Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermoresponsive polymerized gemini dicationic ionic liquid
Yongjun
Men
,
Helmut
Schlaad
*,
Antje
Voelkel
and
Jiayin
Yuan
*
Max Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, Research Campus Golm, D-14424 Potsdam, Germany. E-mail: jiayin.yuan@mpikg.mpg.de; Fax: +49-331-5679502; Tel: +49-331-5679552
First published on 10th February 2014
Abstract
A new type of gemini poly(ionic liquid), poly[(1,8-octanediyl-bis(tri-n-butylphosphonium) 4-styrene sulfonate], was synthesized via the free radical polymerization of a dicationic ionic liquid monomer in DMF. This poly(ionic liquid) presented a lower critical solution temperature (LCST)-type phase transition in aqueous solution. Unusually this phase transition can be observed even at a concentration as low as 0.1 wt%. Additionally, the type and concentration of external salt can influence the cloud point. Copolymerisation of this gemini dicationic ionic liquid monomer with divinylbenzene crosslinker in water led to the formation of a hydrogel, which exhibited a temperature-triggered volume change in water.
Introduction
Thermoresponsive polymers, which usually display a reversible phase transition in their solution at a specific temperature, have received major interest in the past two decades.1–5 Generally speaking, water-soluble thermoresponsive polymers are of special interest with respect to their wide applications in tissue engineering, controlling systems and sensing devices.6–8 The best known examples of these polymers are poly(N-isopropylacrylamide) (PNIPAM) and the poly(meth)acrylamide derivatives with hydrogen-bonding properties and hydrophobic interactions, which exhibit a lower critical solution temperature (LCST) behavior in aqueous solution.6,9–12 Except for zwitterionic polymers, like poly[3-(N-(3-methacrylamidopropyl)-N,N-dimethylammonio)-propanesulfonate], most thermoresponsive polymers themselves are nonionic in bulk.Conventional polyelectrolytes are water-soluble polymers carrying ionizable functionalities along the polymer chain, which have a wide range of applications, such as water purification, oil recovery, pigment removal, paper making, layer-by-layer technology and so on. Environmentally responsive or smart polyelectrolytes are normally considered as pH or salt responsive polymers. Recent research demonstrates that strong polyelectrolytes in solution possess thermoresponsive properties. Aoshima et al. synthesized poly(vinyl ether)s with pendant imidazolium salt moieties, which presented an upper critical solution temperature (UCST) or LCST phase separation in organic solvents of specific polarity.13 By changing the anion to tetrafluoroborate in these polymers, a UCST-type phase separation in water was observed. Ritter et al. first reported that poly[1-butyl-3-vinylimidazolium bis(trifluoromethylsulfonyl)imide], when mixed with cyclodextrin in water, showed a pseudo-LCST effect.14 The cyclodextrin ring complexed with the large-sized hydrophobic Tf2N anion at low temperature to make the polymer soluble in water, and slipped off at high temperature returning the polymer to its hydrophobic state and making it insoluble in water again.
Homopolymers of polyelectrolytes with thermoresponsive properties in aqueous solution were first observed by Ohno's group. They initially studied an ionic liquid (IL), tetra-n-butylphosphonium styrene sulfonate (SS-P), which showed a LCST-type phase transition in water. Surprisingly, polymerization of SS-P yielded a poly(ionic liquid) (PIL) exhibiting similar thermo-sensitive behavior.15,16 Recently, the same group synthesized another strong anionic polyelectrolyte, poly(tributylhexylphosphonium 3-sulfopropyl methacrylate), with LCST-type phase separation in water in a wide temperature range.17 Shortly before the submission of this manuscript, Diamond's group prepared copolymer thermoresponsive hydrogels from these two anionic PILs with several diacrylate crosslinkers of various length of the oligo(ethylene glycol).18 The volume transition temperature of these gels decreased with increasing gel crosslinker concentration.
