One-pot pseudo three-component reaction of nitroketene-N,S-acetals and aldehydes for synthesis of highly functionalized hexa-substituted 1,4-dihydropyridines†
H.
Surya Prakash Rao
* and
A.
Parthiban
Department of Chemistry, Pondicherry University, Puducherry – 605 014, India. E-mail: hspr.che@pondiuni.edu.in; hspr@yahoo.com; Fax: +91-413-2656230; Tel: +91-413-2654411
First published on 5th June 2014
Abstract
We have described the simple, convenient and high yielding one-pot synthesis of a library of highly functionalized hexa-substituted 1,4-dihydropyridines (1,4-DHPs) by 2-aminopyridine catalysed pseudo three-component reaction of nitroketene-N,S-acetals and aldehydes. This domino transformation involves formation of dihydropyridine ring by creation of two C–C bonds and one C–N bond, all of them taking place in a single synthetic operation. As the products precipitate out of the reaction simple filtration is enough to gather the products and thus, there is no need for work-up or column-chromatography. The C6-methylsulfanyl group in the product 1,4-DHPs was substituted with primary and secondary amines to provide 1,4-DHPs with further possibilities in structural diversity. As a demonstration of application of the method we have synthesised an analogue of nitenpyram, a neonicotinoid insecticide.
Introduction
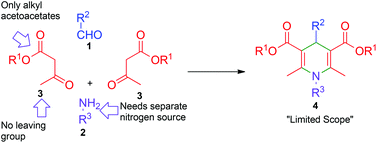
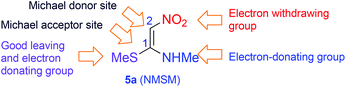
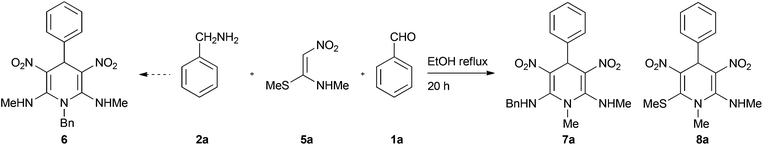
The multicomponent reactions (MCRs) are among the simplest, atom-economic and eco-friendly reactions used for synthesis of complex molecules from readily available bench-top organic chemicals.1 On their way to generation of complex products, MCRs go through cascade reactions resulting in formation of several bonds within a single pot. Owing to such highly desirable reaction features that include chemo- and regioselective formation of the products in high yield and purity, in recent years, MCRs have garnered a great deal of attention for synthesis of structurally diverse and densely functionalized biologically and medicinally important products.2 Classical Hantzsch 1,4-dihydropyridine (1,4-DHP) synthesis in which one unit each of an aldehyde 1, an amine 2 and two units of acetoacetic ester 3, undergo four-component MCRs to provide 1,4-DHPs 4 is a quintessential reaction for synthesis of a large library of six-member nitrogen heterocycles (Scheme 1).3 The 1,4-DHPs are of immense biological importance, as they are analogues of the reactive portion of nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADPH), both of which are cofactors in the enzymes that perform oxidation–reduction reactions.4 Synthetic 1,4-DHPs found extensive medicinal applications5 including use as calcium channel blockers,6 antitumor agents,7 and anti-inflammatory molecules.8 Due to the highly specific activity of 1,4-DHPs as calcium channel blocking agents and ease in bulk-scale synthesis, their derivatives, for example nifedipine, became standard drugs for treatment of coronary heart diseases.9 In addition to medicinal applications, some 1,4-DHPs, like neonicotinoids, exhibit insecticidal activity without being very toxic to humans.10 Apart from the above applications 1,4-DHPs are used as a hydride source in reduction reactions11 and as synthetic intermediates in alkaloid syntheses.12 Owing to diverse applications, 1,4-DHP has become a privileged scaffold and the group has attracted considerable attention for the preparation of structurally diverse six-member nitrogen heterocycles and equally for methodology development.13 Although, Hantzsch 1,4-DHP synthesis is hugely popular, it has some limitations like being restricted to alkyl acetoacetates, the need for a separate nitrogen source, difficulty in further functionalization of the alkyl group, etc. (Scheme 1). Hence, development of new methods by overcoming such difficulties for diversity oriented synthesis of polysubstituted 1,4-DHPs is of great interest.In continuation of our efforts in exploring the chemistry of nitroketene-N,S-acetals,14 we conceived a simple and convenient synthesis of highly functionalized 1,4-DHPs by pseudo MCR of aldehydes and (E)-N-methyl-1-(methylthio)-2-nitroethenamine (N-methyl-S-methyl nitroethylene, NMSM) 5a (Fig. 1) and their analogues. For their simplicity and reactivity pattern (Fig. 1) nitroenamines like NMSM are attractive substrates for synthesis of a variety of heterocyclic compounds.15 Recently, Tobe and co-workers reported the synthesis of C4-substituted 3,5-dinitro-1,4-DHPs, molecules relevant to the present study, by pseudo three-component condensation involving two equivalents of 2-formyl-2-nitroenamine (specifically 2-nitro-3-(propylamino)acrylaldehyde) and one equivalent electron-rich aromatic compound.16 By taking the reactivity pattern of NMSM 5a as enumerated in Fig. 1 into consideration, we performed a Hantzsch type pseudo four-component condensation of two mole equivalents of NMSM 5a with one mole equivalent each of benzaldehyde 1a and benzylamine 2a in refluxing ethanol with the intention to generate 1,4-DHP 6 (Scheme 2). The reaction, surprisingly however, provided its regioisomer 1,4-DHP 7a exclusively. In the formation of 7a benzylamine appeared to replace the methylsulfanyl group located on the initially formed 1,4-DHP 8a. When the reaction of NMSM 5a and benzaldehyde 1a was conducted in the presence of a catalytic amount of benzylamine 2a (10 mol%) the 1,4-DHP 8a was the major product (71%) and 7a was the minor product (9%). We explored the reaction further and present here its scope for the synthesis of a library of 1,4-DHPs 7 and 8 and for the facile synthesis of an analogue of neonicotinoid insecticide.
 | ||
| Scheme 2 The pseudo four-component reaction of aldehyde 1a, NMSM 5a and benzylamine 2a for the synthesis of 1,4-DHP 7a. | ||
Results and discussion
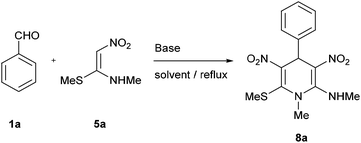
Our initial studies were focused on identification of optimal reaction conditions for the pseudo MCR of benzaldehyde 1a and NMSM 5a to afford 1,4-DHP 8a (Table 1). The reaction did not take place in the absence of a base catalyst (Table 1, entry 1). A series of the reactions was then performed with a catalytic amount (10 mol%) of base which included non-nucleophilic tertiary amines like 4-(dimethylamino)pyridine (DMAP; entry 2), 1,8-diazabicycloundec-7-ene (DBU; entry 3), triethylamine (entry 4) and pyridine (entry 5) in EtOH medium. In each case 1,4-DHP 8a was obtained in poor yield. Although the yield of the desired product 8a improved when secondary amines like piperidine (entry 6), pyrrolidine (entry 7) and L-proline (entry 8) were employed, still the yield was only moderate and much lower than when primary amine bases like benzyl amine (entry 9), 30% aq. solution of ethyl amine (entry 10) or aniline (entry 11) were employed. The real breakthrough appeared when we employed 10 mol% of 2-aminopyridine (2-AP; entry 12) and the reaction provided over 92% yield consistently. The yield of 8a was lower when 4-aminopyridine (entry 13) or inorganic bases, e.g. potassium carbonate (entry 14), were employed. Next, we varied the amount of the 2-AP to evaluate the minimum amount required to furnish 8a in near quantitative yield. The set of experiments (entries 15–17) showed that 10 mol% of 2-AP provides products in high purity in over 92% yield.| Entry | Basea (equivalents) | Solvent | Time (h) | Yield (%) |
|---|---|---|---|---|
| a DMAP: 4-(dimethylamino)pyridine; DBU: 1,8-diazabicycloundec-7-ene; AP: aminopyridine; DMF: N,N-dimethylformamide. | ||||
| 1 | No catalyst | EtOH | 24 | — |
| 2 | DMAP (0.1) | EtOH | 15 | 30 |
| 3 | DBU (0.1) | EtOH | 20 | 10 |
| 4 | Et3N (0.1) | EtOH | 20 | 26 |
| 5 | Pyridine (0.1) | EtOH | 10 | 20 |
| 6 | Piperidine (0.1) | EtOH | 15 | 38 |
| 7 | Pyrrolidine (0.1) | EtOH | 10 | 36 |
| 8 | L-Proline (0.1) | EtOH | 15 | 46 |
| 9 | Benzylamine (0.1) | EtOH | 20 | 71 |
| 10 | Ethyl amine (0.1) | EtOH | 12 | 62 |
| 11 | Aniline (0.1) | EtOH | 15 | 62 |
| 12 | 2-AP (0.1) | EtOH | 5 | 92 |
| 13 | 4-AP (0.1) | EtOH | 10 | 71 |
| 14 | K2CO3 (0.1) | EtOH | 15 | 23 |
| 15 | 2-AP (0.05) | EtOH | 20 | 70 |
| 16 | 2-AP (0.01) | EtOH | 15 | 62 |
| 17 | 2-AP (0.001) | EtOH | 25 | 60 |
| 18 | 2-AP (1) | EtOH | 2 | 92 |
| 19 | 2-AP (0.1) | H2O | 20 | 40 |
| 20 | 2-AP (0.1) | EtOH–H2O | 15 | 75 |
| 21 | 2-AP (0.1) | MeOH | 10 | 85 |
| 22 | 2-AP (0.1) | DMF | 15 | 62 |
| 23 | 2-AP (0.1) | Toluene | 24 | 65 |
With 1 equivalent of 2-AP the product 8a was formed in over 92% yield within 2 h (entry 18), but the product required chromatographic purification. Although the reaction proceeded in various solvents like water (entry 19), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and ethanol (entry 20), methanol (entry 21), dimethylformamide (entry 22) and toluene (entry 23) at respective solvent reflux temperature, refluxing ethanol was selected for optimized reaction conditions. Under these conditions, yellow colored product 8a precipitated from the reaction mixture and simple filtration was enough to collect the spectroscopic grade product.
1 mixture of water and ethanol (entry 20), methanol (entry 21), dimethylformamide (entry 22) and toluene (entry 23) at respective solvent reflux temperature, refluxing ethanol was selected for optimized reaction conditions. Under these conditions, yellow colored product 8a precipitated from the reaction mixture and simple filtration was enough to collect the spectroscopic grade product.
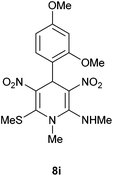
The 1,4-DHP 8a was characterized on the basis of IR, 1H NMR, 13C NMR spectral data and HRMS analysis. The 1H NMR spectrum of 8a displayed a characteristic signal for C4H at 5.94 ppm. The signals located at δ 2.53, 3.09 and 3.42 attributable to SMe, NHMe and NMe, respectively, supported the assigned structure. The 13C NMR spectrum of 8a displayed the anticipated 12 signals out of which four were located in the aliphatic region. The structure of 8i was unequivocally assigned on the basis of single crystal X-ray structure analysis (Fig. 2).
