE could be advantageous in attaining efficient mass transfer in the catalytic reactions. The heterogeneous nature and the stable 3D structure of polyHIPE allow its facile removal from the polymerization mixture and reusability without a significant loss of activity. Moreover, the π-conjugated polyHIPE-based photoinitiator minimizes odor, toxicity and migration problems, which usually accompany the low molar mass photoinitiators.Acknowledgements
The Max Planck Society is acknowledged for financial support. The authors thank Saman Ghasimi for the solid state NMR measurement.References
- K. S. Anseth, S. M. Newman and C. N. Bowman, Biopolymers, 1995, 122, 177–217 CAS.
- J. P. Fisher, D. Dean, P. S. Engel and A. G. Mikos, Annu. Rev. Mater. Res., 2001, 31, 171–181 CrossRef CAS.
- J. G. Kloosterboer, Adv. Polym. Sci., 1988, 84, 1–61 CrossRef CAS.
- Y. Yagci, S. Jockusch and N. J. Turro, Macromolecules, 2010, 43, 6245–6260 CrossRef CAS.
- C. G. Roffey, Photogeneration of Reactive Species for UV Curing, 1997 Search PubMed.
- J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC.
- K. Kalyanasundaram, Coord. Chem. Rev., 1982, 46, 159–244 CrossRef CAS.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. Vonzelewsky, Coord. Chem. Rev., 1988, 84, 85–277 CrossRef CAS.
- M. L. Marin, L. Santos-Juanes, A. Arques, A. M. Amat and M. A. Miranda, Chem. Rev., 2012, 112, 1710–1750 CrossRef CAS PubMed.
- M. Neumann, S. Fuldner, B. Konig and K. Zeitler, Angew. Chem., Int. Ed., 2011, 50, 951–954 CrossRef CAS PubMed.
- H. J. Liu, W. Feng, C. W. Kee, Y. J. Zhao, D. Leow, Y. H. Pan and C. H. Tan, Green Chem., 2010, 12, 953–956 RSC.
- J. Lalevee, M. A. Tehfe, F. Dumur, D. Gigmes, N. Blanchard, F. Morlet-Savary and J. P. Fouassier, ACS Macro Lett., 2012, 1, 286–290 CrossRef CAS.
- S. Jockusch, H. J. Timpe, W. Schnabel and N. J. Turro, J. Phys. Chem. A, 1997, 101, 440–445 CrossRef CAS.
- B. Ganster, U. K. Fischer, N. Moszner and R. Liska, Macromolecules, 2008, 41, 2394–2400 CrossRef CAS.
- N. Moszner, U. K. Fischer, B. Ganster, R. Liska and V. Rheinberger, Dent. Mater., 2008, 24, 901–907 CrossRef CAS PubMed.
- J. Jakubiak, X. Allonas, J. P. Fouassier, A. Sionkowska, E. Andrzejewska, L. A. Linden and J. F. Rabek, Polymer, 2003, 44, 5219–5226 CrossRef CAS.
- M. Degirmenci, A. Onen, Y. Yagci and S. P. Pappas, Polym. Bull., 2001, 46, 443–449 CrossRef CAS.
- J. Jakubiak and J. F. Rabek, Polimery, 1999, 44, 447–461 CAS.
- N. Davidenko, O. Garcia and R. Sastre, J. Biomater. Sci., Polym. Ed., 2003, 14, 733–746 CrossRef CAS PubMed.
- B. Gacal, H. Akat, D. K. Balta, N. Arsu and Y. Yagci, Macromolecules, 2008, 41, 2401–2405 CrossRef CAS.
- G. Temel, B. Aydogan, N. Arsu and Y. Yagci, Macromolecules, 2009, 42, 6098–6106 CrossRef CAS.
- Y. Yagci and A. Onen, J. Macromol. Sci., Part A: Pure Appl.Chem., 1991, 28, 25–29 CrossRef.
- H. Akat, B. Gacal, D. K. Balta, N. Arsu and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2109–2114 CrossRef CAS.
- B. Kiskan, M. Antonietti and J. Weber, Macromolecules, 2012, 45, 1356–1361 CrossRef CAS.
- B. Kiskan, J. S. Zhang, X. C. Wang, M. Antonietti and Y. Yagci, ACS Macro Lett., 2012, 1, 546–549 CrossRef CAS.
- M. Bokhari, R. J. Carnachan, N. R. Cameron and S. A. Przyborski, Biochem. Biophys. Res. Commun., 2007, 354, 1095–1100 CrossRef CAS PubMed.
- N. Dizge, B. Keskinler and A. Tanriseven, Colloids Surf., B, 2008, 66, 34–38 CrossRef CAS PubMed.
- S. J. Pierre, J. C. Thies, A. Dureault, N. R. Cameron, J. C. M. van Hest, N. Carette, T. Michon and R. Weberskirch, Adv. Mater., 2006, 18, 1822–1826 CrossRef CAS.
- F. Su, C. L. Bray, B. Tan and A. I. Cooper, Adv. Mater., 2008, 20, 2663–2666 CrossRef CAS.
- K. Zhang, Z. Vobecka, K. Tauer, M. Antonietti and F. Vilela, Chem. Commun., 2013, 49, 11158–11160 RSC.
- Z. J. Wang, S. Ghasimi, K. Landfester and K. A. I. Zhang, 2014, submitted.
- A. Kohler, D. A. dos Santos, D. Beljonne, Z. Shuai, J. L. Bredas, A. B. Holmes, A. Kraus, K. Mullen and R. H. Friend, Nature, 1998, 392, 903–906 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, monomer synthesis, FT-IR and solid-state 13C/MAS NMR spectra, N2 gas sorption data, additional SEM images and reaction mechanism. See DOI: 10.1039/c4py00323c |
| This journal is © The Royal Society of Chemistry 2014 |