Open Access Article
Open Access ArticleTri-isopropylsilyl thioglycosides as masked glycosyl thiol nucleophiles for the synthesis of S-linked glycosides and glyco-conjugates†
S.
Mandal
and
U. J.
Nilsson
*
Centre for Analysis and Synthesis, Department of Chemistry, Lund University, POB 124, SE-22100, Lund, Sweden. E-mail: ulf.nilsson@chem.lu.se
First published on 9th May 2014
Abstract
Tri-isopropylsilyl thio-glycosides (TIPS S-glycosides) were synthesized through base promoted SN2 substitution of glycosyl halides with TIPS-SH or by Lewis acid promoted glycosylation of TIPS-SH with glycosyl acetates or p-methoxyphenyl glycosides. Various thioglycoside derivatives were obtained in high yields by one-pot fluoride-mediated de-silylation and thiol alkylation with alkyl halides or Michael acceptors of one common TIPS S-glycoside.
Development of glycomimetics is an important area of research due to their potential as biological tools and in pharmaceutical applications.1,2 A large and important class of glycomimetics includes stable analogues in which one or more oxygen atoms, i.e. glycosidic oxygens, have been replaced by e.g. sulfur3,4 or other heteroatoms,5 leading to structures more tolerable towards enzymatic and acidic hydrolysis and thus with enhanced stability in biological environments. Hence, the enhanced hydrolytic stability of thioglycosides3,4 makes them particularly useful as tools in investigation of glycoconjugate functions. Examples include thioglycosides as key mediators for cellular surface recognition and enzyme inhibitors,6 and thioglycosides binding to many lectins,7e.g. the unnatural disaccharide thiodigalactoside binding to galectins with about the same affinity as LacNAc.8 Indeed, thiodigalactoside shows promising immune-promoting properties against tumor cells9 and its derivatives have been shown to attenuate tumor motility,10 macrophage differentiation,11 fibrosis progression,12 hepatitis,13 and pancreatic islet cell apoptosis.14
Furthermore, thioglycosides are being used as versatile donors and thus key precursors in oligosaccharide synthesis, synthesis of glycodendrimers,15,16 sulfur containing glycolipids,4 and S-linked protein glycoconjugates.17,18 In such a use of thioglycosides, the chemical properties of the thioglycoside aglycon part typically influence the reactivity and reaction outcome, that is why methods for easy diversification of the thio-glycoside aglycon are of interest. Thioglycoside synthesis is usually performed by SN2 displacement of a glycosyl halide by a thiol(ate) or by Lewis acid-catalysed glycosylation of a thiol with glycosyl acetates or phenyl O-glycosides.4,5 Additional methods include in situ aminolysis and alkylation of thioacetates,19,20 Michael type addition of 1-thiolates to carbohydrate enones,21 coupling of glycals and thiols in Ferrier-type reactions,22 thiol–ene click reactions,21 ring opening of carbohydrate epoxides with thiols,23 the use of Lawesson's reagent with hemiacetals,24 formation of α-thio-glycosidic linkages from 1,6-anhydro derivatives with bis-(trimethylsilyl) sulfide,25 formation of β-thioglycosidic linkages by the use of Na2S and CS2,4 and Michael type reactions involving a thiirane intermediate.4 In case a collection of thio-glycosides carrying diverse aglycon structures is needed, the majority of these methods would require that a separate (glycosylation) reaction with a thiol was undertaken for each member of the collection. Triisopropylsilyl sulfides are remarkably stable,26 yet mildly de-silylated and alkylated in the presence of fluoride sources.27–29 Triisopropylsilyl thio-glycosides are known and their de-silylation has been demonstrated,30 but have not been evaluated as masked glycosyl thiolate nucleophiles for in situ activation and reaction with electrophiles. Herein, we report on a novel, rapid, and effective synthetic method towards different thio-glycosides in one step within 5 min by reacting electrophiles with readily accessible tri-isopropylsilyl thio-glycosides as common precursors in the presence of tetrabutylammonium fluoride.