In anionic PILs, the cation (such as the tetrabutylphosphonium cation) of the ILs always needs to be synthesized by careful structure design, but in cationic PILs, the corresponding anion, such as styrene sulfonate, is easily obtained from commercially available sodium salts. This means that the hydrophilicity/hydrophobicity of the anion is more easily tunable than that of the cation from a synthetic point of view. Our group synthesized the first cationic PIL, poly(tributyl-4-vinylbenzylphosphonium pentanesulfonate), exhibiting a LCST-type phase transition in aqueous solution.19 The transition temperature was variable in terms of the PIL concentration and externally added salts. These thermoresponsive PILs have demonstrated potentials as “smart” stabilizers for carbon and metal nanoparticles. For example, raising the temperature of a colloidal dispersion of graphene sheets or gold nanoparticles stabilized by these PILs would precipitate out the inorganic colloids at a specific and designable temperature.
The thermoresponsive behavior of PILs has a tight relationship with their chemical structures and hydrophilicity. Until now all the thermoresponsive ILs and PILs reported are monovalent ion molecules or polymers. Gemini (dimeric) ILs are made up of two monomeric ILs connected via the headgroups by a spacer that may be hydrophilic, hydrophobic, flexible, or rigid. Comparing with the corresponding monomeric IL, gemini ILs have a higher surface activity in aqueous solution, and exhibit a much lower critical micelle concentration, and a higher efficiency in reducing the oil–water interfacial tension.
PILs with gemini ions have the potential to present special phenomena due to their thermoresponsive properties. A key factor governing the LCST (and surface tension) is the polymer structure arising from the polymer–water interactions. Therefore, thermoresponsive PILs bearing gemini ions are of special interest, as they might present a different behavior from those made up of monomeric IL monomers.
In this research, we synthesized a gemini dication-based PIL by free radical polymerization in DMF. The PIL with unexpected high solubility is made up of a poly(4-styrene sulfonate) backbone and a gemini dication. This PIL in water showed a LCST-type phase transition, tunable in terms of the polymer concentration and the externally added salts. Dynamic light scattering (DLS) measurement was employed to gain insight into the phase separation process. When synthesized in the presence of a crosslinker divinylbenzene, a thermoresponsive hydrogel was obtained.
Experimental section
Materials
All chemicals were purchased from Aldrich: tetra-n-butylphosphonium bromide (P-Br, 99%), tri-n-butylphosphine (99%), 1,8-dibromooctane (98%), divinylbenzene (technical grade, 80%), sodium 4-styrene sulfonate (SS-Na, technical, ≥90%), and all other chemicals (analytical grade) were used as received. 2,2′-Azobis(2-methylpropionitrile) (AIBN, 98%) was recrystallized from hexane.Synthesis of 1,8-octanediyl-bis(tri-n-butylphosphonium) dibromide (P2-Br)
30.3 g (0.15 mol) of tributylphosphine and 13.6 g (0.05 mol) of 1,8-dibromooctane and 150 mL dry acetone were loaded into a 250 mL round bottomed flask, containing a magnetic stir bar. The mixture was heated to 40 °C for 48 h, and then precipitated into 1.5 L diethyl ether. The solid was filtered off and washed several times with diethyl ether. The resulting white powder was dried in vacuum oven to obtain a final yield of 30.1 g (89%).Synthesis of 1,8-octanediyl-bis(tri-n-butylphosphonium) 4-styrene sulfonate (SS-P2)
