Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aminobenzodione-based polymers with low bandgaps and solvatochromic behavior†
Haichang
Zhang
a,
Saman
Ghasimi
a,
Bernd
Tieke
*a,
Alexander
Schade
b and
Stefan
Spange
b
aDepartment of Chemistry, University of Cologne, Luxemburger Str.116, D-50939 Cologne, Germany. E-mail: tieke@uni-koeln.de
bDepartment of Polymer Chemistry, Chemnitz University of Technology, Strasse der Nationen 62, D-09111 Chemnitz, Germany
First published on 25th March 2014
Abstract
Pd-catalyzed amination polymerization of deep blue aminobenzodifuranone monomer 3-(4-bromo-phenyl)-7-(4-octylaminophenyl)-benzo[1,2-b:4,5-b′]-difuran-2,6-dione (M1) is described, as well as polymerization of symmetric dibromophenyl-benzodifuranone (M2), or the corresponding dibromophenylbenzodipyrrolidone (M3) with N,N′-dialkylated phenylenediamines (M4a,b). The resulting polymers P1–3 exhibit low bandgaps (1.08–1.47 eV), broad UV/vis absorption bands (400–900 nm), and a large solvatochromic shift up to 3140 cm−1 from n-hexane to hexamethylphosphoramide. Multiple linear regression analyses of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max of the solvent-dependent solvatochromic UV/vis absorption bands of M1, P1 and P2a are presented, from which Kamlet–Taft and Catalán solvent parameters were determined. All monomers and polymers exhibit high extinction coefficients up to 8.6 × 104 L mol−1 cm−1 and high photostability, and might be suitable for electronic applications.
max of the solvent-dependent solvatochromic UV/vis absorption bands of M1, P1 and P2a are presented, from which Kamlet–Taft and Catalán solvent parameters were determined. All monomers and polymers exhibit high extinction coefficients up to 8.6 × 104 L mol−1 cm−1 and high photostability, and might be suitable for electronic applications.
Introduction
Molecular D–A (donor–acceptor) systems are of current interest owing to their potential applications in molecular electronic devices,1 nonlinear optics,2 and artificial photosynthetic systems.3 Among all kinds of electron acceptor moieties, benzodifuranone (BDF), benzodipyrrolidone (BDP) and their derivatives because of their quinonoid structure have received much attention recently as promising candidates for electron-deficient materials.4,5 BDF and BDP are high-performance pigments serving as important building blocks for near-infrared (NIR) absorption. They were first developed in the mid-1970s and commercialized as disperse dyes owing to their deep color and high photochemical stability.6 Depending on the substitution pattern, BDFs exhibit red to blue colors.7Monomeric aminobenzodifuranone (ABDF) is a deep blue colored dye with interesting solvatochromic behavior,8 which has attracted our attention. The aminobenzodifuranone chromophore is a typical D–A system, in which aniline as an electron donor is combined with benzodifuranone as an acceptor unit. To our knowledge, only a few articles reported on derivatives of ABDF,8 while polymers based on ABDF are completely unknown.
Experimental section
Materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.
241.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.
600.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (6 ml) under nitrogen. Then, cesium carbonate (310 mg, 0.95 mmol) is added and the mixture is allowed to stir for 48 hours under nitrogen at 90 °C. After completion of the reaction, the dark blue solution is treated with DCM, washed three times with water and once with brine. Then the organic layer is dried over anhydrous magnesium sulfate, and the solvent is removed at reduced pressure. Subsequently the crude product is dissolved in a minimal amount of DCM and precipitated in methanol. The product is obtained as a dark solid (181.2 mg, yield: 63%). 1H NMR (300 MHz, CDCl3) δ ppm: 7.71–7.78 (d, 4H), 6.88–7.06 (d, 2H), 6.90 (s, 4H), 6.71–6.78 (d, 2H), 6.63–6.69 (d, 2H), 3.55–3.65 (d, 2H), 3.03–3.09 (d, 2H), 1.70–1.86 (br, 2H), 1.22–1.49 (m, 16H), 1.06–1.40 (m, 10H), 0.88–0.99 (m, 12H). Molecular weight (GPC, THF): Mw = 5.7 kDa, PD = 1.5. UV/vis (DCM): 709 nm, UV/vis (thin film): 724 nm; ε (709)/L mol−1 cm−1: 16
1) (6 ml) under nitrogen. Then, cesium carbonate (310 mg, 0.95 mmol) is added and the mixture is allowed to stir for 48 hours under nitrogen at 90 °C. After completion of the reaction, the dark blue solution is treated with DCM, washed three times with water and once with brine. Then the organic layer is dried over anhydrous magnesium sulfate, and the solvent is removed at reduced pressure. Subsequently the crude product is dissolved in a minimal amount of DCM and precipitated in methanol. The product is obtained as a dark solid (181.2 mg, yield: 63%). 1H NMR (300 MHz, CDCl3) δ ppm: 7.71–7.78 (d, 4H), 6.88–7.06 (d, 2H), 6.90 (s, 4H), 6.71–6.78 (d, 2H), 6.63–6.69 (d, 2H), 3.55–3.65 (d, 2H), 3.03–3.09 (d, 2H), 1.70–1.86 (br, 2H), 1.22–1.49 (m, 16H), 1.06–1.40 (m, 10H), 0.88–0.99 (m, 12H). Molecular weight (GPC, THF): Mw = 5.7 kDa, PD = 1.5. UV/vis (DCM): 709 nm, UV/vis (thin film): 724 nm; ε (709)/L mol−1 cm−1: 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721.