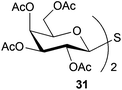
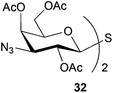
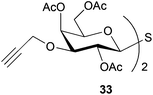
Boron trifluoride etherate-catalysed glycosylation of tri-isopropylsilylthiol (TIPSSH) with anomeric acetates or p-methoxy phenyl glycosides, as well as SN2 displacement of anomeric bromides with TIPSSH in the presence of a base, K2CO3, resulted in stereoselective formation of TIPS thio-glycosides (Scheme 1). Evaluation of reaction conditions for the Lewis acid-mediated activation of glycosyl acetates and p-methoxyphenyl β-glycosides revealed that dichloromethane was a solvent superior to toluene, diethyl ether, and acetonitrile for activation of glycosyl acetates (4, 6a, 8a, 10a), while toluene31 was the best solvent with p-methoxyphenyl β-galactoside as a donor (1). Furthermore, boron trifluoride etherate proved to be the best choice of Lewis acid over TMSOTf. Among different conditions investigated for the displacement of glycosyl bromides (5, 6b, 8b, 10b, 12, 13) under basic conditions, NaH in DMF, Cs2CO3 in DMF, and K2CO3 in acetone, the best results were obtained with K2CO3 in acetone. While reasonable yields were obtained by boron trifluoride etherate activation of glycosyl acetates (4, 6a, 8a, 10a) and p-methoxyphenyl β-galactoside 1, the best yields for formation of TIPS thio-glycosides were obtained using glycosyl bromides (5, 6b, 12,3213![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33) in the presence of a base. For mannose and rhamnose the best yields were achieved with Lewis acid-promoted activation of anomeric acetates (8a and 10a).
33) in the presence of a base. For mannose and rhamnose the best yields were achieved with Lewis acid-promoted activation of anomeric acetates (8a and 10a).
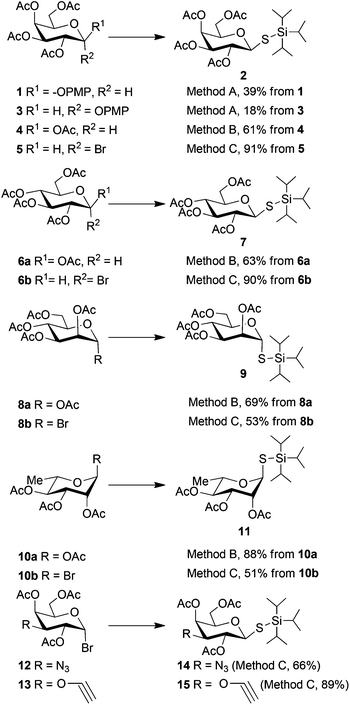 | ||
| Scheme 1 Synthesis of tri-isopropylsilyl thio-glycosides: Method A: BF3·Et2O, TIPSSH, dry toluene. Method B: BF3·Et2O, TIPSSH, dry DCM. Method C: K2CO3, TIPSSH, dry acetone. | ||
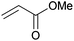
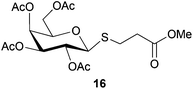
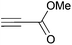
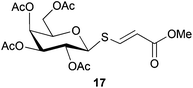
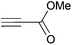
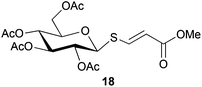
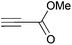
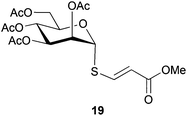
Cleavage and in situ alkylation of STIPS sulfides have been demonstrated to be efficient with fluoride sources.27 Hence, the S–Si bond of the TIPS thioglycosides (2, 7, 9, and 11) was found to be easily transformed into various thioglycosides by activation with fluoride ions in the presence of an electrophile (Michael acceptor, alkyl halide, acid chloride, or glycosyl bromide) in a high-yielding and stereoselective reaction at RT in 5 min on both small and large (gram) scales (Scheme 2). Michael acceptors are known to be highly efficient electrophiles in reaction with thiol(ate) nucleophiles, which proved to also be the case with the conditions for de-silylating and activating TIPS thioglycosides with TBAF (Table 1). Michael additions to methyl propiolate afforded the more stable E-isomer exclusively (compounds 17, 18, and 19; Table 1) according to NMR analysis. Hence, the method is of potential value for the construction of hydrolytically stable glycomimetics via thiolate Michael additions.