27.06 g (0.04 mol) of P2-Br, 20.62 g (0.1 mol) of sodium 4-styrene sulfonate (SS-Na) and 40 mL water were loaded into a 250 mL reactor. The mixture was stirred at room temperature for 30 min. The product was extracted 3 times with CH2Cl2 (50 mL × 3). The organic phases were combined and washed with water (25 mL × 3). After solvent evaporation, the product (SS-P2) was dried at room temperature by high vacuum until constant weight (26.65 g, 75% yield).Synthesis of poly[1,8-octanediyl-bis(tri-n-butylphosphonium) 4-styrene sulfonate], poly(SS-P2)
25 g (0.0283 mol) of SS-P2, 98.6 mg of (0.57 mmol) AIBN and 30 mL DMF were added into a 100 mL flask. The mixture was sealed with a septum and deoxygenated by flushing with argon for 30 min. The solution was stirred at 90 °C for 12 h. The obtained poly(SS-P2) solution was dialyzed against deionized water exhaustively (molecular weight cut off: 3500 Da). After freeze-drying, 17.5 g of product was obtained (70% yield).Synthesis of poly(tri-n-butylphosphonium 4-styrene sulfonate), poly(SS-P)
The synthesis procedures and conditions were kept the same as for poly(SS-P2), using tetra-n-butylphosphonium bromide (P-Br) instead of P2-Br.Measurement of the molar mass of poly(SS-P2)
0.5 g of poly(SS-P2) solution was loaded into a dialysis tube (MWCO 3500 Da) and dialyzed against NaCl solution (10 wt%) for 7 d. The NaCl solution was changed twice per day. Then, the tube was transferred into deionized water to remove free NaCl salt. Dialysis water was refreshed 10 times with an interval of 12 h. The solution after cation exchange (PSS-Na solution) was freeze-dried to obtain a PSS-Na powder. The average molar mass of the PSS-Na was measured by aqueous size exclusion chromatography (SEC) using PSS-Na calibration: Mn = 5270 g mol−1 (dispersity Mw/Mn = 3.2). The number-average molar mass of the corresponding poly(SS-P2) is then calculated by| Mn(poly(SS-P2)) = [M(SS-P2)/M(SS-Na)] × Mn(PSS-Na) = 2.14 × Mn(PSS-Na) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1. The cation exchange to tetra-n-butylphosphonium was performed applying the same procedure.
300 g mol−1. The cation exchange to tetra-n-butylphosphonium was performed applying the same procedure.
Preparation of poly(SS-P2) hydrogel
250 mg (2.83 mmol) of SS-P2, 37 mg (0.283 mmol) of divinylbenzene, 9.69 mg (0.059 mmol) of AIBN and 1 mL water were loaded into a 5 mL tube reactor. The mixture was polymerized at 80 °C for 12 h. The gel phase was separated from the water phase during polymerization. The water in the upper layer was discarded, and the crosslinked poly(SS-P2) hydrogel was obtained at the bottom.Characterization methods
1H-NMR measurements were conducted on a Bruker DPX-400 operating at 400.1 MHz in D2O (signals referenced to δ 4.65 ppm). Size exclusion chromatography (SEC) with simultaneous UV (260 nm) and RI detection was performed with 0.1 N aqueous NaNO3 as the eluent at a flow rate of 1.0 mL min−1 at 25 °C. A PSS-MCX (300 × 8 mm2, 10 μm, 105 Å) column was used as the stationary phase, and the calibration was done with PSS-Na standards (PSS, Mainz, Germany). Temperature dependent UV/Vis measurements were conducted using a T70+ UV/Vis Spectrophotometer (PG Instruments Ltd, Leicestershire, England) equipped with a PTC-2 Peltier temperature controller (temperature control accuracy ±0.5 °C). The measurements were performed with aqueous polymer solutions in quartz cuvettes using an absorbance wavelength of 660 nm; heating rate was 1 °C min−1. Dynamic light scattering (DLS) measurements were performed on an ALV/CGS-3 compact goniometer system, equipped with a 22 mW HeNe laser, at a fixed scattering angle of 90°. Heating/cooling of polymer samples in a thermostat was done in steps of 2 °C from 25 °C to 58 °C. At every temperature, the first measurement was started after 600 s and data were accumulated for 5 × 30 s. Evaluation of data was done using the REPES program.20Results and discussion
