An iterative in silico and modular synthetic approach to aqueous soluble tercyclic α-helix mimetics†
Abstract
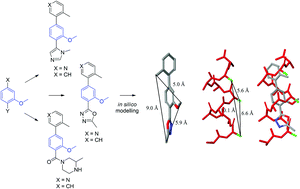
Tercyclic scaffolds, designed to have improved synthetic accessibility and aqueous solubility, were evaluated as structural α-helix mimetics by using an iterative in silico approach. The synthesis of these tercyclic scaffolds was accomplished using a modular synthetic approach by employing functionalised methoxyphenyl units which were readily manipulated to allow the introduction of various nitrogen-based heterocycles. The ability of these scaffolds to mimic the key i, i + 3 and i + 7 residues of a polyalanine α-helix was ratified by in silico studies, X-ray crystallographic and NOESY analysis, and their aqueous solubility was measured by a kinetic turbidimetric method.

 Please wait while we load your content...
Please wait while we load your content...