| Cpd | Electrophile | Thioglycoside product | Yield (%) |
|---|---|---|---|
| a STIPS glycoside (1 eq.), Michael acceptor (1.3 eq.), MeCN (26 mL mmol−1 STIPS glycoside), TBAF (1.2 eq., 1.0 M in THF), 5 min. | |||
| 2 |
|
|
91 |
| 2 |
|
|
93 |
| 7 |
|
|
95 |
| 9 |
|
|
94 |
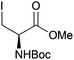
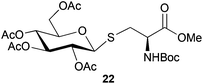
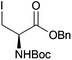
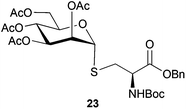
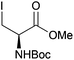
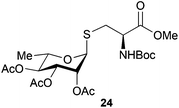
Neoglycopeptides and proteins are important research tools in glycobiology and S-glycosylated cysteine derivatives are useful building blocks for such structures. Reaction of N-Boc-protected β-iodo-L-alanine ester derivatives with TIPS thio-glycosides in the presence of TBAF in acetonitrile at RT for 5 min furnished the corresponding L-cysteine derivatives 20–24 in excellent yields (Table 2). Hence, the method provides an efficient one step protocol with minimal formation of e.g. eliminated by-products.
| Cpd | Electrophile | Thioglycoside product | Yield (%) |
|---|---|---|---|
| a STIPS glycoside (1 eq.), β-iodo-L-alanine ester (1.2 eq.), MeCN (26 mL mmol−1 STIPS glycoside), TBAF (1.2 eq., 1.0 M in THF), 5 min. | |||
| 2 |
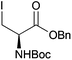
|
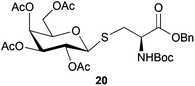
|
91 |
| 2 |
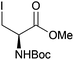
|
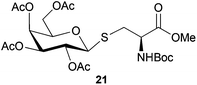
|
93 |
| 7 |
|
|
94 |
| 9 |
|
|
93 |
| 11 |
|
|
94 |
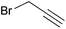
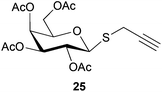
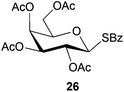
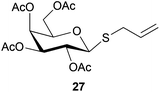
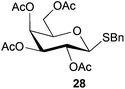
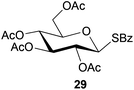
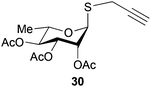
To further explore the method, TIPS thio-β-glycosides were evaluated as masked sulfur nucleophiles with alkyl, acid, and glycosyl halides, including substituted galactosyl bromides (1232 and 1333), under the same reaction conditions as described above (Table 3). All reactions proceeded smoothly to furnish the corresponding alkyl β-thioglycosides 25, 27–28, and 30, glycosyl thio-benzoates 26 and 29, and thiodigalactosides 31–33 in excellent yields. In particular, the examples involving synthesis of thiodigalactoside derivatives 31–33 are illustrative of the high efficiency of the STIPS glycosides in the synthesis of thioglycosides as other methods for synthesis of thiodiglycosides including those using glycosyl thiouronium intermediates as masked glycosyl thiolate nucleophiles provide significantly less clean reactions and substantially lower yields in our hands and in published work by others.34–36 Furthermore, formation of glycosyl thiouronium intermediates commonly requires reaction at higher temperatures with thiourea and in many cases the glycosyl thiouronium intermediates show limited stability upon storage.
