Suguru Yoshida, Takako Nonaka, Takamoto Morita and Takamitsu Hosoya
Org. Biomol. Chem., 2014,12, 7489-7493
DOI:
10.1039/C4OB01654H,
Communication
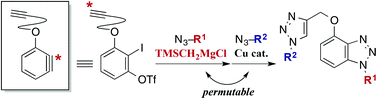
A modular synthetic method for bis- and tris-1,2,3-triazoles that include a benzotriazole structure was developed on the basis of sequential azide–aryne and azide–alkyne cycloadditions. The key to success was efficient halogen–metal exchange reaction-mediated generation of aryne from ortho-iodoaryl triflates bearing a base-sensitive terminal alkyne moiety, which was achieved using trimethylsilylmethyl Grignard reagent.