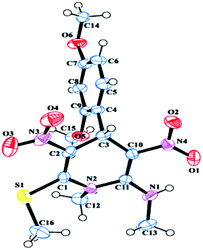 | ||
| Fig. 2 ORTEP diagram of the 4-(2,4-dimethoxyphenyl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8i. | ||
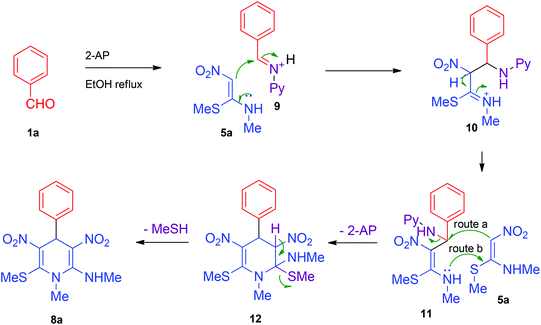
Based on the above results, a plausible mechanism was proposed as shown in Scheme 3. In the first step benzaldehyde 1a reacts with 2-AP to form the iminium ion 9. The iminium ion then reacts with NMSM 5a to generate intermediate 10 which quickly rearranges to the more stable intermediate 11 where the nitroketene-N,S-acetal substructure has been restored. The intermediate 11 reacts with one more unit of NMSM 5a to generate intermediate 12 and 2-AP. Finally, an intramolecular elimination of methanethiol furnishes 1,4-DHP 8a. Formation of iminium ion 9 as the initial intermediate is indicated by the fact that primary amines efficiently promote the pseudo MCR (Table 1). Nucleophilic base 2-AP appears to have the unique ability to form an imine by reacting with benzaldehyde 1a but not displace the methylsulfanyl group in 8a. The reaction of intermediate 11 with a second unit of NMSM 5a could go through several pathways, like formation of C–C bond (route a, Scheme 3) ahead of C–N bond formation (route b, Scheme 3) or the other way. Although confirmative evidences are not available at present, we presume that route a is more feasible than b (Scheme 3) as nucleophilic primary amines like benzyl amine did not react with NMSM to provide 6 or 7a as the primary product (Scheme 2). The reaction of intermediate 11 with 5a could go through an aza-Diels–Alder pathway. However, taking into consideration the low reactivity of NMSM 5a towards Diels–Alder reactions17 we assume this pathway is unlikely.
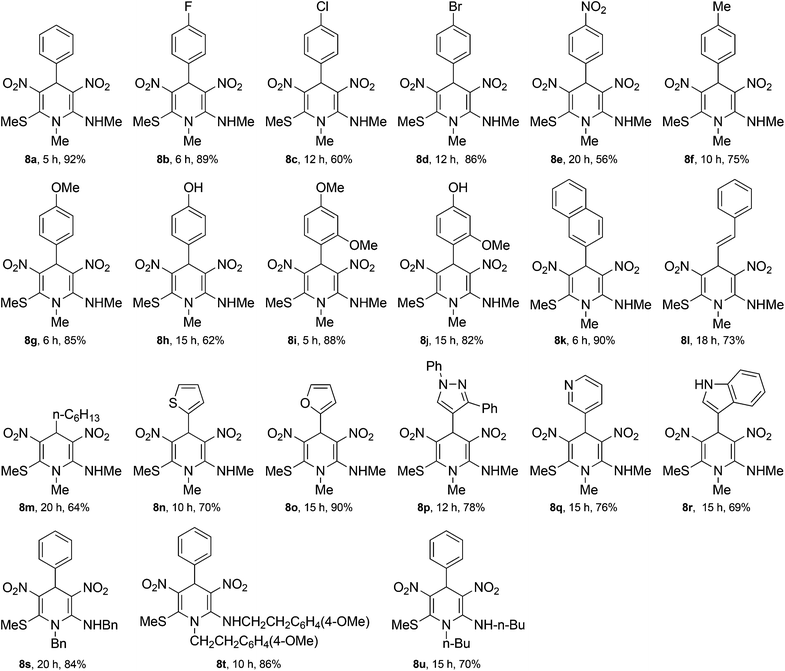
The 1,4-DHPs 8 have two potential areas for development of structural diversity, namely the aldehyde and the N-alkyl groups, which can be exploited for preparation of a library of two-dimensional matrix. We employed the optimized reaction conditions for construction of a small library of 1,4-DHPs 8a–u (Table 2, Fig. 3). Initially, variations were incorporated in the benzaldehyde ring by placing electron-withdrawing or electron-donating groups in the C4 position (Table 2, entries 2–8) and the products 8b–h were obtained in good yields. The results indicated that the electron-donating or withdrawing groups had little influence on the outcome of the reaction. Introduction of steric effects at the C2-position of benzaldehyde (entries 9–10) in the form of OMe group did not have any effect on the formation of 1,4-DHPs 8i–j. Similarly, naphthaldehyde (entry 11) provided the 1,4-DHP 8k without much difficulty. This methodology was tested with cinnamaldehyde (entry 12) for the formation of 1,4-DHP 8l and the results indicated that the α,β-unsaturation does not have much influence. An aliphatic aldehyde, e.g. hexanal (entry 13), furnished 1,4-DHP 8m in good yield. The pseudo MCR was next performed with various heterocyclic aromatic aldehydes like thiophene-2-carbaldehyde 1n, furan-2-carbaldehyde 1o, 1,3-diphenyl-1H-pyrazole-4-carbaldehyde 1p, pyridine-3-carbaldehyde 1q and indole-3-carbaldehyde 1r (entries 14–18) to result in the corresponding 1,4-DHPs 8n–r in good yield.
| Entry | R1 in 1 | R2 in 5 | 1,4-DHP 8 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a General conditions: 1 (1 equivalent), 5 (2 equivalents) and 2-aminopyridine (0.1 equivalent) in refluxing ethanol. b Isolated yield after triturating with cold (0–5 °C) ethanol. | |||||
| 1 | Ph | Me | 8a | 5 | 92 |
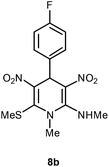
| 2 | p-FC6H4 | Me | 8b | 6 | 89 |
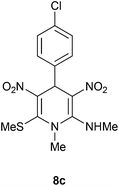
| 3 | p-ClC6H4 | Me | 8c | 12 | 60 |
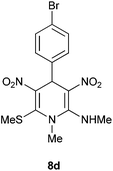
| 4 | p-BrC6H4 | Me | 8d | 12 | 86 |
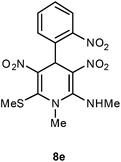
| 5 | o-O2NC6H4 | Me | 8e | 20 | 56 |
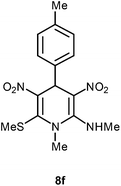
| 6 | p-MeC6H4 | Me | 8f | 10 | 75 |
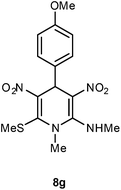
| 7 | p-MeOC6H4 | Me | 8g | 6 | 85 |
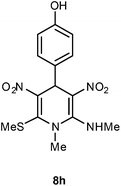
| 8 | p-HOC6H4 | Me | 8h | 15 | 62 |
| 9 | o,p-(MeO)2C6H3 | Me | 8i | 5 | 88 |
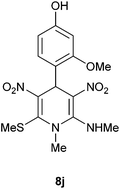
| 10 | p-HO,o-MeOC6H3 | Me | 8j | 15 | 82 |
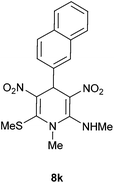
| 11 | Naphthalene-2-yl | Me | 8k | 6 | 90 |
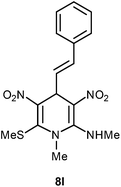
| 12 | Styryl | Me | 8l | 18 | 73 |
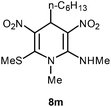
| 13 | Pentyl | Me | 8m | 20 | 64 |
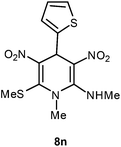
| 14 | Thiophen-2-yl | Me | 8n | 10 | 70 |
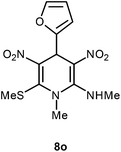
| 15 | Furan-2-yl | Me | 8o | 15 | 90 |
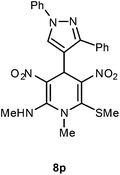
| 16 | 1,3-Biphenyl-1H-pyrazol-4-yl | Me | 8p | 12 | 78 |
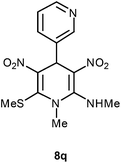
| 17 | Pyridine-3-yl | Me | 8q | 15 | 76 |
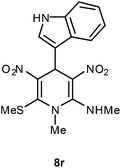
| 18 | Indol-3-yl | Me | 8r | 15 | 69 |
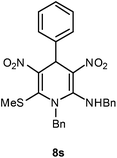
| 19 | Ph | Bn | 8s | 20 | 84 |
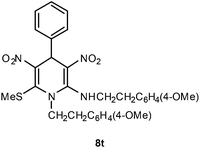
| 20 | Ph | 4-MeOC6H4CH2CH2 | 8t | 10 | 86 |
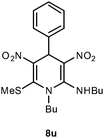
| 21 | Ph | n-Butyl | 8u | 15 | 70 |
After demonstrating the ease of synthesis of 1,4-DHPs 8b–r by incorporating various changes in the aldehyde portion, we have taken up the synthesis of 1,4-DHPs where diversity is built into N-alkyl group. The reaction of nitroketene-N,S-acetals with N-benzyl 5b, N-4-methoxyphenylethyl 5c and n-butyl 5d groups afforded the corresponding 1,4-DHPs 8s–u (entries 19–21) without much difficulty. However, the reaction with the nitroketene-N,S-acetal with N-phenyl group did not furnish the desired 1,4-DHP, possibly due to steric repulsion that gets inbuilt when the product is formed. Spectroscopic data of 1,4-dihydropyridines 8b–u compared well with those of the parent compound 8a.
