 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of tertiary propargylamines via a rationally designed multicomponent reaction of primary amines, formaldehyde, arylboronic acids and alkynes†
Jiayi
Wang
ab,
Qiaoying
Shen
a,
Pinzhen
Li
a,
Yanqing
Peng
a and
Gonghua
Song
*a
aShanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237, P. R. China. E-mail: ghsong@ecust.edu.cn; Fax: +86-21-64252603; Tel: +86-21-64253140
bShanghai Key Laboratory of Catalysis Technology for Polyolefins, Shanghai Research Institute of Chemical Industry, Shanghai, 200062, P. R. China
First published on 17th June 2014
Abstract
A novel approach for the synthesis of tertiary propargylamines is achieved through a Cu(OAc)2-catalyzed multicomponent reaction of primary amines, formaldehyde, arylboronic acids and alkynes, where a combination of PBM and A3-coupling reactions is involved in this new multicomponent reaction.
Multicomponent reactions (MCR), generally with high selectivity, flexibility and atom economy, are among the most powerful synthetic strategies to access diverse complex structures from small molecular compounds,1 and a variety of new MCRs have been developed.2,3 Among them, the combination of MCR (MCR2), which combines two different types of MCRs in a single process, has gained considerable attention.4 However, the Ugi reaction as well as isonitriles are indispensably involved in most of the MCR2 cases.2a,c,4 Therefore, the development of novel MCR2 without the Ugi reaction is highly valuable.
The three-component reaction of aldehydes, amines and alkynes (A3-coupling) provides an efficient strategy to the synthesis of propargylamines, which are often useful key intermediates and building blocks for the preparation of many biologically active compounds.5,6 A number of metal catalysts, such as Au salts,7a Ag salts,7b FeCl3/Fe2O3/Fe3O4,7c,d InCl3,7e Cu salts,7f,i and so on,5,6b have been applied for this reaction via C–H activation of terminal alkynes. Meanwhile, the Petasis borono–Mannich (PBM) reaction of aldehydes, amines and boronic acids, developed by Petasis in 1993,8 has attracted considerable attention in the synthesis of diverse α-hydroxyl amines, α-amino acids and nitrogen-containing heterocycles.9,10 Realizing both amines and aldehydes are involved in A3-coupling and PBM reactions, and a secondary amine is generally preferable to a primary one in A3 reaction;5,7 it is possible to develop a novel multicomponent reaction in which the secondary amines produced from PBM reaction could serve as the amine component in further A3-coupling to construct the final propargylamines. Thus, a novel five-component MCR2 of PBM and A3-coupling reactions has been accomplished and reported herein (Scheme 1).
At the outset, CuI was chosen as the catalyst to screen the effect of solvents on the model reaction of aniline, formaldehyde, phenylboronic acid and phenylacetylene at 80 °C for 24 h. The nature of the solvent significantly affected the reaction (Table 1). Among the solvents screened, 1,2-dichloroethane (DCE) was found to be the most suitable solvent for the combination of PBM and A3-coupling reactions with a desired product yield of 86% (Table 1, entry 1). Toluene was inferior and generated the corresponding product in 72% yield (Table 1, entry 2), whereas acetonitrile and 1,4-dioxane afforded lower yields of the desired products (Table 1, entries 3 and 4). Tetrahydrofuran (THF), ethanol and water delivered less than 5% yield (Table 1, entries 5–7), and no desired product was achieved when the reactions were performed in N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) (Table 1, entries 8 and 9). When the reaction was performed in the absence of a solvent, a lower yield of 22% was obtained (Table 1, entry 10), and no desired product was detected without the catalyst (Table 1, entry 11).