721.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (3.2 ml) under nitrogen. Then, cesium carbonate (198 mg, 0.60 mmol) is added and the mixture is allowed to stir for 48 hours under nitrogen at 90 °C. After completion of the reaction, the dark solution is treated with DCM, washed three times with water and once with brine. Then the organic layer is dried over anhydrous magnesium sulfate and the solvent is removed at reduced pressure. Subsequently, the crude product is dissolved in a minimal amount of DCM and precipitated in methanol. The product is obtained as a dark blue solid (156.5 mg, yield: 54%). 1H NMR (300 MHz, CDCl3) δ ppm: 7.70–7.76 (d, 4), 7.28 (s, 2H), 6.96–7.02 (d, 4H), 6.61–6.66 (d, 2H), 6.51–6.59 (d, 2H), 3.58 (s, 2H), 3.03 (s, 2H), 2.04 (s, 2H), 1.12–1.43 (br, 64H), 0.88–0.93 (br, 12H). Molecular weight (GPC, THF): Mw = 9.7 kDa, PD = 1.7. UV/vis (DCM): 725 nm, UV/vis (thin film): 741 nm; ε (725)/L mol−1 cm−1: 23
1) (3.2 ml) under nitrogen. Then, cesium carbonate (198 mg, 0.60 mmol) is added and the mixture is allowed to stir for 48 hours under nitrogen at 90 °C. After completion of the reaction, the dark solution is treated with DCM, washed three times with water and once with brine. Then the organic layer is dried over anhydrous magnesium sulfate and the solvent is removed at reduced pressure. Subsequently, the crude product is dissolved in a minimal amount of DCM and precipitated in methanol. The product is obtained as a dark blue solid (156.5 mg, yield: 54%). 1H NMR (300 MHz, CDCl3) δ ppm: 7.70–7.76 (d, 4), 7.28 (s, 2H), 6.96–7.02 (d, 4H), 6.61–6.66 (d, 2H), 6.51–6.59 (d, 2H), 3.58 (s, 2H), 3.03 (s, 2H), 2.04 (s, 2H), 1.12–1.43 (br, 64H), 0.88–0.93 (br, 12H). Molecular weight (GPC, THF): Mw = 9.7 kDa, PD = 1.7. UV/vis (DCM): 725 nm, UV/vis (thin film): 741 nm; ε (725)/L mol−1 cm−1: 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911.
911.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762.
762.