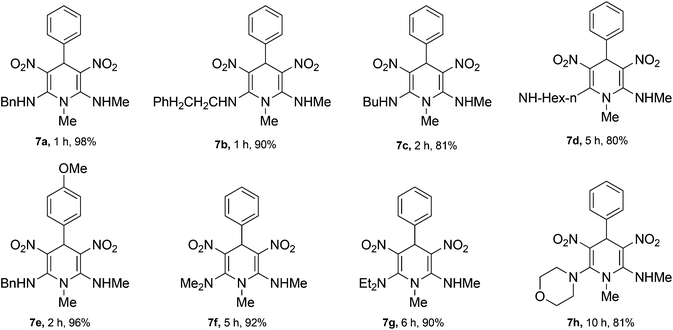
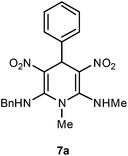
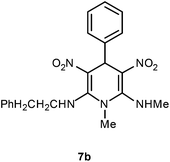
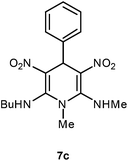
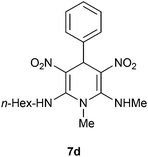
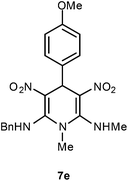
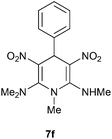
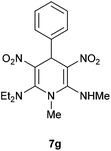
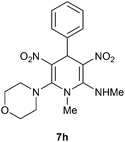
The 1,4-dihydropyridines 8 possess a highly labile SMe group, which can be replaced with a variety of nucleophiles by SNV substitution.18 For the present study, we have selected to replace the C6 methylsulfanyl group in 8a with a variety of aliphatic primary and secondary amines. Thus, the reaction of 1,4-DHP 8a with 1 equivalent of primary amines like benzyl amine 2a, phenylethyl amine 2b, n-butyl amine 2c, and n-hexyl amine 2d in refluxing EtOH afforded the corresponding 1,4-DHPs 7a–d in good yields (Scheme 4, Table 3, entries 1–4). The above experiments showed that it is possible to synthesise a library of 1,4-DHPs that possess three areas of structural diversity, derived from the aldehydes 1, amine in N,S-acetals 5 and amines 2 (Scheme 4). To demonstrate this premise on one example, the 1,4-DHP 8g where the C4-aryl ring is derived from 4-methoxybenzaldehyde was subjected to reaction with benzylamine and the product 7e was isolated without any difficulty (Table 3, entry 5). Although the reaction took longer, the methylsulfanyl group in 1,4-DHP 8a, could be replaced with secondary amines like N,N-dimethylamine 2e, N,N-diethylamine 2f and morpholine 2g afforded the corresponding 1,4-DHPs 7f–h in good yields (Table 3, entries 6–8). The library of 1,4-DHPs of type 7 that was prepared in this study is shown in Fig. 4. Although it is possible to prepare dihydropyridines like 7via a pseudo four-component MCR of one equivalent of primary amine, two equivalents of NMSM and one equivalent of the aldehyde, we found that the transformation is restricted to reactive and high boiling primary amines like benzyl amine. For other primary amines like butyl and hexylamine or secondary amines like piperidine the reaction was slow and low yielding.
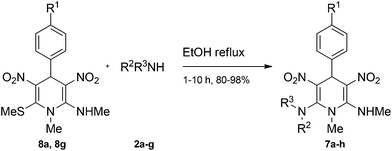 | ||
| Scheme 4 Synthesis of 1,4-dihydropyridines (7a–h) by nucleophilic displacement of the C6 methylsulfanyl group in 8a with primary or secondary amines and 8g with benzylamine. | ||
| Entry | R1 | R2 in 2 | R3 in 2 | Product | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | H | Bn | H | 7a | 1 | 98 |
| 2 | H | Phenylethyl | H | 7b | 1 | 90 |
| 3 | H | n-Bu | H | 7c | 2 | 81 |
| 4 | H | n-Hexyl | H | 7d | 5 | 80 |
| 5 | OMe | Bn | H | 7e | 2 | 96 |
| 6 | H | Me | Me | 7f | 5 | 92 |
| 7 | H | Et | Et | 7g | 6 | 82 |
| 8 | H | Morpholine | H | 7h | 10 | 81 |
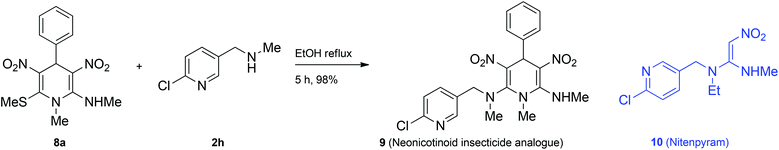
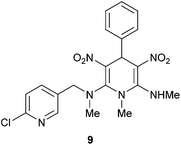
To demonstrate an application of the present study, we designed a synthesis of neonicotinoid analogue 9 (Scheme 5). Neonicotinoids, for example nitenpyram 10 are a group of newer class of insecticides used in agriculture and flea control in domestic animals.19 They exhibit lower toxicity in mammals than in insects compared to organochlorides, organophosphates and carbamates.20 Although neonicotinoids have become hugely popular, there are some concerns about their activity against humans and also on human-friendly insects like honey bees.21 Moreover acquisition of resistance to the neonicotinoid insecticides is on the rise.22 Therefore there is a requirement to design suitable analogues with favourable properties. Chuanwen and co-workers synthesized a few 1,4-DHPs incorporating the nitenpyram structural motif and showed that when the nitro group and the secondary amine are locked in a cis configuration, the insecticide activity against the common pest Aphis medicaginis is better.23 We have taken up synthesis of the neonicotinoid analogue 9 to demonstrate an application of our newly developed pseudo MCR for the synthesis of 1,4-DHPs. The reaction of 8a with 1-(6-chloropyridin-3-yl)-N-methylmethanamine 2h furnished the neonicotinoid analogue 9 in excellent yield. The structure of 9 was assigned on the basis of spectroscopic data and confirmed by single crystal X-ray diffraction analysis (Fig. 5, given in the Experimental section). Interestingly, the room-temperature 1H NMR spectrum of 9 showed broad peaks for methylene hydrogens. On heating to 80 °C the spectrum became clear (see ESI†). This result indicated slow rotation around the C2–N bond at room temperature owing to steric hindrance between ArCH2NMe and NO2 groups.
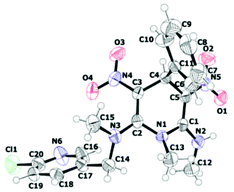 | ||
| Fig. 5 ORTEP diagram of the N2-((6-chloropyridin-3-yl)methyl)-N2,N6,1-trimethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 9. | ||
Conclusion
In summary, we have discovered and described new pseudo three-component reactions for regio- and chemoselective, high-yielding and experimentally convenient one-pot synthesis of diversely functionalized hexa-substituted 1,4-dihydropyridines 8 from readily available aldehydes 1 and nitroketene-N,S-acetals 5. The primary amine 2-AP acted as a tailor-made catalyst for the pseudo MCR. Conveniently the product 1,4-DHPs precipitated out of the reaction and simple filtration was enough to isolate pure products. Nucleophilic displacement of the C6 methylsulfanyl group in 8 with different primary and secondary amines afforded 1,4-dihydropyridines 7 with additional structural diversity. The newly developed protocol allowed a convenient and high-yielding synthesis of neonicotinoid insecticide analogue 9.Experimental
General experimental
All melting points were uncorrected and were determined using open-ended capillary tubes on VEEGO VMP-DS instrument. All the reactions and chromatographic separations were observed by thin layer chromatography. Glass plates coated with silica gel (60–120 mesh SRL chemicals) were employed for thin layer chromatography (TLC). Infra Red (IR) spectra were recorded as KBr pellets on a Nicolet-6700 spectrometer. 1H NMR (400 MHz), 13C NMR (100 MHz) and DEPT-135 spectra were recorded for (CDCl3) or (DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions on a BrukerAvance 400 spectrometer with tetramethylsilane (TMS) as internal standard; J values are in Hz. 1H NMR data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet), coupling constant, integration. High resolution mass spectra were recorded on a Water Q-TOF micro mass spectrometer using electron spray ionization mode. The X-ray diffraction measurements were performed at 298 K on Oxford CrysAlis CCD area detector system equipped with a graphite monochromator and a Mo-Kα fine-focus sealed tube (λ = 0.71073 Å). Benzaldehydes and amines was purchased from Sigma Aldrich Chemicals Private Limited and (E)-N-methyl-1-(methylthio)-2-nitroethenamine NMSM 5a was gifted by Shasun Chemical Company, Chennai. We synthesized NMSM derivatives like (E)-N-benzyl-1-(methylthio)-2-nitroethenamine 5b, (E)-N-(4-methoxyphenethyl)-1-(methylthio)-2-nitroethenamine 5c and (E)-N-(1-(methylthio)-2-nitrovinyl)butan-1-amine 5d in our laboratory.
1) solutions on a BrukerAvance 400 spectrometer with tetramethylsilane (TMS) as internal standard; J values are in Hz. 1H NMR data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet), coupling constant, integration. High resolution mass spectra were recorded on a Water Q-TOF micro mass spectrometer using electron spray ionization mode. The X-ray diffraction measurements were performed at 298 K on Oxford CrysAlis CCD area detector system equipped with a graphite monochromator and a Mo-Kα fine-focus sealed tube (λ = 0.71073 Å). Benzaldehydes and amines was purchased from Sigma Aldrich Chemicals Private Limited and (E)-N-methyl-1-(methylthio)-2-nitroethenamine NMSM 5a was gifted by Shasun Chemical Company, Chennai. We synthesized NMSM derivatives like (E)-N-benzyl-1-(methylthio)-2-nitroethenamine 5b, (E)-N-(4-methoxyphenethyl)-1-(methylthio)-2-nitroethenamine 5c and (E)-N-(1-(methylthio)-2-nitrovinyl)butan-1-amine 5d in our laboratory.
General procedure for synthesis of 1,4-dihydropyridines 8
A solution of aldehydes (1.0 equiv.), NMSM (2.0 equiv.) and 2-aminopyridine (0.1 equiv.) in ethanol (3 mL) were mixed and stirred at 80 °C until the reaction was complete, as monitored by thin-layer chromatography (TLC, hexanes–EtOAc, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The reaction mixture was cooled to room temperature and the resulting solid was filtered off and recrystallized from dichloromethane and hexane to obtain pure products 8.
2). The reaction mixture was cooled to room temperature and the resulting solid was filtered off and recrystallized from dichloromethane and hexane to obtain pure products 8.
Representative procedure for preparation of hexa substituted 1,4-dihydropyridines 8a
In a round-bottomed flask a solution of benzaldehyde 1a (105 mg, 0.94 mmol) NMSM 5a (279 mg, 1.88 mmol) and 10 mol% of 2-aminopyridine (10 mg, 0.09 mmol) in ethanol (3 mL) were mixed and stirred at 80 °C until the reaction was complete, as monitored by thin-layer chromatography (TLC, hexanes–EtOAc, 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After 5 h yellow solid was obtained which was filtered to afford N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a. Good crystals were obtained by recrystallization with a solution of dichloromethane–hexane (9
2). After 5 h yellow solid was obtained which was filtered to afford N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a. Good crystals were obtained by recrystallization with a solution of dichloromethane–hexane (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). Yield (292 mg 92%; mp 201 °C; IR Data (ν) (KBr) 3057, 2995, 2928, 1631, 1497, 1440, 1358, 1244, 1069, 721 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
3 v/v). Yield (292 mg 92%; mp 201 °C; IR Data (ν) (KBr) 3057, 2995, 2928, 1631, 1497, 1440, 1358, 1244, 1069, 721 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.05 (s, 1H), 7.28 (t, J = 10.8 Hz, 2H), 7.23 (d, J = 7.2 Hz, 1H), 7.12 (d, J = 7.1 Hz, 2H), 5.94 (s, 1H), 3.42 (s, 3H), 3.09 (d, J = 5.2 Hz, 3H), 2.53 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.05 (s, 1H), 7.28 (t, J = 10.8 Hz, 2H), 7.23 (d, J = 7.2 Hz, 1H), 7.12 (d, J = 7.1 Hz, 2H), 5.94 (s, 1H), 3.42 (s, 3H), 3.09 (d, J = 5.2 Hz, 3H), 2.53 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 156.3 (C), 154.8 (C), 139.9 (C), 137.6 (C), 129.1 (CH), 127.8 (CH), 126.9 (CH), 113.2 (C), 43.2 (NMe), 40.8 (NHMe), 32.1 (CH), 16.4 (SMe); HRMS (ESI) Calcd for C14H16N4O4SNa [M + Na] 359.0790 amu, found 359.0791 amu.