Synthesis and characterization of gemini poly(ionic liquid)
As outlined in Scheme 1, quaternization reaction of tri-n-butylphosphine with 1,8-dibromooctane at 40 °C in acetone was employed to prepare the quaternary phosphonium bromide compound 1,8-octanediyl-bis(tri-n-butylphosphonium) dibromide (P2-Br). In this structure, two phosphonium bromide units are bridged via an octyl spacer. After exchange of the bromide anion in P2-Br with the sulfonate in sodium 4-styrene sulfonate (SS-Na), a polymerizable gemini dicationic IL SS-P2, containing two 4-styrene sulfonate anions was obtained. The final product poly(SS-P2) used in this work was synthesized via conventional free radical polymerization of SS-P2 in DMF at 90 °C using AIBN (0.4 wt% with respect to monomer) as the initiator.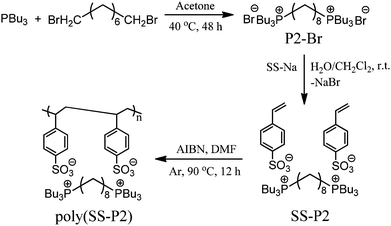 | ||
| Scheme 1 The chemical structure and synthetic route to a PIL prepared from a gemini dicationic IL. SS-Na: sodium 4-styrene sulfonate. | ||
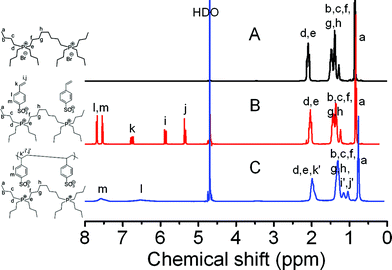
Proton nuclear magnetic resonance (1H-NMR) spectra of these compounds in D2O (Fig. 1) were recorded to confirm the chemical structures of the products at each synthetic step. Fig. 1A displays the 1H-NMR spectrum of P2-Br. The methyl protons (−CH3) at the end of the butyl chain appear at 0.9 ppm, the methylene protons (–CH2–) next to the phosphonium at 2.0 ppm, and all other methylene protons at 1.2–1.6 ppm. After anion exchange to incorporate the sulfonate anion, the peaks of the alkyl chains at 0.8–2.0 ppm on the phosphonium cation are not affected (Fig. 1B). The newly introduced peaks are attributed to the 4-styrene sulfonate anion, namely the phenyl ring peaks at 7.6 and 7.7 ppm, and the well-resolved three vinyl proton signals at 5.4, 5.9, and 6.7 ppm. The integral ratio of the –CH2– protons next to the phosphonium cation (d and e in Fig. 1B) to the phenyl protons (i and m in Fig. 1B) is 2.0, identical to the theoretical value of SS-P2, which confirms the complete exchange between bromide and styrene sulfonate. Following the polymerization, the three peaks from the vinyl protons vanished, and the newly formed backbone protons overlap with the cation alkyl protons at 1.1–1.4 ppm. Thus, 1H-NMR spectra verify the successful synthesis of the gemini dicationic IL and the corresponding PIL.
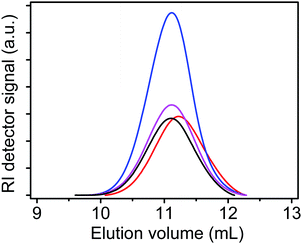
Poly(SS-P2) was further characterized by aqueous size exclusion chromatography (SEC). Fig. 2 shows the SEC traces of poly(SS-P2), poly(SS-P), and also PSS-Na and poly(SS-P) obtained via cation exchange of poly(SS-P2) with NaCl and P-Br, respectively. It can be observed that the poly(SS-P2) and its cation-exchanged products, PSS-Na and poly(SS-P), have almost identical elution volumes and thus hydrodynamic sizes. This may suggest that the cations of all three samples are exchanged by sodium when dissolved at 0.15 wt% in 0.1 M NaNO3 solution (eluent). Hence, the absolute number-average molar mass (Mn) of poly(SS-P2) can be calculated, based on a PSS-Na calibration, to be 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1 (see Experimental section), which corresponds to 25 styrene sulfonate units. Interestingly, poly(SS-P2) prepared from SS-P2 has a higher molar mass than poly(SS-P) prepared from SS-P under otherwise identical polymerization conditions. This might be caused by the dicationic monomer structure of SS-P2, having two styrene sulfonate units in very close proximity (high local monomer concentration).