Methods
Results and discussion
In this article, we report on the novel monomer M1 and four aminobenzodione-based π-conjugated polymers. M1 was prepared according to Scheme 1 which is very soluble in common organic solvents and suitable for Buchwald–Hartwig amination and Stille, Heck and Suzuki coupling because of the bromine and alkylamino end groups.The polymers consist of amino groups as electron-donating units, and BDF or BDP as electron-accepting units causing low bandgaps, broad UV/vis absorption and high photostability. BDF/BDP-based monomers M2 and M3, and the N,N′-dialkylaminobenzene monomer M4 were prepared according to the literature.4a,5a,9 The polymers were synthesized by Buchwald–Hartwig amination using Pd2(dba)3 as the catalyst and X-Phos as the ligand (Scheme 2).10
 | ||
| Scheme 2 Synthesis and structure of polymers P1–3. (i) Pd2(dba)3, X-Phos, Cs2CO3 (t-BuOK for P3), DMF–toluene (2/1) (dioxane for P1 and P3), N2. | ||
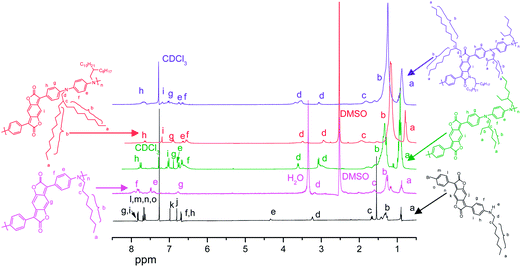
The polymers were characterized by 1H NMR spectroscopy (Fig. 1) and gel permeation chromatography (GPC). The signals around 0.8–3.3 ppm originate from the alkyl groups, while the signals around 6.72–7.96 are typical for the protons of the core of BDF and the phenyl groups attached to the core. For M1, the chemical shifts at 4.37 ppm originate from the protons attached to the amino group while these signals disappear for the polymers due to polymerization. The number-average molecular weights were found to be 5.9 kDa (PDI: 2.2, P1), 5.7 kDa (PDI: 1.5, P2a), 9.7 kDa (PDI: 1.7, P2b) and 10.1 kDa (PDI: 1.5, P3), respectively.
Optical properties
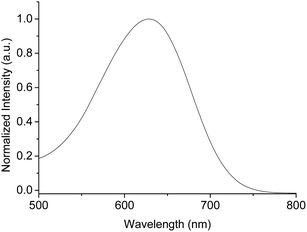
In Fig. 2, the UV/vis spectrum of M1 in dichloromethane solution is shown. M1 exhibits a broad UV/vis absorption band with a maximum at 628 nm, the extinction coefficient of the maximum being 6.5 × 104 L mol−1 cm−1. The maximum of M1 is 121 nm red-shifted compared with M2 in dichloromethane since the alkylamino unit is a powerful donor, which shifts the UV/vis absorption to a longer wavelength. M1 is readily soluble in common organic solvents such as toluene, N,N-dimethylformamide and so on, in contrast to M2, which is not very soluble in toluene or dichloromethane, and therefore not suitable for Suzuki coupling. This is firstly due to the presence of the amino group, which can form a highly polar resonance structure and interact with adjacent solvent molecules (see a suggested mechanism in S4†), and secondly the alkyl substituent of the amino group favors the solubility.In Fig. 3, the UV/vis absorption spectra of the polymers in dichloromethane solution and as thin films are shown. All polymers are deep colored, the extinction coefficients of the strongest bands being 1.7–3.1 × 104 L mol−1 cm−1. The UV/vis absorption spectra of P2a and P2b in dichloromethane exhibit broad bands with maxima at 709 and 724 nm. The maximum of P2b is slightly red-shifted compared with P2a, since P2b has a higher molecular weight and a more strongly extended π-system. The UV/vis absorption spectrum of P3 in dichloromethane exhibits a strong maximum at 623 nm. Compared with P2a and P2b, the maximum of P1 is blue-shifted (645 nm), giving rise to weaker D–A interactions between the alkylamino groups and the benzodifuranone core. In P2a and P2b enhanced intramolecular charge transfer (ICT) is due to the presence of the N,N′-dialkyl-1,4-phenylenediamine unit with a more powerful donor ability favoring the red shifted absorption. The red shift is stronger than those of the polymers containing the thienyl-benzodione chromophore (88 nm for P1, 152 nm for P2a, 167 nm for P2b and 58 nm for P3 in dichloromethane, respectively) since the alkylamino group is a more powerful electron donor than the thienyl group.4a,5a All polymers show a very broad UV/vis absorption from 400 to 900 nm, which matches the solar photon most intense flux (that is in the 400–800 nm range)11 in thin films with maxima between 638 and 725 nm (Fig. 2 and Table 1). All UV/vis absorption spectra of films are red shifted when compared to dichloromethane solution spectra. The strongest red-shift was found for P1 with 63 nm. This indicates a gain of planar conformation and/or the presence of π–π interchain association in the solid state. The optical HOMO–LUMO energy gaps estimated from the onset of absorption for films are 1.19–1.47 eV.