1) δ 156.3 (C), 154.8 (C), 139.9 (C), 137.6 (C), 129.1 (CH), 127.8 (CH), 126.9 (CH), 113.2 (C), 43.2 (NMe), 40.8 (NHMe), 32.1 (CH), 16.4 (SMe); HRMS (ESI) Calcd for C14H16N4O4SNa [M + Na] 359.0790 amu, found 359.0791 amu.
Following the representative procedure, the solution of 4-fluorobenzaldehyde 1b (202 mg, 1.62 mmol), NMSM 5a (454 mg, 3.29 mmol) and 10 mol% of 2-aminopyridine (15 mg, 0.161 mmol in ethanol (3 mL) afforded 4-(4-fluorophenyl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8b. Yield (400 mg, 89%); mp 230 °C; IR Data (ν) (KBr) 3067, 2934, 1630, 1486, 1440, 1393, 1299, 1193, 1069, 802 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.06 (s, 1H), 7.38–7.32 (m, 1H), 7.07–7.02 (m, 1H), 6.98 (d, J = 7.8 Hz, 1H), 6.89 (td, J = 9.8, 2.9 Hz, 1H), 5.98 (s, 1H), 3.45 (s, 3H), 3.10 (d, J = 5.3 Hz, 3H), 2.56 (s, 3H),13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.06 (s, 1H), 7.38–7.32 (m, 1H), 7.07–7.02 (m, 1H), 6.98 (d, J = 7.8 Hz, 1H), 6.89 (td, J = 9.8, 2.9 Hz, 1H), 5.98 (s, 1H), 3.45 (s, 3H), 3.10 (d, J = 5.3 Hz, 3H), 2.56 (s, 3H),13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 162.2 (C), 155.5 (C), 142.5 (C), 136.6 (C), 130.8 (CH), 122.5 (CH), 114.4 (C), 113.7 (C), 42.8 (NMe), 39.9 (NHMe), 31.8 (CH), 16.1 (SMe); HRMS (ESI) Calcd for C14H15FN4O4SNa [M + Na] 377.0696 amu, found 377.0694 amu.
1) δ 162.2 (C), 155.5 (C), 142.5 (C), 136.6 (C), 130.8 (CH), 122.5 (CH), 114.4 (C), 113.7 (C), 42.8 (NMe), 39.9 (NHMe), 31.8 (CH), 16.1 (SMe); HRMS (ESI) Calcd for C14H15FN4O4SNa [M + Na] 377.0696 amu, found 377.0694 amu.
Following the representative procedure, the solution of 4 chlorobenzaldehyde 1c (255 mg, 1.78 mmol), NMSM 5a (498 mg, 3.57 mmol) and 10 mol% of 2-aminopyridine (20 mg, 0.17 mmol) in ethanol (3 mL) afforded 4-(4-chlorophenyl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8c. Yield (250 mg, 60%); mp 213 °C; IR Data (ν) (KBr) 3188, 3067, 2996, 2932, 1626, 1580, 1448, 1392, 1361, 1322, 1283, 1070, 778 cm−1. 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.05 (s, 1H), 7.32 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.5 Hz, 2H), 5.96 (s, 1H), 3.45 (s, 3H), 3.11 (d, J = 5.2 Hz, 3H), 2.57 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.05 (s, 1H), 7.32 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.5 Hz, 2H), 5.96 (s, 1H), 3.45 (s, 3H), 3.11 (d, J = 5.2 Hz, 3H), 2.57 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.7 (C), 154.8 (C), 138.5 (C), 136.7 (C), 132.2 (C), 128.6 (CH), 128.4 (CH), 112.5 (C), 42.7 (NMe), 39.9 (NHMe), 31.7 (CH), 16.0 (SMe); HRMS (ESI) Calcd for C14H15ClN4O4SNa [M + Na] 393.0400 amu, found 393.0400 amu.
1) δ 155.7 (C), 154.8 (C), 138.5 (C), 136.7 (C), 132.2 (C), 128.6 (CH), 128.4 (CH), 112.5 (C), 42.7 (NMe), 39.9 (NHMe), 31.7 (CH), 16.0 (SMe); HRMS (ESI) Calcd for C14H15ClN4O4SNa [M + Na] 393.0400 amu, found 393.0400 amu.
Following the representative procedure, the solution of 4-bromo benzaldehyde 1d (101 mg, 0.54 mmol), NMSM 5a (173 mg, 1.08 mmol) and 10 mol% of 2-aminopyridine (12 mg, 0.05 mmol) in ethanol (3 mL) afforded 4-(4-bromophenyl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8d. Yield (180 mg, 86%); mp 219 °C; IR Data (ν) (KBr) 3198, 3068, 2995, 2933, 1627, 1484, 1361, 1281, 1239, 1192, 1068, 775 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.04 (s, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H), 5.94 (s, 1H), 3.44 (s, 3H), 3.10 (s, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.04 (s, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H), 5.94 (s, 1H), 3.44 (s, 3H), 3.10 (s, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.7 (C), 154.8 (C), 138.9 (C), 136.6 (C), 131.6 (CH), 128.8 (CH), 120.6 (C), 112.4 (C), 42.7 (NMe), 39.9 (NHMe), 31.7 (CH), 16.0 (SMe); HRMS (ESI) Calcd for C14H15BrN4O4SNa [M + Na] 436.9895 amu, found 436.9897 amu.
1) δ 155.7 (C), 154.8 (C), 138.9 (C), 136.6 (C), 131.6 (CH), 128.8 (CH), 120.6 (C), 112.4 (C), 42.7 (NMe), 39.9 (NHMe), 31.7 (CH), 16.0 (SMe); HRMS (ESI) Calcd for C14H15BrN4O4SNa [M + Na] 436.9895 amu, found 436.9897 amu.
Following the representative procedure, the solution of 2-nitrobenzaldehyde 1e (204 mg, 1.32 mmol), NMSM 5a (353 mg, 2.64 mmol) and 10 mol% of 2-aminopyridine (26 mg, 0.13 mmol) in ethanol (3 mL) afforded N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-(2-nitrophenyl)-1,4-dihydropyridin-2-amine 8e. Yield (250 mg, 56%); mp 226 °C; IR Data (ν) (KBr) 2994, 2934, 1628, 1568, 1529, 1492, 1367, 1277, 1162, 1068, 719 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.15 (s, 1H), 7.80 (dd, J = 8.0, 1.1 Hz, 1H), 7.67 (td, J = 7.9, 1.2 Hz, 1H), 7.45 (t, J = 11.5 Hz, 1H), 7.26 (dd, J = 8.0, 1.2 Hz, 1H), 6.49 (s, 1H), 3.59 (s, 3H), 3.12 (d, J = 3.1 Hz, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.15 (s, 1H), 7.80 (dd, J = 8.0, 1.1 Hz, 1H), 7.67 (td, J = 7.9, 1.2 Hz, 1H), 7.45 (t, J = 11.5 Hz, 1H), 7.26 (dd, J = 8.0, 1.2 Hz, 1H), 6.49 (s, 1H), 3.59 (s, 3H), 3.12 (d, J = 3.1 Hz, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 156.0 (C), 152.5 (C), 149.1 (C), 136.2 (C), 133.4 (CH), 132.6 (C), 129.6 (CH), 128.8 (CH), 124.3 (CH), 112.4 (CH), 42.9 (NMe), 36.6 (NHMe), 32.0 (CH), 16.1 (SMe); HRMS (ESI) Calcd for C14H15N5O6SNa [M + Na] 404.0641 amu, found 404.0639 amu.
1) δ 156.0 (C), 152.5 (C), 149.1 (C), 136.2 (C), 133.4 (CH), 132.6 (C), 129.6 (CH), 128.8 (CH), 124.3 (CH), 112.4 (CH), 42.9 (NMe), 36.6 (NHMe), 32.0 (CH), 16.1 (SMe); HRMS (ESI) Calcd for C14H15N5O6SNa [M + Na] 404.0641 amu, found 404.0639 amu.
Following the representative procedure, the solution of 4 methyl benzaldehyde 1f (502 mg, 4.16 mmol), NMSM 5a (798 mg, 8.33 mmol) and 10 mol% of 2-aminopyridine (41 mg, 0.41 mmol) in ethanol (5 mL) afforded N,1-dimethyl-6(methylthio)-3,5-dinitro-4-(p-tolyl)-1,4-dihydropyridin-2-amine 8f. Yield (1.1 g, 75%); mp 214 °C; IR Data (ν) (KBr) 3176, 2999, 2927, 1630, 1489, 1443, 1389, 1358, 1197, 1168, 1069, 777 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 9.96 (s, 1H), 7.04 (d, J = 8.0 Hz, 2H), 6.98 (d, J = 8 Hz, 2H), 5.92 (s, 1H), 3.39 (s, 3H), 3.09 (s, 3H), 2.49 (d, J = 9.2 Hz, 3H), 2.25 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 9.96 (s, 1H), 7.04 (d, J = 8.0 Hz, 2H), 6.98 (d, J = 8 Hz, 2H), 5.92 (s, 1H), 3.39 (s, 3H), 3.09 (s, 3H), 2.49 (d, J = 9.2 Hz, 3H), 2.25 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.7 (C), 152.8 (C), 137.9 (C), 136.8 (C), 136.4 (C), 129.1 (CH), 126.4 (CH), 113.1 (C), 42.5 (NMe), 40.2 (NHMe), 31.6 (CH), 20.6 (CH3), 15.9 (SMe); HRMS (ESI) Calcd for C15H18N4O4SNa [M + Na] 373.0946 amu, found 373.0945 amu.
1) δ 155.7 (C), 152.8 (C), 137.9 (C), 136.8 (C), 136.4 (C), 129.1 (CH), 126.4 (CH), 113.1 (C), 42.5 (NMe), 40.2 (NHMe), 31.6 (CH), 20.6 (CH3), 15.9 (SMe); HRMS (ESI) Calcd for C15H18N4O4SNa [M + Na] 373.0946 amu, found 373.0945 amu.
Following the representative procedure, the solution of 4-methoxybenzaldehyde 1g (252 mg, 1.83 mmol), NMSM 5a (531 mg, 3.67 mmol) and 10 mol% of 2-aminopyridine (21 mg, 0.18 mmol) in ethanol (3 mL) afforded 4-(4-methoxyphenyl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8g. Yield (570 mg, 85%); mp 203 °C; IR Data (ν) (KBr) 3192, 2994, 2935, 1624, 1497, 1363, 1287, 1249, 1174, 1064 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 9.96 (s, 1H), 7.05 (d, J = 8.4 Hz, 2H), 6.79 (d, J = 8.4 Hz, 2H), 5.91 (s, 1H), 3.72 (s, 3H), 3.43 (s, 3H), 3.12 (d, J = 5.2 Hz, 3 H), 2.58 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 9.96 (s, 1H), 7.05 (d, J = 8.4 Hz, 2H), 6.79 (d, J = 8.4 Hz, 2H), 5.91 (s, 1H), 3.72 (s, 3H), 3.43 (s, 3H), 3.12 (d, J = 5.2 Hz, 3 H), 2.58 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 158.4 (C), 155.6 (C), 152.2 (C), 138.1 (C), 131.4 (CH), 127.6 (CH), 113.8 (C), 113.3 (C), 54.6 (OMe), 42.4 (NMe), 40.1 (NHMe), 31.4 (CH), 15.8 (SMe); HRMS (ESI) Calcd for C15H18N4O5SNa [M + Na] 389.0896 amu, found 389.0894 amu.
