One molecule of ionic liquid and tert-alcohol on a polystyrene-support as catalysts for efficient nucleophilic substitution including fluorination†
Abstract
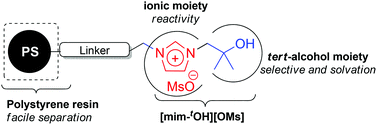
The tert-alcohol and ionic liquid solvents in one molecule [mim-tOH][OMs] was immobilized on polystyrene and reported to be a highly efficient catalyst in aliphatic nucleophilic substitution using alkali metal salts. Herein, we investigated the catalytic activity of a new structurally modified polymer-supported tert-alcohol functionalized imidazolium salt catalyst in nucleophilic substitution of 2-(3-methanesulfonyloxypropyoxy)naphthalene as a model substrate with various metal nucleophiles. The tert-alcohol moiety of the ionic liquid with a hexyl chain distance from polystyrene had a better catalytic activity compared to the other resin which lacked an alkyl linker and tert-alcohol moiety. We found that the maximum [mim-tOH][OMs] loading had the best catalytic efficacy among the tested polystyrene-based ionic liquids (PSILs) in nucleophilic fluorination. The catalytic efficiency of the PS[him-tOH][OMs] as a phase transfer catalyst (PTC) was determined by carrying out various nucleophilic substitutions using the corresponding alkali metal salts from the third to sixth periodic in CH3CN or tert-BuOH media. The scope of this protocol with primary and secondary polar substrates containing many heteroatoms is also reported. This PS[him-tOH][OMs] catalyst not only enhances the reactivity of alkali metal salts and reduces the formation of by-products but also affords high yield with easy isolation.

 Please wait while we load your content...
Please wait while we load your content...