300 g mol−1 (see Experimental section), which corresponds to 25 styrene sulfonate units. Interestingly, poly(SS-P2) prepared from SS-P2 has a higher molar mass than poly(SS-P) prepared from SS-P under otherwise identical polymerization conditions. This might be caused by the dicationic monomer structure of SS-P2, having two styrene sulfonate units in very close proximity (high local monomer concentration).
LCST-type phase transition behavior
Monomer SS-P2 and poly(SS-P2) were both soluble in water at room temperature (<25 °C) at a concentration of 2 wt%, yet solutions turned turbid upon heating. Ultraviolet-visible (UV-vis) spectroscopy (operated at λ = 660 nm) was employed to determine the cloud point temperatures (Tcp), defined here as the temperature at which transmission dropped to 80%. The turbidity curves of SS-P2 (Fig. 3) show that the Tcp increases upon dilution: Tcp = 6 °C (at 2 wt%), 33 °C (1 wt%), and 37 °C (0.5 wt%). The Tcp is higher than the boiling point of water at an even lower concentration of 0.2 wt%. It should be noted that at 2 wt%, the Tcp value is 26 °C, lower than for its corresponding mono-cation IL, tetra-n-butylphosphonium 4-styrene sulfonate (SS-P), at the same concentration, as reported previously.16,21 Evidently, connecting two phosphonium cations into a gemini dication strongly influences the solution and phase separation behavior. | ||
| Fig. 3 Turbidity curves of SS-P2 at concentrations of 2 wt% (black), 1 wt% (red), 0.5 wt% (blue), and 0.2 wt% (magenta) in water. | ||
Concentration-dependent cloud point temperatures, i.e. phase transitions, were also observed for poly(SS-P2), see Fig. 4A. The Tcp increases from 35 °C (10 wt%) to 38 °C (2 wt%) and further up to 51 °C (0.1 wt%), hence the Tcp variation is more sensitive at lower concentrations. The phase transition is always sharp and hysteresis is very narrow, e.g. just 0.8 °C (2 wt%) and 0.4 °C (0.5 wt%) (Fig. 4B). The values for poly(SS-P2) are thus significantly lower than for poly(SS-P), made up of the monovalent IL monomer.21 Notably, poly(SS-P2) exhibits a higher phase separation temperature (43 °C at 0.5 wt%), i.e. it is more hydrophilic, than the monomer SS-P2 (36 °C at 0.5 wt%). It appears that the shielding of hydrophobic styrene units from the water phase is better achieved in a polymer chain structure rather than in a (non-aggregated) monomer.
 | ||
| Fig. 4 (A) Plot of the cloud point temperature vs. poly(SS-P2) concentration. (B) Turbidity curves of poly(SS-P2) at 2 wt% (blue) and 0.5 wt% (red) in water for 3 cycles. | ||
Turbidity measurements can only detect macroscopic phase separations, that is when the agglomerates are already very large (>1 μm) and/or concentration (density) is sufficiently high. For insights into the transformation processes on the nanometer level, we conducted temperature-dependent dynamic light scattering (DLS) measurements. Results, i.e. hydrodynamic radii (Rh) of particles, for poly(SS-P2) at 0.1 and 0.5 wt% in water are displayed in Fig. 5. At room temperature, both (optically clear) solutions contain molecularly dissolved polymer chains as well as a tiny fraction (<0.1% in number) of aggregates with Rh < 100 nm. Upon heating, the primary soluble aggregates continually grow. For the 0.5 wt% solution, a sudden increase of aggregate size from 180 nm to >600 nm occurs at 44 °C. This temperature is close to the Tcp (43 °C) as determined by turbidity measurement. At higher temperatures a decrease in aggregate size is observed which is due to the sedimentation of the particles out of the scattering volume. For the more dilute sample (0.1 wt%), on the other hand, DLS only detects a continuous growth of particles and no sharp transition until 60 °C. The Tcp of this sample as determined by turbidity measurement is 51 °C, corresponding to an aggregate size of 130 nm in Fig. 5. Here, the aggregates, though stable in solution, are large enough to scatter the visible light and turn the original solution to a turbid dispersion. Upon cooling back to room temperature, both samples clear again. The solutions contain molecularly dissolved chains and small aggregates, as before, demonstrating the reversibility of the phase transition.
