An efficient synthesis of methyl 2-cyano-3,12-dioxoursol-1,9-dien-28-oate (CDDU-methyl ester): analogues, biological activities, and comparison with oleanolic acid derivatives†
Abstract
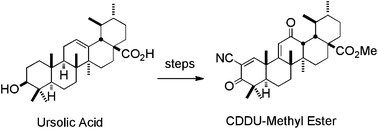
An efficient synthesis of methyl 2-cyano-3,12-dioxoursol-1,9-dien-28-oate (CDDU-methyl ester) from commercially available ursolic acid, which features an oxidative ozonolysis-mediated C-ring enone formation, and provides the first access to ursolic acid-derived cyano enone analogues with C-ring activation. These new ursolic acid analogues show potent biological activities, with potency of approximately five-fold less than the corresponding oleanolic acid derivatives.

 Please wait while we load your content...
Please wait while we load your content...