| Entry | Solvent | Yieldb (%) |
|---|---|---|
| a Reaction conditions: aniline (1 mmol), formaldehyde (40% aqueous solution) (2.2 mmol), phenylboronic acid (1.05 mmol), phenylacetylene (1.2 mmol), CuI (10 mol%), solvent (3 mL), 80 °C, 24 h. b Yields were determined by GC using an internal standard. c Without catalyst. | ||
| 1 | DCE | 86 |
| 2 | Toluene | 72 |
| 3 | 1,4-Dioxane | 12 |
| 4 | MeCN | 32 |
| 5 | THF | <5 |
| 6 | EtOH | <5 |
| 7 | H2O | <5 |
| 8 | DMF | 0 |
| 9 | DMSO | 0 |
| 10 | Neat | 22 |
| 11 | DCE | 0c |
We next investigated the catalytic activity of various copper salts (Table 2). Cu(OAc)2 was found to be the most effective catalyst with a high yield of 96% (Table 2, entry 6). Interestingly, except for CuOAc/Cu(OAc)2, Cu(I) catalysts showed better catalytic activities than Cu(II) catalysts (Table 2, entries 1–12). Cu(OAc)2 was therefore adopted for the optimization of other reaction conditions. As to the temperature, 80 °C was found to be optimal. The reactions at lower temperatures generated less product (Table 2, entries 13 and 14). Lowering the catalyst load amount to 5 mol%, or reducing the reaction time to 12 h also resulted in decrease of yield (Table 2, entries 15 and 16).
| Entry | Catalyst | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: aniline (1 mmol), formaldehyde (40% aqueous solution) (2.2 mmol), phenylboronic acid (1.05 mmol), phenylacetylene (1.2 mmol), catalyst (10 mol%), DCE (3 mL) at a temperature indicated in the table, 12–24 h. b Yields were determined by GC using an internal standard. c 5 mol% of Cu(OAc)2 was used. | ||||
| 1 | CuI | 80 | 24 | 86 |
| 2 | CuBr | 80 | 24 | 89 |
| 3 | CuCl | 80 | 24 | 71 |
| 4 | CuOAc | 80 | 24 | 89 |
| 5 | Cu2O | 80 | 24 | 68 |
| 6 | Cu(OAc)2 | 80 | 24 | 96 |
| 7 | CuBr2 | 80 | 24 | 46 |
| 8 | CuCl2 | 80 | 24 | 51 |
| 9 | CuSO4 | 80 | 24 | 68 |
| 10 | CuO | 80 | 24 | 54 |
| 11 | Cu | 80 | 24 | 65 |
| 12 | Cu(CF3SO3)2 | 80 | 24 | 32 |
| 13 | Cu(OAc)2 | 25 | 24 | <5 |
| 14 | Cu(OAc)2 | 60 | 24 | 72 |
| 15 | Cu(OAc)2 | 80 | 24 | 62c |
| 16 | Cu(OAc)2 | 80 | 12 | 71 |
On the basis of the optimized reaction conditions (Table 2, entry 6), the scope of this five-component reaction was evaluated (Table 3). In general, for all components in this reaction, electron-donating substituents (–MeO and –Me) on the phenyl ring lead to higher yields than electron-withdrawing groups (–Cl and –F) (Table 3, entries 1–7, 13–17 and 19–23). The reaction almost ceased when a reactant with a highly electron deficient substituent, such as 4-nitroaniline or 4-(trifluoromethyl)phenylboronic acid was adopted (Table 3, entries 8 and 18). For the amine component, substituted anilines delivered the corresponding products in 60–92% yields (Table 3, entries 1–7). Aliphatic amines, such as phenylmethanamine and butan-1-amine, also delivered desired products in satisfactory yields (Table 3, entries 9 and 10). Furthermore, the successful application of methyl 2-aminoacetate and methyl 2-aminopropanoate in this MCR2 (Table 3, entries 11 and 12) might provide a complementary approach to the functionalization of NH2-terminal amino acid esters or peptides.7h,i As for the alkyne component, in addition to the success of phenylacetylenes (Table 3, entries 1–7, 9–17 and 19–23), aliphatic alkynes also worked smoothly and desired products were obtained in moderate yields with prolonged reaction time of 48 h (Table 3, entries 24–26).