It has earlier been observed that the addition of foreign salts can shift the Tcp of thermoresponsive PILs in a wide range.21 Normally, Tcp will increase upon adding more hydrophilic salts and decrease upon adding more hydrophobic salts. For instance, the Tcp of a solution of poly(SS-P) increases upon the addition of KBr and decreases upon the addition of P-Br due to different hydrophobicity of the cations. Ohno et al.17 also demonstrated that a buffer solution containing KH2PO4/K2HPO4 could decrease the Tcp of another anionic PIL, poly(tributylhexylphosphonium 3-sulfopropyl methacrylate). In the present system, the gemini dication-based poly(SS-P2) shows a similar behavior. Fig. 6A depicts the turbidity curves of the aqueous solution of poly(SS-P2) at 0.5 wt% at various concentrations of KBr. The Tcp of poly(SS-P2) increases from 43 °C to 46 °C at a very low KBr concentration of about 0.01 M, and further increases to 55 °C, 64 °C and 73 °C for [KBr] = 0.1 M, 0.2 M and 0.3 M, respectively. At all these conditions, the turbidity curves dropped sharply at their Tcp. No clouding, i.e. Tcp > 95 °C, is observed at 0.5 M KBr.
Other salts like SS-P2, P2-Br, and P-Br were also applied at the same polymer concentration (Fig. 6B). Addition of the more hydrophobic gemini diphosphonium (P22+) cation lowers the Tcp more effectively than the tetra-n-butylphosphonium (P+) cation, for instance to below 31 °C at 0.02 M SS-P2. In fact, the addition of P-Br seems to have no effect on cloud point temperature due to a balanced contribution between the anion exchange effect and salting out effect. From this point of view the solution behavior of poly(SS-P2) can be varied via the choice of salt additives (like the Hofmeister salt effect).6
Thermoresponsive hydrogel
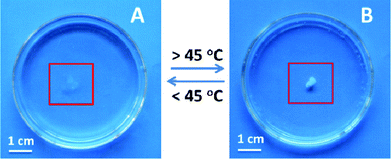
It was expected (or hoped) that the polymerization of SS-P2 in DMF would produce a gel, the chains being crosslinked by ionic interactions through the gemini diphosphonium cation. Possible explanations for the failure of crosslinking could be the low degree of polymerization (25) and the dynamic ionic bonding between the PSS backbone and the P2 dication. A poly(SS-P2) gel could be produced by polymerization of SS-P2 using divinylbenzene (10 mol% with regard to SS-P2 monomer) as crosslinking agent and AIBN as initiator in water at 80 °C. At this high temperature and concentration, SS-P2 separates from water forming a two-phase mixture (cf.Fig. 3) and polymerization proceeds under heterophase conditions. The produced poly(SS-P2) gel, which settles at the bottom of the reaction vessel, is dialyzed against water for 72 h (to remove unreacted monomer), isolated, and dried in vacuum (1 × 10−3 mbar) at 100 °C.The water content in the hydrogel was determined to be 92.4 wt%. The solid content in the gel is 7.6 wt%, a concentration at which the Tcp of poly(SS-P2) should be around 36 °C (very close to human body temperature). Considering the hydrophobic divinylbenzene fraction, the Tcp is expected to drop even below 36 °C. However, the transformation temperature of the hydrogel state was recognized to be 45 °C (Fig. 7). It appears that the polymer chains in a crosslinked state might have a different physical environment from the free polymer chains in solution. Crosslinking of poly(SS-P2) chains, however, seems to increase the cloud point temperature.
