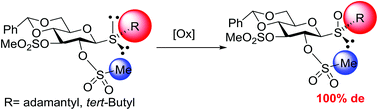
A detailed study on the diastereoselective oxidation of 1-thio-β-D-glucopyranosides is reported. It has been shown that the sense and the degree of stereochemical outcome of the oxidation are highly dependent on the substituent of the sulfur and on the protective group of the C2–OH. In the case of thioglycosides with a bulky aglycone, the mesylation of C2–OH has a significant effect on the stereochemical outcome of the oxidation, affording the usually less favoured RS sulfoxide as a single diastereoisomer. The absolute configuration of the final sulfinyl glycosides was ascertained by NMR analysis and corroborated by X-ray crystallography.