| Entry | R1 | Ar | R2 | Yieldb (%) |
|---|---|---|---|---|
| a A mixture of aniline 1 (1 mmol), formaldehyde 2 (40% aqueous solution) (2.2 mmol), arylboronic acid 3 (1.05 mmol), alkyne 4 (1.2 mmol), Cu(OAc)2 (10 mol%) and DCE (3 mL) was stirred at 80 °C for 24 h. b Isolated yields. c The reaction time is 48 h. | ||||
| 1 | Ph | Ph | Ph | 92 |
| 2 | p-(MeO)–Ph | Ph | Ph | 91 |
| 3 | p-Me–Ph | Ph | Ph | 78 |
| 4 | p-Cl–Ph | Ph | Ph | 60 |
| 5 | p-F–Ph | Ph | Ph | 66 |
| 6 | o-Me–Ph | Ph | Ph | 74 |
| 7 | m-Me–Ph | Ph | Ph | 86 |
| 8 | p-NO2–Ph | Ph | Ph | 0 |
| 9 | Bn | Ph | Ph | 90 |
| 10 | n-Butyl | Ph | Ph | 71c |
| 11 |

|
Ph | Ph | 64c |
| 12 |
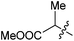
|
Ph | Ph | 60c |
| 13 | Ph | p-(MeO)–Ph | Ph | 95 |
| 14 | Ph | p-Me–Ph | Ph | 87 |
| 15 | Ph | p-Cl–Ph | Ph | 61 |
| 16 | Ph | p-F–Ph | Ph | 60 |
| 17 | Ph | o-(MeO)–Ph | Ph | 79 |
| 18 | Ph | p-CF3–Ph | Ph | 0 |
| 19 | p-(MeO)–Ph | p-(MeO)–Ph | Ph | 86c |
| 20 | Ph | Ph | p-Me–Ph | 82 |
| 21 | Ph | p-Cl–Ph | p-Me–Ph | 61c |
| 22 | p-(MeO)–Ph | p-(MeO)–Ph | p-Me–Ph | 82 |
| 23 | Ph | Ph | p-Cl–Ph | 70 |
| 24 | Ph | Ph | n-Butyl | 82c |
| 25 | Ph | Ph | n-Pentyl | 78c |
| 26 | Ph | Ph |

|
40c |
A tentative mechanism for this five-component reaction is proposed in Scheme 2. The reaction of primary amine 1, formaldehyde 2, and arylboronic acid 3 afforded a secondary amine 6via the PBM reaction,8,9,11 which was detected by GC-MS throughout the reaction. The secondary amine 6 might further react with formaldehyde 2 to produce an iminium intermediate B. The copper acetylide intermediate, generated from alkyne 4 and Cu(OAc)2, reacted with iminium B to give the corresponding propargylamine 5 and regenerated the copper catalyst for further reactions.5,7 Thus, this five-component reaction involved a MCR2 of PBM and A3-coupling reactions.
In conclusion, we have developed a novel approach to the synthesis of tertiary propargylamines via a rationally designed Cu-catalyzed multicomponent reaction of primary amines, formaldehydes, arylboronic acids and alkynes. The combination of PBM and A3-coupling reactions provides an efficient and fast one-pot approach to the tertiary propargylamines. Both aromatic and aliphatic amines and alkynes are applicable. Furthermore, the MCR2 also provided a complementary pathway to the functionalization of NH2-terminal amino acid esters and peptides.
Financial support for this work from the National Basic Research Program of China (973 program) (grant no. 2010CB126101), the National Natural Science Foundation of China (grant 20972052) and the Shanghai Key Laboratory of Catalysis Technology for Polyolefins (LCTP-201301) are gratefully acknowledged.
Notes and references
- For recent reviews, see: (a) J. E. Biggs-Houck, A. Younai and J. T. Shaw, Curr. Opin. Chem. Biol., 2010, 14, 371 CrossRef CAS PubMed; (b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed; (c) C. M. Marson, Chem. Soc. Rev., 2012, 41, 7712 RSC; (d) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427 CrossRef CAS PubMed.
- For selected reviews, see: (a) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed; (b) B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed; (c) S. Brauch, S. S. Van Berkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948 RSC.
- T. J. Montavon, J. R. Cabrera-Pardo, M. Mrksich and S. A. Kozmin, Nat. Chem., 2012, 4, 45 CrossRef CAS PubMed.
- For selected examples, see: (a) A. Dömling and I. Ugi, Angew. Chem., Int. Ed. Engl., 1993, 32, 563 CrossRef; (b) N. Elders, D. Van der Born, L. J. D. Hendrickx, B. J. J. Timmer, A. Krauce, E. Janssen, F. J. J. De Kanter, E. Ruijter and R. V. A. Orru, Angew. Chem., Int. Ed., 2009, 48, 5856 CrossRef CAS PubMed; (c) S. Brauch, L. Gabriel and B. Westermann, Chem. Commun., 2010, 46, 3387 RSC.