Conclusions
In summary, this report presents an efficient and convenient one-pot protocol for stereoselective synthesis of thioglycoside derivatives by room temperature and one-pot TBAF-mediated de-silylation and nucleophilic reactions of storage-stable TIPS thio-glycosides towards Michael acceptors, as well as alkyl, acyl, and glycosyl halides in high yields.Acknowledgements
The authors thank The Swedish Research Council (grant no. 621-2009-5326) and the European Community's Seventh Framework Program (FP7-2007-2013) under grant agreement no. HEALTH-F2-2011-256986 (PANACREAS) for financial support. SM thanks The Swedish Institute for providing a postdoctoral scholarship.Notes and references
- B. Ernst and J. L. Magnani, Nat. Rev. Drug Discovery, 2009, 8, 661–677 CrossRef CAS PubMed
.
- M. von Itzstein, Curr. Opin. Struct. Biol., 2008, 18, 558–566 CrossRef CAS PubMed
.
- Z. J. Witczak and J. M. Culhane, Appl. Microbiol. Biotechnol., 2005, 69, 237–244 CrossRef CAS PubMed
.
- K. Pachamuthu and R. R. Schmidt, Chem. Rev., 2006, 106, 160–187 CrossRef CAS PubMed
.
- L. Szilágyi and O. Varela, Curr. Org. Chem., 2006, 10, 1745–1770 CrossRef
.
- Z. J. Witczak and D. Boryczewski, Bioorg. Med. Chem. Lett., 1998, 8, 3265–3268 CrossRef CAS
.
- F. Schweizer and O. Hindsgaul, Curr. Opin. Chem. Biol., 1999, 3, 291–298 CrossRef CAS
.
- H. Leffler and S. H. Barondes, J. Biol. Chem., 1986, 22, 10119–10126 Search PubMed
.
- K. A. Stannard, P. M. Collins, K. Ito, E. M. Sullivan, S. A. Scott, E. Gabutero, I. Darren Grice, P. Low, U. J. Nilsson, H. Leffler, H. Blanchard and S. J. Ralph, Cancer Lett., 2010, 299, 95–110 CrossRef CAS PubMed
.
- T. Delaine, I. Cumpstey, L. Ingrassia, M. Le Mercier, P. Okechukwu, H. Leffler, R. Kiss and U. J. Nilsson, J. Med. Chem., 2008, 51, 8109–8114 CrossRef CAS PubMed
.
- A. C. MacKinnon, S. L. Farnworth, P. S. Hodkinson, N. C. Henderson, K. M. Atkinson, H. Leffler, U. J. Nilsson, C. Haslett, S. J. Forbes and T. Sethi, J. Immunol., 2008, 180, 2650–2658 CrossRef CAS
.
- A. C. Mackinnon, M. A. Gibbons, S. L. Farnworth, H. Leffler, U. J. Nilsson, T. Delaine, A. J. Simpson, S. J. Forbes, N. Hirani, J. Gauldie and T. Sethi, Am. J. Respir. Crit. Care Med., 2012, 185, 537–546 CrossRef CAS PubMed
.
- V. Volarevic, M. Milovanovic, B. Ljujic, N. Pejnovic, N. Arsenijevic, U. Nilsson, H. Leffler and M. L. Lukic, Hepatology, 2012, 55, 1954–1964 CrossRef CAS PubMed
.
- T. Saksida, I. Nikolic, M. Vujicic, U. J. Nilsson, H. Leffler, M. L. Lukic, I. Stojanovic and S. Stosic-Grujicic, J. Cell Physiol., 2013, 228, 1568–1576 CrossRef CAS PubMed
.
- M. Kohn, J. M. Benito, C. Ortiz Mellet, T. K. Lindhorst and J. M. Garcia Fernandez, ChemBioChem, 2004, 5, 771–777 CrossRef PubMed
.
- M. Gingras, Y. M. Chabre, M. Roy and R. Roy, Chem. Soc. Rev., 2013, 42, 4823–4841 RSC
.