1) δ 158.4 (C), 155.6 (C), 152.2 (C), 138.1 (C), 131.4 (CH), 127.6 (CH), 113.8 (C), 113.3 (C), 54.6 (OMe), 42.4 (NMe), 40.1 (NHMe), 31.4 (CH), 15.8 (SMe); HRMS (ESI) Calcd for C15H18N4O5SNa [M + Na] 389.0896 amu, found 389.0894 amu.
Following the representative procedure, the solution of 4-hydroxybenzaldehyde 1h (510 mg, 2.04 mmol), NMSM 5a (605 mg, 4.09 mmol) and 10 mol% of 2-aminopyridine (20 mg, 0.20 mmol) in ethanol (3 mL) afforded 4-(1-methyl-2-(methylamino)-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-4-yl)phenol 8h. Yield (240 mg, 62%); mp 221 °C; IR Data (ν) (KBr) 3244, 3014, 2940, 1624, 1511, 1478, 1394, 1353, 1322, 1278, 1239, 1195, 1162, 1122 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 9.99 (s, 1H), 9.25 (s, 1H), 6.90 (d, J = 8.8 Hz, 2H), 6.66 (dd, J = 6.6, 1.8 Hz, 2H), 5.85 (s, 1H), 3.41 (s, 3H), 3.09 (d, J = 5.6 Hz, 3H), 2.51 (s, 3 H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 9.99 (s, 1H), 9.25 (s, 1H), 6.90 (d, J = 8.8 Hz, 2H), 6.66 (dd, J = 6.6, 1.8 Hz, 2H), 5.85 (s, 1H), 3.41 (s, 3H), 3.09 (d, J = 5.6 Hz, 3H), 2.51 (s, 3 H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 156.8 (C), 155.8 (C), 152.6 (C), 138.3 (C), 129.8 (C), 127.6 (CH), 115.5 (CH), 113.5 (C), 42.6 (NMe), 39.7 (NHMe), 31.6 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C14H16N4O5SNa [M + Na] 375.0739 amu, found 375.0738 amu.
1) δ 156.8 (C), 155.8 (C), 152.6 (C), 138.3 (C), 129.8 (C), 127.6 (CH), 115.5 (CH), 113.5 (C), 42.6 (NMe), 39.7 (NHMe), 31.6 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C14H16N4O5SNa [M + Na] 375.0739 amu, found 375.0738 amu.
Following the representative procedure, the solution of 2,4-dimethoxy benzaldehyde 1i (501 mg, 3.01 mmol), NMSM 5a (891 mg, 6.02 mmol) and 10 mol% of 2-aminopyridine (25 mg, 6.02 mmol) in ethanol (5 mL) afforded 4-(2,4-dimethoxyphenyl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8i. Yield (1.1 g, 88%); mp 204 °C; IR Data (ν) (KBr) 3165, 2959, 2836, 1628, 1485, 1391, 1362, 1205, 1165, 1047, 770 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.12 (s, 1H), 6.69 (d, J = 2.3 Hz, 1H), 6.49 (d, J = 2.4 Hz, 1H), 6.41 (dd, J = 8.4, 2.3 Hz, 1H) 5.86 (s, 1H), 3.73 (s, 3 H), 3.70 (s, 3H), 3.38 (s, 3H), 3.12 (d, J = 5.2 Hz, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.12 (s, 1H), 6.69 (d, J = 2.3 Hz, 1H), 6.49 (d, J = 2.4 Hz, 1H), 6.41 (dd, J = 8.4, 2.3 Hz, 1H) 5.86 (s, 1H), 3.73 (s, 3 H), 3.70 (s, 3H), 3.38 (s, 3H), 3.12 (d, J = 5.2 Hz, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 159.9 (C), 158.1 (C), 156.8 (C), 148.3 (C), 137.9 (C), 129.5 (CH), 118.4 (C), 111.3 (C), 104.4 (CH), 98.8 (CH), 55.3 (OMe), 54.9 (OMe), 41.8 (NMe), 37.9 (NHMe), 31.8 (CH), 15.6 (SMe); HRMS (ESI) Calcd for C16H20N4O6SNa [M + Na] 419.1001 amu, found 419.1002 amu.
1) δ 159.9 (C), 158.1 (C), 156.8 (C), 148.3 (C), 137.9 (C), 129.5 (CH), 118.4 (C), 111.3 (C), 104.4 (CH), 98.8 (CH), 55.3 (OMe), 54.9 (OMe), 41.8 (NMe), 37.9 (NHMe), 31.8 (CH), 15.6 (SMe); HRMS (ESI) Calcd for C16H20N4O6SNa [M + Na] 419.1001 amu, found 419.1002 amu.
Empirical formula, C16H20N4O6S; formula weight, 396.41; crystal colour, light yellow; crystal dimensions a = 7.6554(3) Å, b = 9.9905(4) Å, c = 12.4133(5) Å; α = 84.570(4), β = 88.088(3), γ = 67.987(4); crystal system, triclinic; V = 876.22(7) A3; space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; Z = 2; Dcalcd = 1.495 g cm−3; F(000) = 414.0; R(I ≥ 2σ1) = 0.1010, wR2 = 0.2675. Detailed X-ray crystallographic data was available from the Cambridge Crystallographic Data Centre (for compound 8i CCDC 992572; see Fig. 2).
; Z = 2; Dcalcd = 1.495 g cm−3; F(000) = 414.0; R(I ≥ 2σ1) = 0.1010, wR2 = 0.2675. Detailed X-ray crystallographic data was available from the Cambridge Crystallographic Data Centre (for compound 8i CCDC 992572; see Fig. 2).
Following the representative procedure, the solution of 4-hydroxy-2-methoxybenzaldehyde 1j (502 mg, 3.28 mmol), NMSM 5a (973 mg, 6.57 mmol) and 10 mol% of 2-aminopyridine (30 mg, 0.32 mmol) in ethanol (5 mL) afforded 3-methoxy-4-(1-methyl-2-(methylamino)-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-4-yl)phenol 8j. Yield (980 mg, 82%); mp 229 °C; IR Data (ν) (KBr) 3442, 3014, 2934, 1629, 1484, 1445, 1363, 1324, 1241, 1193, 741 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.03 (s, 1H), 8.84 (s, 1H), 6.68 (d, J = 8 Hz, 1H), 6.62 (d, J = 1.6 Hz, 1H), 6.50 (dd, J = 8, 1.6 Hz, 1H), 5.90 (s, 1H), 3.74 (s, 3H) 3.44(s, 3H), 3.12 (d, J = 5.2 Hz, 3H), 2.54 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.03 (s, 1H), 8.84 (s, 1H), 6.68 (d, J = 8 Hz, 1H), 6.62 (d, J = 1.6 Hz, 1H), 6.50 (dd, J = 8, 1.6 Hz, 1H), 5.90 (s, 1H), 3.74 (s, 3H) 3.44(s, 3H), 3.12 (d, J = 5.2 Hz, 3H), 2.54 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.8 (C), 153.3 (C), 147.5 (C), 146.1 (C), 137.9 (C), 130.4 (CH), 118.8 (CH), 115.5 (C), 113.2 (CH), 110.7 (C), 55.4 (OMe), 42.6 (NMe), 39.9 (NHMe), 31.6 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C15H18N4O6SNa [M + Na] 405.0845 amu, found 405.0847 amu.
1) δ 155.8 (C), 153.3 (C), 147.5 (C), 146.1 (C), 137.9 (C), 130.4 (CH), 118.8 (CH), 115.5 (C), 113.2 (CH), 110.7 (C), 55.4 (OMe), 42.6 (NMe), 39.9 (NHMe), 31.6 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C15H18N4O6SNa [M + Na] 405.0845 amu, found 405.0847 amu.
Following the representative procedure, the solution of 2-naphthaldehyde 1k (501 mg, 3.20 mmol) NMSM 5a (948 mg, 6.41 mmol) and 10 mol% of 2-aminopyridine (30 mg, 0.32 mmol) and in ethanol (5 mL) afforded N,1-dimethyl-6-(methylthio)-4-(naphthalen-2-yl)-3,5-dinitro-1,4-dihydropyridin-2-amine 8k. Yield (1.1 g, 90%); mp 217 °C; IR Data (ν) (KBr) 3421, 3200, 3049, 2928, 1629, 1576, 1481, 1446, 1370, 1290, 1245, 1195, 1068 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.11 (s, 1H), 7.87 (dd, J = 21.8, 7.9 Hz, 3H), 7.63 (s, 1H), 7.47 (dd, J = 9.1, 5.3 Hz, 2H), 7.32 (d, J = 8.4 Hz, 1H), 6.20 (s, 1H), 3.50 (s, 3H), 3.14 (d, J = 3.9 Hz, 3H), 2.58 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.11 (s, 1H), 7.87 (dd, J = 21.8, 7.9 Hz, 3H), 7.63 (s, 1H), 7.47 (dd, J = 9.1, 5.3 Hz, 2H), 7.32 (d, J = 8.4 Hz, 1H), 6.20 (s, 1H), 3.50 (s, 3H), 3.14 (d, J = 3.9 Hz, 3H), 2.58 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.9 (C), 154.7 (C), 137.2 (C), 137.1 (C), 132.8 (C), 132.2 (C), 128.6 (CH), 127.7 (CH), 127.3 (CH), 126.2 (CH), 125.9 (CH), 125.1 (CH), 124.9 (CH), 112.8 (C), 42.8 (NMe), 40.6 (CH), 31.8 (NHMe), 16.1 (SMe); HRMS (ESI) Calcd for C18H18N4O4SNa [M + Na] 409.0946 amu, found 409.0946 amu.
1) δ 155.9 (C), 154.7 (C), 137.2 (C), 137.1 (C), 132.8 (C), 132.2 (C), 128.6 (CH), 127.7 (CH), 127.3 (CH), 126.2 (CH), 125.9 (CH), 125.1 (CH), 124.9 (CH), 112.8 (C), 42.8 (NMe), 40.6 (CH), 31.8 (NHMe), 16.1 (SMe); HRMS (ESI) Calcd for C18H18N4O4SNa [M + Na] 409.0946 amu, found 409.0946 amu.
Following the representative procedure, the solution of cinnamaldehyde 1l (203 mg, 1.51 mmol), NMSM 5a (448 mg, 3.03 mmol) and 10 mol% of 2-aminopyridine (14 mg, 0.15 mmol) in ethanol (5 mL) afforded (E)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-styryl-1,4-dihydropyridin-2-amine 8l. Yield (400 mg, 73%); mp 206 °C; IR Data (ν) 3436, 3241, 3009, 2926, 1621, 1549, 1511, 1442, 1390, 1360, 1270, 1244, 1190, 1115 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.02 (s, 1H), 7.40 (d, J = 7.2 Hz, 2H), 7.28 (t, J = 10.8 Hz, 2H). 7.20 (t, J = 10.4 Hz, 1H), 6.36 (d, J = 15.6 Hz, 1H), 6.05 (dd, J = 15.6, 6.8 Hz, 1H), 5.48 (d, J = 6.8 Hz, 1H), 3.44 (s, 3H), 3.20 (s, 3H), 2.55 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.02 (s, 1H), 7.40 (d, J = 7.2 Hz, 2H), 7.28 (t, J = 10.8 Hz, 2H). 7.20 (t, J = 10.4 Hz, 1H), 6.36 (d, J = 15.6 Hz, 1H), 6.05 (dd, J = 15.6, 6.8 Hz, 1H), 5.48 (d, J = 6.8 Hz, 1H), 3.44 (s, 3H), 3.20 (s, 3H), 2.55 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.8 (C), 155.0 (C), 136.2 (C), 135.9 (C), 130.2 (CH), 128.2 (CH), 127.5 (CH), 126.3 (CH), 125.8 (CH), 119.9 (C), 42.6 (NMe), 38.4 (NHMe), 31.4 (CH), 15.7 (SMe); HRMS (ESI) Calcd for C16H18N4O4SNa [M + Na] 385.0946 amu, found 385.0942 amu.