- (a) C. Wei, L. Zhang and C. J. Li, Synlett, 2004, 1472 CAS; and references cited therein; (b) W. J. Yoo, L. Zhao and C. J. Li, Aldrichimica Acta, 2011, 44, 43 CAS.
- (a) T. Naota, H. Takays and S. I. Murahashi, Chem. Rev., 1998, 98, 2599 CrossRef CAS PubMed; (b) V. A. Peshkov, O. P. Pereshivko and E. V. Van der Eycken, Chem. Soc. Rev., 2012, 41, 3790 RSC.
- For selected examples, see: (a) C. Wei and C. J. Li, J. Am. Chem. Soc., 2003, 125, 9584 CrossRef CAS PubMed; (b) C. Wei, Z. Li and C. J. Li, Org. Lett., 2003, 5, 4473 CrossRef CAS PubMed; (c) P. Li, Y. Zhang and L. Wang, Chem. – Eur. J., 2009, 15, 2045 CrossRef CAS PubMed; (d) T. Zeng, W. W. Chen, C. M. Cirtius, A. Moores, G. H. Song and C. J. Li, Green Chem., 2010, 12, 570 RSC; (e) Y. Zhang, P. Li, M. Wang and L. Wang, J. Org. Chem., 2009, 74, 4364 CrossRef CAS PubMed; (f) C. Wei and C. J. Li, J. Am. Chem. Soc., 2002, 124, 5638 CrossRef CAS PubMed; (g) C. Wei, J. T. Mague and C. J. Li, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5749 CrossRef CAS PubMed; (h) N. Uhlig and C. J. Li, Org. Lett., 2012, 14, 3000 CrossRef CAS PubMed; (i) N. Sharma, U. K. Shama, N. M. Mishram and E. V. Van der Eycken, Adv. Synth. Catal., 2014, 356, 1029 CrossRef CAS.
- (a) N. A. Petasis and I. Akritopoulou, Tetrahedron Lett., 1993, 34, 583 CrossRef CAS; (b) N. A. Petasis, A. Goodman and I. A. Zavialov, Tetrahedron, 1997, 53, 16463 CrossRef CAS; (c) N. A. Petasis and I. A. Zavialov, J. Am. Chem. Soc., 1997, 119, 445 CrossRef CAS.
- N. R. Candeias, F. Montalbano, P. M. S. D. Cal and P. M. P. Gois, Chem. Rev., 2010, 110, 6169 CrossRef CAS PubMed ; and references therein.
- For selected examples, see: (a) G. Muncipinto, P. N. Moquist, S. L. Schreiber and S. E. Schaus, Angew. Chem., Int. Ed., 2011, 50, 8172 CrossRef CAS PubMed; (b) S. T. Le Quement, T. Flagstad, R. J. T. Mikkelsen, M. R. Hansen, M. C. Givskov and T. E. Nielsen, Org. Lett., 2012, 14, 640 CrossRef CAS PubMed; (c) W. Y. Han, Z. J. Wu, X. Zhang and W. C. Yuan, Org. Lett., 2012, 14, 976 CrossRef CAS PubMed; (d) M. S. T. Morin, Y. Lu, D. A. Black and B. A. Arndtsen, J. Org. Chem., 2012, 77, 2013 CrossRef CAS PubMed; (e) E. Ascic, S. T. Le Quement, M. Ishoey, M. Daugaard and T. E. Nielsen, ACS Comb. Sci., 2012, 14, 253 CrossRef CAS PubMed; (f) D. A. Mundal, K. E. Lutz and R. J. Thomson, J. Am. Chem. Soc., 2012, 134, 5782 CrossRef CAS PubMed; (g) Y. Li and M. H. Xu, Org. Lett., 2012, 14, 2062 CrossRef CAS PubMed; (h) R. Frauenlob, C. García, G. A. Bradshaw, H. M. Burke and E. Bergin, J. Org. Chem., 2012, 77, 4445 CrossRef CAS PubMed; (i) P. Ghosal and A. K. Shaw, J. Org. Chem., 2012, 77, 7627 CrossRef CAS PubMed.
- J. Wang, P. Li, Q. Shen and G. Song, Tetrahedron Lett., 2014, 55, 3888 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, spectroscopic data. See DOI: 10.1039/c4ob01055h |
| This journal is © The Royal Society of Chemistry 2014 |