- G. M. Watt and G. J. Boons, Carbohydr. Res., 2004, 339, 181–193 CrossRef CAS PubMed
.
- N. Floyd, B. Vijayakrishnan, J. R. Koeppe and B. G. Davis, Angew. Chem., Int. Ed., 2009, 48, 7798–7802 CrossRef CAS PubMed
.
- U. J. Nilsson, E. J.-L. Fournier, E. J. Fryz and O. Hindsgaul, Comb. Chem. High Throughput Screening, 1999, 2, 335–352 CAS
.
- U. J. Nilsson, E. J.-L. Fournier and O. Hindsgaul, Bioorg. Med. Chem., 1998, 6, 1563–1575 CrossRef CAS
.
- Z. J. Witczak, Phosphorus, Sulfur Silicon Relat. Elem., 2013, 188, 413–417 CrossRef CAS
.
- D. Ellis, S. E. Norman and H. Osborn, Tetrahedron, 2008, 64, 2832–2854 CrossRef CAS PubMed
.
- V. E. Manzano, M. L. Uhrig and O. Varela, J. Org. Chem., 2008, 73, 7224–7235 CrossRef CAS PubMed
.
- G. J. L. Bernardes, D. P. Gamblin and B. G. Davis, Angew. Chem., Int. Ed., 2006, 45, 4007–4011 CrossRef CAS PubMed
.
- X. Zhu, R. T. Dere, J. Jiang, L. Zhang and X. Wang, J. Org. Chem., 2011, 76, 10187–10197 CrossRef CAS PubMed
.
- E. I. Miranda, M. J. Díaz, I. Rosado and J. A. Soderquist, Tetrahedron Lett., 1994, 35, 3221–3224 CrossRef CAS
.
- A. Rane, E. Miranda and J. Soderquist, Tetrahedron Lett., 1994, 35, 3225–3226 CrossRef CAS
.
- J. J. Marugan, C. Manthey, B. Anaclerio, L. Lafrance, T. B. Lu, T. Markotan, K. A. Leonard, C. Crysler, S. Eisennagel, M. Dasgupta and B. Tomczuk, J. Med. Chem., 2005, 48, 926–934 CrossRef CAS PubMed
.
- X. Billot, A. Chateauneuf, N. Chauret, D. Denis, G. Greig, M. C. Mathieu, K. M. Metters, D. M. Slipetz and R. N. Young, Bioorg. Med. Chem. Lett., 2003, 13, 1129–1132 CrossRef CAS
.
- D. P. Galonić, W. A. van der Donk and D. Y. Gin, Chem. – Eur. J., 2003, 9, 5997–6006 CrossRef PubMed
.
- Z. Zhang and G. Magnusson, Carbohydr. Res., 1996, 295, 41–55 CrossRef CAS
.
- T. L. Lowary and O. Hindsgaul, Carbohydr. Res., 1994, 251, 33–67 CrossRef CAS
.
-
U. J. Nilsson, H. Leffler, B. Mukhopadhyay and V. K. Rajput, PCT Int. Appl., WO 2013110704 A1 20130801, 2013 Search PubMed
.
- E. S. H. El Ashry, L. F. Awad, H. M. A. Hamid and A. I. Atta, J. Carbohydr. Chem., 2005, 24, 745–753 CrossRef CAS
.
- P. Tiwari, G. Agnihotri and A. K. Misra, J. Carbohydr. Chem., 2005, 24, 723–732 CrossRef CAS
.
- M. Van Scherpenzeel, E. E. Moret, L. Ballell, R. M. J. Liskamp, U. J. Nilsson, H. Leffler and R. J. Pieters, ChemBioChem, 2009, 10, 1724–1733 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and copies of 1H and 13C NMR of all compounds and COSY and HMQC for selected compounds. See DOI: 10.1039/c4ob00741g |
| This journal is © The Royal Society of Chemistry 2014 |