| Polymers | λ max [nm] | Extinct. coeff. ε (λmax) [L mol−1 cm−1] | E optg /Eecgc [eV] | k [h−1] | |
|---|---|---|---|---|---|
| In DCM | As thin films | ||||
| a Photostability was determined upon irradiation with a 200 W Hg lamp. b Optical bandgap Eoptg was measured at the onset of absorption of the polymer film (Eoptg = 1240/λabs,onset eV). c E ecg = EHOMO − ELUMO, −ELUMO = Eonset(red) + 4.8 eV and −EHOMO = Eonset(ox) + 4.8 eV, where Eonset(red) and Eonset(ox) are the onset potentials of the oxidation and reduction processes vs. ferrocene. | |||||
| P1 | 645 | 708 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.19/1.19 | 0.11 |
| P2a | 709 | 724 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721 721 |
1.31/1.23 | 0.26 |
| P2b | 725 | 741 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 911 |
1.20/1.08 | 0.33 |
| P3 | 623 | 638 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762 762 |
1.47/1.45 | 0.68 |
Electrochemical properties
The electrochemical properties of the four polymers were investigated by cyclic voltammetry. The conditions of the measurement are described in the Experimental part. The HOMO and LUMO energy levels of the polymers were estimated from the onset of the oxidation and reduction curves, respectively (see Fig. 4). It can be seen that anodic oxidation of polymers sets in at low potentials of 0.32 V (P1), 0.17 V (P2a), 0.15 V (P2b) and 0.89 V (P3), respectively. Two to three anodic waves with maxima between 0.5 and 1.2 V occur, which can be ascribed to the formation of cation radicals and dications. The reductive cycles of all polymers exhibit two reversible cathodic waves, which originate from the reduction of the quinonoid to a benzoic structure. This can possibly be explained with a stabilizing negative charge of the oxygen atoms in the carbonyl groups of both the lactone groups in the benzodione units. | ||
| Fig. 4 Cyclic voltammograms of polymers as thin films deposited on the ITO surface. Solution: 0.1 M TBAPF6/acetonitrile. Potential calculated versus ferrocene. Scan rate: 100 mV s−1; T = 20 °C. | ||
All polymers show low LUMO (−3.79 to −4.24 eV) and HOMO levels (−4.95 to −5.81 eV). Due to the low LUMO energy level of the polymers, good electron injection and ambient stabilities of OFET devices can be expected.5b The polymers exhibit quite low HOMO–LUMO bandgaps (1.08 to 1.45 eV). In Table 1, optical and bandgap data of the polymers are compiled.
Photostability properties
The photostability was studied by exposing toluene solutions of the polymers to a 200 W Hg-lamp at a distance of 20 cm and measuring the decrease of the optical absorption vs. time. In Fig. 5a, UV/vis absorption spectra of P2b in toluene are shown before and after irradiation for different time periods. Corresponding spectra of M2, M3, P1, P2a and P3 are shown in Fig. S2 and S3.† The plots of ln(At/A0) (At,0 = absorbance at time t and time t = 0) vs. time for irradiation of polymers lead to nearly straight lines. From the initial slope the rate constants (k) of the photoreaction were derived (Fig. 5 and Table 1). As can be seen from Fig. 5, P2a and P2b are more stable than P1 since the N,N′-dialkyl-1,4-phenylenediamine unit exhibits a more powerful donor ability than the alkylamino unit. Probably the strong donor–acceptor character of these polymers prevents energy transfer from the amino groups to the BDF core and thus contributes to a higher photostability. Compared with P2a, P3 is less stable, which can be ascribed to the fact that the monomer BDP chromophore is less stable than BDF in UV-light (see S2†). In general, polyiminobenzodiones are less stable than isoDPP-based conjugated polymers12 or 1,10-naphthodifuranone-based polymers,4d,13 but more stable than benzodifuranone-based polymers4d or DPP-14 and DTPP-based polymers.15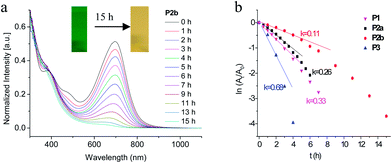 | ||
| Fig. 5 UV/vis absorption spectra of P2b before and after irradiation in toluene with a 200 W Hg-lamp and ln(At/A0) vs. time for determination of rate constants (k) for polymers. | ||
Solvatochromic properties
M1, P1 and P2a were chosen to study the solvatochromic properties. Positive solvatochromic behavior can be attributed to a number of specific and non-specific solute–solvent interactions as summarized in the simplified Kamlet–Taft equation (eqn (1)),16 where s, a, and b are solvent independent coefficients, and π*,17aα17b and β17c reflect the general solvent dipolarity/polarizability, its specific H-bond donating (HBD) ability, and its specific H-bond accepting (HBA) ability, respectively, which may or may not oppose one another in terms of solvatochromic contributions.![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 is the longest wavelength UV/vis absorption maximum of the compound measured in a particular solvent where π* = α = β = 0 (e.g. cyclohexane).8a
max,0 is the longest wavelength UV/vis absorption maximum of the compound measured in a particular solvent where π* = α = β = 0 (e.g. cyclohexane).8a![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max = max = ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 + aα + bβ + sπ* max,0 + aα + bβ + sπ* | (1) |
Until now, empirical polarity scales have always described the polarizability and dipolarity of the solvent together in one parameter. The first and only successful attempt to separate polarizability and dipolarity was suggested by Catalán with the introduction of solvent acidity (SA),18 solvent basicity (SB),19 solvent polarizability (SP)20 and solvent dipolarity (SdP)21 (eqn (2)).