Conclusion
Soluble poly[1,8-octanediyl-bis(tri-n-butylphosphonium) 4-styrene sulfonate] (poly(SS-P2)) was synthesized via free radical polymerization of the IL monomer SS-P2 in DMF. This polymer contains a PSS backbone and a gemini quaternary diphosphonium cation. In aqueous solution it shows a LCST-type phase transition, which is tunable in terms of polymer concentration and externally added salts. Uniquely, this polymer shows a detectable cloud point (Tcp) at a rather low concentration (0.1 wt%) and a very narrow hysteresis (<1 °C), which has not been observed in previously reported PILs with a LCST-type phase transition. A thermoresponsive poly(SS-P2) hydrogel could also be prepared with the aid of divinylbenzene as crosslinking agent.Acknowledgements
The authors thank the Max Planck Society for financial support. YM would like to acknowledge a scholarship from the China Scholarship Council.Notes and references
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- I. Dimitrov, B. Trzebicka, A. H. E. Müller, A. Dworak and C. B. Tsvetanov, Prog. Polym. Sci., 2007, 32, 1275–1343 CrossRef CAS.
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173–1222 CrossRef CAS.
- C. Weber, R. Hoogenboom and U. S. Schubert, Prog. Polym. Sci., 2012, 37, 686–714 CrossRef CAS.
- J. W. Robinson, C. Secker, S. Weidner and H. Schlaad, Macromolecules, 2013, 46, 580–587 CrossRef CAS.
- N. ten Brummelhuis, C. Secker and H. Schlaad, Macromol. Rapid Commun., 2012, 33, 1690–1694 CrossRef CAS PubMed.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC.
- Y. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, J. Am. Chem. Soc., 2005, 127, 14505–14510 CrossRef CAS PubMed.
- E. Karjalainen, N. Chenna, P. Laurinmaki, S. J. Butcher and H. Tenhu, Polym. Chem., 2013, 4, 1014–1024 RSC.
- N. Weber, J. Texter and K. Tauer, Macromol. Symp., 2011, 302, 224–234 CrossRef CAS.
- J. Niskanen, C. Wu, M. Ostrowski, G. G. Fuller, S. Hietala and H. Tenhu, Macromolecules, 2013, 46, 2331–2340 CrossRef CAS.
- Y. Men, M. Drechsler and J. Yuan, Macromol. Rapid Commun., 2013, 34, 1721–1727 CrossRef CAS PubMed.
- H. Yoshimitsu, A. Kanazawa, S. Kanaoka and S. Aoshima, Macromolecules, 2012, 45, 9427–9434 CrossRef CAS.
- S. Amajjahe and H. Ritter, Macromolecules, 2008, 41, 3250–3253 CrossRef CAS.
- Y. Kohno and H. Ohno, Aust. J. Chem., 2011, 64, 1560–1567 CrossRef CAS.
- Y. Kohno and H. Ohno, Aust. J. Chem., 2012, 65, 91–94 CrossRef CAS.
- Y. Kohno, Y. Deguchi and H. Ohno, Chem. Commun., 2012, 48, 11883–11885 RSC.
- B. Ziolkowski and D. Diamond, Chem. Commun., 2013, 49, 10308–10310 RSC.
- Y. Men, H. Schlaad and J. Yuan, ACS Macro Lett., 2013, 2, 456–459 CrossRef CAS.
- J. Jakes, Collect. Czech. Chem. Commun., 1995, 60, 1781–1797 CrossRef CAS.
- Y. Men, X.-H. Li, M. Antonietti and J. Yuan, Polym. Chem., 2012, 3, 871–873 RSC.
| This journal is © The Royal Society of Chemistry 2014 |