1) δ 155.8 (C), 155.0 (C), 136.2 (C), 135.9 (C), 130.2 (CH), 128.2 (CH), 127.5 (CH), 126.3 (CH), 125.8 (CH), 119.9 (C), 42.6 (NMe), 38.4 (NHMe), 31.4 (CH), 15.7 (SMe); HRMS (ESI) Calcd for C16H18N4O4SNa [M + Na] 385.0946 amu, found 385.0942 amu.
Following the representative procedure, the solution of hexanaldehyde 1m (101 mg, 1.00 mmol), NMSM 5a (296 mg, 2.00 mmol) and 10 mol% of 2-aminopyridine (20 mg, 0.20 mmol) in ethanol (3 mL) afforded N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-pentyl-1,4-dihydropyridin-2-amine 8m. Yield (220 mg, 64%); mp 195 °C; IR Data (ν) 3445, 3202, 3167, 2928, 2851, 1629, 1491, 1442, 1353, 1322, 1240, 1193, 1167, 1102, 1059 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.98 (d, J = 4.8 Hz, 1H), 4.97 (t, J = 9.2 Hz, 1H), 3.36 (s, 3H), 3.12 (d, J = 5.2 Hz, 3H), 2.44 (s, 3H), 1.48–1.42 (m, 2H), 1.23–1.11 (m, 6H), 0.82 (t, J = 11.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.5 (C), 150.3 (C), 139.9 (C), 114.9 (C), 42.6 (NMe), 36.2 (NHMe), 34.2 (CH2), 31.7 (CH2), 31.5 (CH), 25.6 (CH2), 22.6 (CH2), 16.4 (SMe), 14.1 (Me); HRMS (ESI) Calcd for C13H22N4O4SNa [M + Na] 353.1259 amu, found 353.1258 amu.
Following the representative procedure, the solution of thiophene-2-carbaldehyde 1n (202 mg, 1.78 mmol) NMSM 5a (501 mg, 3.56 mmol) and 10 mol% of 2-aminopyridine (20 mg, 0.17 mmol) in ethanol (3 mL) afforded N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-(thiophen-2-yl)-1,4-dihydropyridin-2-amine 8n. Yield (410 mg, 70%); mp 209 °C; IR Data (ν) (KBr) 3419, 3196, 2994, 2927, 1627, 1576, 1480, 1427, 1392, 1363, 1287, 1158, 1066, 812 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 9.98 (s, 1H), 7.32 (dd, J = 5.0, 1.2 Hz, 1H), 6.92 (dd, J = 5.10, 3.5 Hz, 1H), 6.83 (t, 2.1 Hz, 1H), 6.21 (s, 1H), 3.40 (s, 3H), 3.10 (d, J = 4.9 Hz, 3H), 2.57 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 9.98 (s, 1H), 7.32 (dd, J = 5.0, 1.2 Hz, 1H), 6.92 (dd, J = 5.10, 3.5 Hz, 1H), 6.83 (t, 2.1 Hz, 1H), 6.21 (s, 1H), 3.40 (s, 3H), 3.10 (d, J = 4.9 Hz, 3H), 2.57 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.5 (C), 143.1 (C), 136.6 (C), 126.9 (CH), 124.8 (CH), 124.2 (CH), 112.9 (C), 42.7 (NMe), 36.1 (CH), 31.7 (NHMe), 16.0 (SMe); HRMS (ESI) Calcd for C12H14N4O4S2Na [M + Na] 365.0354 amu, found 365.0354 amu.
1) δ 155.5 (C), 143.1 (C), 136.6 (C), 126.9 (CH), 124.8 (CH), 124.2 (CH), 112.9 (C), 42.7 (NMe), 36.1 (CH), 31.7 (NHMe), 16.0 (SMe); HRMS (ESI) Calcd for C12H14N4O4S2Na [M + Na] 365.0354 amu, found 365.0354 amu.
Following the representative procedure, the solution of furfuraldehyde 1o (502 mg, 5.31 mmol), NMSM 5a (801 mg, 10.63 mmol) and 10 mol% of 2-aminopyridine (49 mg, 0.53 mmol) in ethanol (5 mL) afforded 4-(furan-2-yl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8o. Yield (990 mg, 90%); mp 200 °C; IR Data (ν) Data 3197, 3110, 2945, 1635, 1569, 1569, 1487, 1442, 1393, 1301, 1278, 1156, 1066, 1010, 777 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 9.95 (s, 1H), 7.46 (s, 1H), 6.32 (s, 1H), 6.16 (s, 1H), 6.05 (s, 1H), 3.38 (s, 3H), 3.12 (d, J = 2.4 Hz, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 9.95 (s, 1H), 7.46 (s, 1H), 6.32 (s, 1H), 6.16 (s, 1H), 6.05 (s, 1H), 3.38 (s, 3H), 3.12 (d, J = 2.4 Hz, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.8 (C), 155.1 (C), 150.7 (C), 142.2 (C), 135.0 (CH), 110.72 (CH), 110.2 (CH), 106.1 (C), 42.4 (NMe), 34.8 (NHMe), 31.3 (CH), 15.8 (SMe); HRMS (ESI) Calcd for C12H14N4O5SNa [M + Na] 349.0583 amu, found 349.0582 amu.
1) δ 155.8 (C), 155.1 (C), 150.7 (C), 142.2 (C), 135.0 (CH), 110.72 (CH), 110.2 (CH), 106.1 (C), 42.4 (NMe), 34.8 (NHMe), 31.3 (CH), 15.8 (SMe); HRMS (ESI) Calcd for C12H14N4O5SNa [M + Na] 349.0583 amu, found 349.0582 amu.
Following the representative procedure, the solution of 1,3-diphenyl-1H-pyrazole-4-carbaldehyde 1p (101 mg, 0.40 mmol), NMSM 5a (202 mg, 0.80 mmol) and 10 mol% of 2-aminopyridine (7 mg, 0.040 mmol) in ethanol (3 mL) afforded 4-(1,3-diphenyl-1H-pyrazol-4-yl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8p. Yield (110 mg, 78%); mp 216 °C; IR Data (ν) (KBr) 3125, 1622, 1534, 1493, 1450, 1372, 1300, 1276, 1242, 1174, 1202, 1118, 1065, 785 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.10 (s, 1H), 8.12 (s, 1H), 7.83 (dd, J = 8.6, 1.0 Hz, 2H), 7.69–7.67 (m, 2H), 7.47–7.43 (m, 5H), 7.28 (t, J = 11.1 Hz, 1H), 6.18 (s, 1H), 3.46 (s, 3H), 3.08 (d, J = 5.2 Hz, 3H), 2.49 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.10 (s, 1H), 8.12 (s, 1H), 7.83 (dd, J = 8.6, 1.0 Hz, 2H), 7.69–7.67 (m, 2H), 7.47–7.43 (m, 5H), 7.28 (t, J = 11.1 Hz, 1H), 6.18 (s, 1H), 3.46 (s, 3H), 3.08 (d, J = 5.2 Hz, 3H), 2.49 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.7 (C), 152.1 (C), 150.9 (C), 139.2 (C), 137.7 (C), 133.3 (C), 129.1 (CH), 128.2 (CH), 127.9 (CH), 127.8 (CH), 126.9 (CH), 126.1 (CH), 120.4 (C), 118.4 (CH), 112.9 (C), 41.9 (NMe), 39.9 (NHMe), 31.8 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C23H22N6O4SNa [M + Na] 501.1321 amu, found 501.1321 amu.
1) δ 155.7 (C), 152.1 (C), 150.9 (C), 139.2 (C), 137.7 (C), 133.3 (C), 129.1 (CH), 128.2 (CH), 127.9 (CH), 127.8 (CH), 126.9 (CH), 126.1 (CH), 120.4 (C), 118.4 (CH), 112.9 (C), 41.9 (NMe), 39.9 (NHMe), 31.8 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C23H22N6O4SNa [M + Na] 501.1321 amu, found 501.1321 amu.
Following the representative procedure, the solution of pyridine-3-carbaldehyde 1q (251 mg, 2.35 mmol), NMSM 5a (501 mg, 4.71 mmol) and 10 mol% of 2-aminopyridine (31 mg, 0.23 mmol) in ethanol (3 mL) afforded N,1′-dimethyl-6′-(methylthio)-3′,5′-dinitro-1′,4′-dihydro-[3,4′-bipyridin]-2′-amine 8q. Yield (600 mg, 76%); mp 149 °C; IR Data (ν) (KBr) 3443, 3204, 3069, 2931, 2810, 2742, 1693, 1631, 1476, 1446, 1394, 1299, 1281, 794 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.09 (s, 1H), 8.43 (td, J = 7.9, 1.9 Hz, 2H), 7.56–7.53 (m, 1H), 7.34–7.30 (m, 1H), 5.94 (s, 1H), 3.52 (s, 3H), 3.14 (s, 3H), 2.59 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.09 (s, 1H), 8.43 (td, J = 7.9, 1.9 Hz, 2H), 7.56–7.53 (m, 1H), 7.34–7.30 (m, 1H), 5.94 (s, 1H), 3.52 (s, 3H), 3.14 (s, 3H), 2.59 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.8 (C), 155.5 (C), 148.4 (C), 148.2 (C), 136.1 (CH), 135.1 (CH), 134.4 (CH), 123.7 (CH), 112.1 (C), 42.9 (NMe), 40.1 (NHMe), 31.7 (CH), 16.1 (SMe); HRMS (ESI) Calcd for C13H15N5O4SNa [M + Na] 360.0742 amu, found 360.0744 amu.
1) δ 155.8 (C), 155.5 (C), 148.4 (C), 148.2 (C), 136.1 (CH), 135.1 (CH), 134.4 (CH), 123.7 (CH), 112.1 (C), 42.9 (NMe), 40.1 (NHMe), 31.7 (CH), 16.1 (SMe); HRMS (ESI) Calcd for C13H15N5O4SNa [M + Na] 360.0742 amu, found 360.0744 amu.