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max = max = ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 + aSA + bSB + dSP + eSdP max,0 + aSA + bSB + dSP + eSdP | (2) |
The three compounds show the shortest wavelength UV/vis absorption band in n-hexane, and exhibit the strongest bathochromic shift in DMSO (for M1, P1 and P2a, Δ values (λmax,pol − λmax,nonpol) of 1700 cm−1, 2400 cm−1, and 1180 cm−1, respectively). Both equations show good accuracy for M1 with r > 0.9 (eqn (2) in Table 2 and eqn (1) in Table S2†). It is shown that especially the HBA ability of the solvents (β or SB) causes a strong bathochromic shift (b < 0). This can be easily explained by H-bonding between the NH-group in the main chain and HBA-solvents. The influence of the β-term of the solvents on the bathochromic shift of M1 represents the interactions of HBA capacity solvents with the proton of the N–H group, which increases the +M-effect and therefore strengthening of the aromatic push–pull system occurs. HBD-solvents only exhibit a small effect typical for benzodifuranone-based dyes.8b–d A weak bathochromic shift can be recognized due to the interaction with the carbonyl groups (a < 0). Polarizability and dipolarity cause a bathochromic shift (π*, SP and SdP < 0), respectively. The solvatochromic range of M1 is 1700 cm−1 from n-hexane to DMSO. Hexamethylphosphoramide (HMPA) has not been included in the correlation, since it causes an unexpectedly high bathochromic shift of the UV/vis absorption maximum.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0 cyclohexane, number of solvents (n), correlation coefficient (r), standard deviation (sd), and significance (f) of the calculated solvatochromism of the model compounds M1, P1 and P2a
max,0 cyclohexane, number of solvents (n), correlation coefficient (r), standard deviation (sd), and significance (f) of the calculated solvatochromism of the model compounds M1, P1 and P2a
| Compound | Catalán equation | Correlation data | |||||||
|---|---|---|---|---|---|---|---|---|---|
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max,0
max,0
|
a | b | d | e | n | r | sd | f | |
| M1 | 18.537 | −0.582 | −1.158 | −2.726 | −0.570 | 32 | 0.95 | 0.156 | <0.0001 |
| P1 | 20.284 | −0.534 | −1.191 | −5.009 | −1.246 | 33 | 0.93 | 0.284 | <0.0001 |
| P2a | 16.629 | 0.137 | −0.428 | −2.452 | −0.541 | 33 | 0.88 | 0.168 | <0.0001 |
Since, in P1, the N–H function is substituted, the effect of HBA-ability (b) should be decreased, but this was not observed. The remaining influence of the HBA-ability of the solvent on the polymeric dye might be ascribed to an N–H end group, for example. Especially for short-chain polymers a visible influence of end groups is conceivable. The influences of H-bonding (a + b) are almost unchanged. The most dominant effect on the solvatochromic behavior is caused by interaction with solvents of different dipolarity/polarizability which is reflected in the large coefficient s in eqn (1) or coefficients d and e in eqn (2).8d The dipolar structures of dyes with an enlarged π-system usually are stabilized within the molecule rather than by interaction with a solvent molecule. To our surprise, the effect of polarizability and dipolarity of P1 increases significantly compared with M1 (the rate SP/SdP remains unchanged). This could firstly be ascribed to the fact that P1 exhibits poor solubility in some solvents, if the chain length gets larger. In those solvents only very short chains, probably dimers or trimers, are measured by UV/vis spectroscopy. Secondly, the presence of N–H end groups found by the unchanged high influence of SB, speaks for such short chains. The solvatochromic shift of P1 from n-hexane to HMPA is 3140 cm−1, and from n-hexane to DMSO is 2400 cm−1, which are much larger than that of M1.
