JieXiang Yin, Theresa Mekelburg and Christopher Hyland
Org. Biomol. Chem., 2014,12, 9113-9115
DOI:
10.1039/C4OB01786B,
Communication
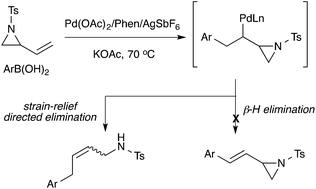
The palladium(II)-catalysed addition of arylboronic acids to vinylaziridines has been developed. This reaction proceeds via an insertion/ring-opening process to provide (Z)-allylsulfonamides preferentially. This stereoselectivity is complimentary to existing methods that typically proceed via a SN2′ mechanism to yield (E)-allylsulfonamides. Electron-deficient arylboronic acids were the optimum substrates for this reaction, while electron-donating groups on the aromatic ring of the boronic acids resulted in moderate yields.