Following the representative procedure, the solution of indole-3-carbaldehyde 1r (101 mg, 0.68 mmol), NMSM 5a (202 mg, 1.37 mmol) and 10 mol% of 2-aminopyridine (12 mg, 0.06 mmol) in ethanol (3 mL) afforded 4-(1H-indol-3-yl)-N,1-dimethyl-6-(methylthio)-3,5-dinitro-1,4-dihydropyridin-2-amine 8r. Yield (180 mg, 69%); mp 229 °C; IR Data (ν) (KBr) 3419, 1619, 1456, 1364, 1230, 1166, 1114, 780 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.93 (s, 1H), 9.93 (s, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 7.06 (t, 16.7 Hz, 1H), 6.98–6.92 (m, 2H), 6.28 (s, 1H), 3.40 (s, 3H), 3.08 (s, 3H), 2.53 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.93 (s, 1H), 9.93 (s, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 7.06 (t, 16.7 Hz, 1H), 6.98–6.92 (m, 2H), 6.28 (s, 1H), 3.40 (s, 3H), 3.08 (s, 3H), 2.53 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 156.0 (C), 152.5 (C), 138.5 (C), 136.5 (CH), 125.4 (C), 122.0 (C), 121.3 (CH), 118.9 (CH), 118.8 (CH), 114.4 (C), 113.4(C), 111.5 (CH), 41.9 (NMe), 39.9 (NHMe), 31.8 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C16H17N5O4SNa [M + Na] 398.0899 amu, found 398.0898 amu.
1) δ 156.0 (C), 152.5 (C), 138.5 (C), 136.5 (CH), 125.4 (C), 122.0 (C), 121.3 (CH), 118.9 (CH), 118.8 (CH), 114.4 (C), 113.4(C), 111.5 (CH), 41.9 (NMe), 39.9 (NHMe), 31.8 (CH), 15.9 (SMe); HRMS (ESI) Calcd for C16H17N5O4SNa [M + Na] 398.0899 amu, found 398.0898 amu.
Following the representative procedure, the solution of benzaldehyde 1a (102 mg, 0.94 mmol), (E)-N-benzyl-1-(methylthio)-2-nitroethenamine 5b (402 mg, 1.88 mmol) and 10 mol% of 2-aminopyridine (20 mg, 0.09 mmol) in ethanol (3 mL) afforded N,1-dibenzyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8s. Yield (250 mg, 84%); mp 224 °C; IR Data (ν) 3060, 3033, 2924, 1614, 1415, 1376, 1294, 1133, 963, 755 cm−1; 1H NMR (400 MHz, CDCl3) δ 10.13 (t, J = 8.4 Hz, 1H), 7.44–7.36 (m, 5H), 2.26 (s, 1H), 7.15–7.03 (m, 5H), 6.94 (t, J = 11.6 Hz, 2H), 6.43 (d, J = 7.2 Hz, 2H), 6.16 (s, 1H), 5.08 (d, J = 14.4 Hz, 1H), 4.87 (d, J = 14.4 Hz, 1H), 4.73–4.71 (m, 2H), 2.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 154.1 (C), 146.6 (C), 140.9 (C), 138.4 (C), 136.0 (C), 133.8 (C), 129.6 (CH), 129.5 (CH), 129.3 (CH), 128.8 (C), 128.6 (CH), 127.1 (CH), 126.9 (CH), 117.2 (C), 56.9 (CH2), 50.4 (CH2), 41.3 (CH), 16.9 (SMe); HRMS (ESI) Calcd for C26H24N4O4SNa [M + Na] 511.1416 amu, found 511.1418 amu.
Following the representative procedure, the solution of benzaldehyde 1a (202 mg, 1.88 mmol), (E)-N-(4-methoxyphenethyl)-1-(methylthio)-2-nitroethenamine 5c (501 mg, 3.77 mmol) and 10 mol% of 2-aminopyridine (42 mg, 0.37 mmol) in ethanol (3 mL) afforded N,1-bis(4-methoxyphenethyl)-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8t. Yield (550 mg, 86%); mp 210 °C; IR Data (ν) 3128, 3064, 2997, 2935, 1634, 1487, 1359, 1282, 1067, 818, 770 cm−1; 1H NMR (400 MHz, CDCl3 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 9.81 (s, 1H), 7.26–6.75 (m, 13H) 6.40 (s, 1H),3.80 (td, J = 18.3, 4.94 Hz, 1H), 3.60 (d, J = 10.8 Hz, 6H), 3.53–3.50 (m, 3H), 2.94–2.90 (m, 2H), 2.38 (s, 3H), 2.18 (td, J = 18, 4.98 Hz, 1H), 1.94 (dd, J = 17.5, 4.9 Hz, 1H); 13C NMR (100 MHz, CDCl3 + CCl4, 1
1) δ 9.81 (s, 1H), 7.26–6.75 (m, 13H) 6.40 (s, 1H),3.80 (td, J = 18.3, 4.94 Hz, 1H), 3.60 (d, J = 10.8 Hz, 6H), 3.53–3.50 (m, 3H), 2.94–2.90 (m, 2H), 2.38 (s, 3H), 2.18 (td, J = 18, 4.98 Hz, 1H), 1.94 (dd, J = 17.5, 4.9 Hz, 1H); 13C NMR (100 MHz, CDCl3 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 159.0 (C), 158.8 (C), 154.6 (C), 139.9 (C), 139.6 (C), 129.8 (CH), 129.5 (CH), 128.9 (CH), 128.6 (CH), 127.8 (CH), 126.3 (CH), 116.1 (CH), 114.6 (CH), 114.3 (CH), 55.5 (OMe), 55.4 (OMe), 55.4 (CH2), 48.3 (CH2), 40.1 (CH2), 36.0 (CH2), 34.3 (CH), 17.2 (SMe); HRMS (ESI) Calcd for C30H32N4O6SNa [M + Na] 599.6940 amu, found 599.1942 amu.
1) δ 159.0 (C), 158.8 (C), 154.6 (C), 139.9 (C), 139.6 (C), 129.8 (CH), 129.5 (CH), 128.9 (CH), 128.6 (CH), 127.8 (CH), 126.3 (CH), 116.1 (CH), 114.6 (CH), 114.3 (CH), 55.5 (OMe), 55.4 (OMe), 55.4 (CH2), 48.3 (CH2), 40.1 (CH2), 36.0 (CH2), 34.3 (CH), 17.2 (SMe); HRMS (ESI) Calcd for C30H32N4O6SNa [M + Na] 599.6940 amu, found 599.1942 amu.
Following the representative procedure, the solution of benzaldehyde 1a (101 mg, 0.94 mmol), (E)-N-(1-(methylthio)-2-nitrovinyl)butan-1-amine 5d (322 mg, 1.88 mmol) and 10 mol% of 2-aminopyridine (20 mg, 0.18 mmol) in ethanol (3 mL) afforded N,1-dibutyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8u. Yield (220 mg, 70%); mp 225 °C; IR Data (ν) 3445, 2962, 2929, 2872, 1623, 1493, 1422, 1372, 1237, 1207, 1182, 1160, 740 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.91 (d, J = 3.6 Hz, 1H), 7.26–7.20 (m, 3H), 7.06–7.04 (dd, J = 7.3, 1.0 Hz, 2H), 6.41 (s, 1H), 3.74–3.34 (m, 1H), 3.33–3.32 (m, 2H), 3.30–3.27 (m, 1H), 2.42 (s, 3H), 1.72–1.46 (m, 2H), 1.46 (s, 1H), 1.46–1.44 (m, 2H), 1.03–0.94 (m, 6H), 0.64 (t, J = 10.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 155.1 (C), 149.6 (C), 139.99 (C), 139.91 (C), 128.8 (CH), 127.6 (CH), 126.4 (CH), 115.9 (C), 54.2 (CH2), 46.9 (CH2), 40.0 (NMe), 32.2 (CH2), 31.0 (CH2), 19.9 (CH2), 19.8 (CH2), 16.9 (SMe), 13.6 (Me), 13.3 (Me); HRMS (ESI) Calcd for C20H28N4O4SNa [M + Na] 443.1729 amu, found 443.1729 amu.
General procedure for synthesis of 1,4-dihydropyridine 7
A solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine (1 equiv.) and aliphatic amine (1 equiv.) in ethanol (5 mL) were mixed and stirred at 80 °C until the reaction was complete, as monitored by thin-layer chromatography (TLC). The reaction mixture was cooled to room temperature and the resulting solid was filtered off and recrystallized from dichloromethane and hexane to obtain pure products 7.In a round-bottomed flask a solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (502 mg, 1.48 mmol) and benzyl amine 2a (159 mg, 1.48 mmol) in ethanol (5 mL) were mixed and stirred at 80 °C until the reaction was complete, as monitored by thin-layer chromatography (TLC, hexanes–EtOAc, 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After 1 h white solid was obtained which was filtered to afford N2-benzyl-N6,1-dimethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 7a. Yield (510 mg, 98%); mp 218 °C; IR Data (ν) 3023, 2995, 2945, 2837, 1639, 1504, 1463, 1375, 1287, 1155, 1052, 699 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
3). After 1 h white solid was obtained which was filtered to afford N2-benzyl-N6,1-dimethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 7a. Yield (510 mg, 98%); mp 218 °C; IR Data (ν) 3023, 2995, 2945, 2837, 1639, 1504, 1463, 1375, 1287, 1155, 1052, 699 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.44 (s, 1H), 10.20 (s, 1H), 7.41–7.38 (m, 4H), 7.35 (d, J = 3.4 Hz, 1H), 7.33–7.25 (m, 2H), 7.21 (t, J = 12.3 Hz, 3H), 5.90 (s, 1H), 4.78–4.72 (m, 2H), 3.38 (s, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.44 (s, 1H), 10.20 (s, 1H), 7.41–7.38 (m, 4H), 7.35 (d, J = 3.4 Hz, 1H), 7.33–7.25 (m, 2H), 7.21 (t, J = 12.3 Hz, 3H), 5.90 (s, 1H), 4.78–4.72 (m, 2H), 3.38 (s, 3H), 2.56 (s, 3H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.8 (C), 155.2 (C), 141.5 (C), 136.8 (C), 128.7 (CH), 128.4 (CH), 127.8 (CH), 127.3 (CH), 126.8 (CH), 126.7 (CH), 114.8 (C), 114.1 (C), 48.5 (NMe), 42.2 (CH2), 38.5 (CH), 31.7 (NHMe); HRMS (ESI) Calcd for C20H21N5O4Na [M + Na] 418.1491 amu, found 418.1494 amu.
1) δ 155.8 (C), 155.2 (C), 141.5 (C), 136.8 (C), 128.7 (CH), 128.4 (CH), 127.8 (CH), 127.3 (CH), 126.8 (CH), 126.7 (CH), 114.8 (C), 114.1 (C), 48.5 (NMe), 42.2 (CH2), 38.5 (CH), 31.7 (NHMe); HRMS (ESI) Calcd for C20H21N5O4Na [M + Na] 418.1491 amu, found 418.1494 amu.