For P2a, the influence of HBA-solvents is smaller, since the N–H-functionality is not present anymore. The residual effect may point to an N–H-end group again. The influence of polarizability and dipolarity remains unchanged compared with M1. The solvatochromic shift is decreased compared with those of M1 and P1a. From n-hexane to HMPA it is 1200 cm−1, and from n-hexane to DMSO it is 1180 cm−1.
Conclusions
In this article, the deep blue monomer M1 and four π-conjugated polymers based on aminobenzodione are described. M1 is very soluble in common organic solvents and suitable for Buchwald amination and Stille and Suzuki coupling because of the bromine and alkylamino substituent groups. The polymers exhibit quite low bandgaps (1.08–1.47 eV), high photostability and a large solvatochromic shift up to 3140 cm−1. Furthermore, the polymers show broad UV/vis absorption bands in a range from 400 to 900 nm with high extinction coefficients of 1.7 to 3.1 × 104 L mol−1 cm−1. This matches well with the solar photon most intense flux. The broad absorption in the visible region of the spectra, combined with high color depth, high photostability and a low bandgap render aminobenzodione-based polymers interesting as building blocks for optoelectronic materials.Acknowledgements
Financial support from BASF Schweiz AG, Basle, Switzerland, China Scholarship Council (CSC) and German Academic Exchange Service (DAAD) is gratefully acknowledged. Dr P. Hayoz from BASF Schweiz AG, Basle is kindly thanked for helpful discussions.References
- (a) T. Limori, T. Naito and N. Ohta, J. Phys. Chem. C, 2009, 113, 4654–4661 CrossRef; (b) H. Kim, W. A. Goddard, S. S. Jang, W. R. Dichtel, J. R. Heath and J. F. Stoddart, J. Phys. Chem. A, 2009, 113, 2136–2143 CrossRef CAS PubMed.
- (a) S. R. Marder, B. Kippelen, A. K.-Y. Jen and N. Peyghambarian, Nature, 1997, 388, 845–851 CrossRef CAS; (b) C.-G. Liu, W. Guan, P. Song, L.-K. Yan and Z.-M. Su, Inorg. Chem., 2009, 48, 6548–6554 CrossRef CAS PubMed.
- (a) Photoinduced Electron Transfer, ed. M. A. Fox and M. Chanon, Elsevier, Amsterdam, 1998 Search PubMed; (b) H. Kurreck and M. Huber, Angew. Chem., 1995, 107, 927–947 CrossRef.
- (a) K. Zhang and B. Tieke, Macromolecules, 2011, 44, 4596–4599 CrossRef CAS; (b) P. A. Ulmann, H. Tanaka, Y. Matsuo, Z. Xiao, I. Soga and E. Nakamura, Phys. Chem. Chem. Phys., 2011, 13, 21045–21049 RSC; (c) K. Zhang, B. Tieke, J. C. Forgie, F. Vilela and P. J. Skabara, Macromolecules, 2012, 45, 743–750 CrossRef CAS; (d) H. Zhang and B. Tieke, Polym. Chem., 2014, 5, 646–652 RSC.
- (a) W. Cui, J. Yuen and F. Wudl, Macromolecules, 2011, 44, 7869–7873 CrossRef CAS; (b) P. Deng, L. Liu, S. Ren, H. Li and Q. Zhang, Chem. Commun., 2012, 48, 6960–6962 RSC; (c) W. Hong, C. Guo, Y. Li, Y. Zheng, C. Huang, S. Lu and A. Facchetti, J. Mater. Chem., 2012, 22, 22282–22289 RSC.
- (a) O. Annen, R. Egli, R. Hasler, B. Henzi, H. Jakob and P. Matzinger, Rev. Prog. Color. Relat. Top., 1987, 17, 72–85 CrossRef CAS; (b) C. W. Greenhalgh, J. L. Carey and D. F. Newton, Dyes Pigm., 1980, 1, 103–120 CrossRef CAS.
- (a) C. W. Greenhalgh, J. L. Carey and D. F. Newton, J. Soc. Dyers Colour., 1994, 110, 178–184 CrossRef CAS; (b) S. J. Bentley and D. J. Milner, Synth. Commun., 1996, 26, 95–100 CrossRef CAS; (c) Z. Cai, J. Gao, X. Li and B. Xiang, Opt. Commun., 2007, 272, 503–508 CrossRef CAS; (d) G. Hallas and C. Yoon, Dyes Pigm., 2001, 48, 107–119 CrossRef CAS.
- (a) A. A. Gorman, M. G. Huthings and P. D. Wood, J. Am. Chem. Soc., 1996, 118, 8497–8498 CrossRef CAS; (b) S. Spange, S. Prause, E. Vilsmeier and W. R. Thiel, J. Phys. Chem. B, 2005, 109, 7280–7289 CrossRef CAS PubMed; (c) A. Oehlke, K. Hofmann and S. Spange, New J. Chem., 2006, 30, 533–536 RSC; (d) K. Hofmann, S. Brumm, C. Mende, K. Nagel, A. Seifert, I. Roth, D. Schaarschmidt, H. Lang and S. Spange, New J. Chem., 2012, 36, 1655 RSC.
- D. J. Zillman, G. C. Hincapie, M. R. Savari, F. G. Mizori and T. E. Cole, Tetrahedron Lett., 2010, 51, 3033–3036 CrossRef CAS.
- D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27–50 RSC.
- P. K. Nayak, G. G. Belmonte, A. Kahn, J. Bisquert and D. Cahen, Energy Environ. Sci., 2012, 5, 6022–6039 CAS.
- H. Zhang, I. Welterlich, J.-M. Neudoerfl, B. Tieke, C. Yang, X. Chen and W. Yang, Polym. Chem., 2013, 4, 4682–4689 RSC.
- H. Zhang, J.-M. Neudoerfl and B. Tieke, Macromolecules, 2013, 46, 5842–5849 CrossRef CAS.
- T. Beyerlein and B. Tieke, Macromol. Rapid Commun., 2000, 21, 182–189 CrossRef CAS.
- I. Welterlich and B. Tieke, Polym. Chem., 2013, 4, 3755–3764 RSC.
- (a) C. Reichardt, Chem. Rev., 1994, 94, 2319–2385 CrossRef CAS; (b) P. Mueller, Pure Appl. Chem., 1993, 22, 409–416 Search PubMed.
- (a) M. J. Kamlet, T. N. Hall, J. N. Hall, J. Boykin and R. Taft, J. Org. Chem., 1979, 44, 2599–2604 CrossRef CAS; (b) R. W. Taft and M. J. Kamlet, J. Am. Chem. Soc., 1976, 98, 2866–2894 CrossRef; (c) M. J. Kamlet and R. W. Taft, J. Am. Chem. Soc., 1976, 98, 377–383 CrossRef CAS.
- (a) J. Catalán and C. Díaz, Liebigs Ann., 1997, 1941–1949 CrossRef; (b) J. Catalán and C. Díaz, Eur. J. Org. Chem., 1999, 885–891 CrossRef; (c) J. Catalán, V. López and P. Pérez, Liebigs Ann., 1995, 793–795 CrossRef.
- (a) J. Catalán, C. Díaz, V. López, P. Pérez, J.-L. G. d. Paz and J. G. Rodríguez, Liebigs Ann., 1996, 1785–1794 CrossRef; (b) J. Catalán, J. Palomar, C. Díaz and J.-L. G. d. Paz, J. Phys. Chem. A, 1997, 101, 5183–5189 CrossRef.
- J. Catalán and H. Hopf, Eur. J. Org. Chem., 2004, 4694–4702 CrossRef.
- J. Catalán, J. Phys. Chem. B, 2009, 113, 5951–5960 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, the 1H NMR spectrum of 4-(octyl amino)phenyl tartronic acid, UV/vis absorption spectra of M2 and M3 and polymers P1, P2a and P3, before and after irradiation in toluene, and solvatochromic data. See DOI: 10.1039/c3py01702h |
| This journal is © The Royal Society of Chemistry 2014 |