Following the representative procedure, the solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (501 mg, 1.48 mmol) and phenylethyl amine 2b (182 mg, 1.48 mmol) in ethanol (5 mL) afforded N2,1-dimethyl-3,5-dinitro-N6-phenethyl-4-phenyl-1,4-dihydropyridine-2,6-diamine 7b. Yield (600 mg, 90%). mp 220 °C; IR Data (ν) 3607, 3518, 1493, 1450, 1408, 1377, 737 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.18 (s, 1H), 10.08 (s, 1H), 7.29–7.26 (m, 4H), 7.24 (d, J = 1.2 Hz, 2H), 7.19 (d, J = 7.2 Hz, 2H), 7.13 (d, J = 7.2 Hz, 2H), 5.83 (s, 1H), 3.75–3.74 (m, 2H), 3.28 (s, 3H), 3.01 (s, 3H), 2.50–2.49 (m, 2H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1
1) δ 10.18 (s, 1H), 10.08 (s, 1H), 7.29–7.26 (m, 4H), 7.24 (d, J = 1.2 Hz, 2H), 7.19 (d, J = 7.2 Hz, 2H), 7.13 (d, J = 7.2 Hz, 2H), 5.83 (s, 1H), 3.75–3.74 (m, 2H), 3.28 (s, 3H), 3.01 (s, 3H), 2.50–2.49 (m, 2H); 13C NMR (100 MHz, DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 155.8 (C), 155.5 (C), 141.5 (C), 137.8 (C), 128.7 (CH), 128.3 (CH), 128.3 (CH), 126.7 (CH), 126.6 (CH), 126.5 (CH), 114.5 (C), 113.8 (C), 46.8 (CH2), 43.1 (NMe), 40.2 (CH2), 38.4 (CH), 35.6 (NHMe); HRMS (ESI) Calcd for C21H23N5O4Na [M + Na] 432.1648 amu, found 432.1648 amu.
1) δ 155.8 (C), 155.5 (C), 141.5 (C), 137.8 (C), 128.7 (CH), 128.3 (CH), 128.3 (CH), 126.7 (CH), 126.6 (CH), 126.5 (CH), 114.5 (C), 113.8 (C), 46.8 (CH2), 43.1 (NMe), 40.2 (CH2), 38.4 (CH), 35.6 (NHMe); HRMS (ESI) Calcd for C21H23N5O4Na [M + Na] 432.1648 amu, found 432.1648 amu.
Following the representative procedure, the solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (202 mg, 0.59 mmol) and butyl amine 2c (43 mg, 0.59 mmol) in ethanol (3 mL) afforded N2-butyl-N6,1-dimethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 7c. Yield (180 mg, 81%); mp 215 °C; IR Data (ν) 3148, 2954, 2868, 1638, 1498, 1466, 1369, 1284, 1284, 1159, 1052, 732 cm−1; 1H NMR (400 MHz, CDCl3) δ 10.03 (d, J = 4.4 Hz, 2H), 7.26–7.17 (m, 5H), 6.01 (s, 1H), 3.45–3.29 (m, 4H), 2.98 (d, J = 5.2 Hz, 3H), 1.70–1.65 (m, 3H), 1.46–1.40 (m, 2H), 0.95 (t, J = 10.9 Hz, 3H); 13C NMR (100 MHz, CDCl3 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 156.2 (C), 155.4 (C), 140.9 (C), 128.7 (CH), 127.42 (CH), 127.40 (CH), 116.0 (C), 115.9 (C), 45.6 (NMe), 42.6 (CH2), 38.9 (CH2), 31.9 (CH), 31.7 (CH2), 20.1 (NHMe), 13.7 (Me); HRMS (ESI) Calcd for C17H23N5O4Na [M + Na] 384.1648 amu, found 384.1646 amu.
1) δ 156.2 (C), 155.4 (C), 140.9 (C), 128.7 (CH), 127.42 (CH), 127.40 (CH), 116.0 (C), 115.9 (C), 45.6 (NMe), 42.6 (CH2), 38.9 (CH2), 31.9 (CH), 31.7 (CH2), 20.1 (NHMe), 13.7 (Me); HRMS (ESI) Calcd for C17H23N5O4Na [M + Na] 384.1648 amu, found 384.1646 amu.
Following the representative procedure, the solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (251 mg, 0.74 mmol) and hexylamine 2d (76 mg, 0.74 mmol) in ethanol (3 mL) afforded N2-hexyl-N6,1-dimethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 7d. Yield (250 mg, 80%); mp 208 °C; IR Data (ν) 3146, 3007, 2930, 2859, 1641; 1H NMR (400 MHz, CDCl3) δ 10.11 (s, 2H), 7.33 (d, J = 4 Hz, 2H), 7.27 (d, J = 4 Hz, 3H), 6.09 (s, 1H), 3.50–3.39 (m, 4H), 3.06 (d, J = 4.8 Hz, 3H), 1.78–1.73 (m, 3H), 1.48–1.33 (m, 6H), 0.96 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 156.2 (C), 155.3 (C), 140.9 (C), 128.7 (CH), 127.42 (CH), 127.40 (CH), 116.0 (C), 115.9 (CH), 45.9 (NMe), 42.5 (CH2), 38.9 (CH2), 31.7 (CH2), 31.3 (CH2), 29.9 (CH2), 26.6 (NHMe), 22.5 (CH2), 14.1 (Me); HRMS (ESI) Calcd for C19H27N5O4Na [M + Na] 412.1961 amu, found 412.1960 amu.
1) δ 156.2 (C), 155.3 (C), 140.9 (C), 128.7 (CH), 127.42 (CH), 127.40 (CH), 116.0 (C), 115.9 (CH), 45.9 (NMe), 42.5 (CH2), 38.9 (CH2), 31.7 (CH2), 31.3 (CH2), 29.9 (CH2), 26.6 (NHMe), 22.5 (CH2), 14.1 (Me); HRMS (ESI) Calcd for C19H27N5O4Na [M + Na] 412.1961 amu, found 412.1960 amu.
Following the representative procedure, the solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (102 mg, 0.27 mmol) benzyl amine 2a (35 mg, 0.27 mmol) in ethanol (2 mL) afforded N2-benzyl-4-(4-methoxyphenyl)-N6,1-dimethyl-3,5-dinitro-1,4-dihydropyridine-2,6-diamine 7e. Yield (120 mg, 96%); mp 198 °C; IR Data (ν) 3444, 3002, 2952, 2923, 1638, 1507, 1469, 1370, 1161, 1194, 1054, 779 cm−1; 1H NMR (400 MHz, DMSO-d6 + CCl4, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 10.41 (s, 1H), 10.17 (s, 1H), 7.40–7.31 (m, 5H), 7.09 (d, J = 8.5 Hz, 2H), 6.80 (d, J = 8.6 Hz, 2H), 5.82 (s, 1H), 4.78–4.66 (m, 2H), 3.71 (s, 3H), 3.37 (s, 3H), 3.23 (s, 3H), 2.93 (d, J = 5.2 Hz, 3H); 13C NMR (DMSO-d6 + CCl4, 1
1) δ 10.41 (s, 1H), 10.17 (s, 1H), 7.40–7.31 (m, 5H), 7.09 (d, J = 8.5 Hz, 2H), 6.80 (d, J = 8.6 Hz, 2H), 5.82 (s, 1H), 4.78–4.66 (m, 2H), 3.71 (s, 3H), 3.37 (s, 3H), 3.23 (s, 3H), 2.93 (d, J = 5.2 Hz, 3H); 13C NMR (DMSO-d6 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 158.1 (C), 155.7 (C), 155.1 (C), 136.8 (C), 133.3 (C), 128.6 (CH), 127.7 (CH), 127.6 (CH), 127.2 (CH), 115.0 (CH), 114.3 (C), 113.7 (C), 54.9 (OMe), 48.4 (CH2). 42.1 (NMe), 37.7 (CH), 31.6 (NHMe). HRMS (ESI) Calcd for C21H23N5O5Na [M + Na] 448.1597 amu, found 448.1594 amu.
1) δ 158.1 (C), 155.7 (C), 155.1 (C), 136.8 (C), 133.3 (C), 128.6 (CH), 127.7 (CH), 127.6 (CH), 127.2 (CH), 115.0 (CH), 114.3 (C), 113.7 (C), 54.9 (OMe), 48.4 (CH2). 42.1 (NMe), 37.7 (CH), 31.6 (NHMe). HRMS (ESI) Calcd for C21H23N5O5Na [M + Na] 448.1597 amu, found 448.1594 amu.
In a round-bottomed flask a solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (201 mg, 0.59 mmol) and N,N-dimethylamine 2e (24 mg, 1.48 mmol) in ethanol (3 mL) were mixed and stirred at 80 °C until the reaction was complete, as monitored by thin-layer chromatography (TLC, hexanes–EtOAc, 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After 5 h yellow solid was obtained which was filtered to afford N2,N2,N6, 1-tetramethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 7f. Yield (220 mg, 92%); mp 220 °C; IR Data (ν) 3023, 2995, 2945, 2837, 1639, 1504, 1463, 1375, 1287, 1155, 1052, 699 cm−1; 1H NMR (400 MHz, DMSO-d6) δ 10.29 (s, 1H), 7.28 (t, J = 7.2 Hz, 2H), 7.20 (t, J = 7.1 Hz, 1H), 7.14 (d, J = 7.5 Hz, 2H), 6.0 (s, 1H), 3.33 (s, 3H), 3.11 (d, J = 4.8 Hz, 3H), 2.88 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 156.4 (C), 156.3 (C), 142.4 (C), 128.7 (CH), 126.8 (CH), 125.8 (CH), 116.4 (C), 114.2 (C), 40.8 (NMe), 40.15 (CH), 32.06 (NHMe), 39.52 (Me); HRMS (ESI) Calcd for C15H19N5O4Na [M + Na] 356.1335 amu, found 356.1338 amu.
3). After 5 h yellow solid was obtained which was filtered to afford N2,N2,N6, 1-tetramethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 7f. Yield (220 mg, 92%); mp 220 °C; IR Data (ν) 3023, 2995, 2945, 2837, 1639, 1504, 1463, 1375, 1287, 1155, 1052, 699 cm−1; 1H NMR (400 MHz, DMSO-d6) δ 10.29 (s, 1H), 7.28 (t, J = 7.2 Hz, 2H), 7.20 (t, J = 7.1 Hz, 1H), 7.14 (d, J = 7.5 Hz, 2H), 6.0 (s, 1H), 3.33 (s, 3H), 3.11 (d, J = 4.8 Hz, 3H), 2.88 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 156.4 (C), 156.3 (C), 142.4 (C), 128.7 (CH), 126.8 (CH), 125.8 (CH), 116.4 (C), 114.2 (C), 40.8 (NMe), 40.15 (CH), 32.06 (NHMe), 39.52 (Me); HRMS (ESI) Calcd for C15H19N5O4Na [M + Na] 356.1335 amu, found 356.1338 amu.
Following the representative procedure, the solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (202 mg, 0.59 mmol) and N,N-diethylamine 2f (44 mg, 0.59 mmol) in ethanol (3 mL) afforded N2,N2-diethyl-N6,1-dimethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 7g. Yield (200 mg, 90%); mp 215 °C; IR Data (ν) 3025, 3000, 2982, 2840, 1666, 1516, 1472, 1386, 1290, 1170, 1062, 700 cm−1; 1H NMR (400 MHz, DMSO-d6) δ 10.26 (s, 1H), 7.30–7.25 (m, 2H), 7.22–7.18 (m, 2H), 7.16–7.12 (m, 2H), 5.98 (s, 1H), 3.44–3.35 (m, 4H), 3.34 (s, 3H), 3.12 (s, 3H), 3.10 (s, 3H), 1.10 (d, J = 6.9 Hz, 6H); (t, J = 7.2 Hz, 2H), 7.20 (t, J = 7.1 Hz, 1H), 7.14 (d, J = 7.5 Hz, 2H), 6.0 (s, 1H), 3.33 (s, 3H), 3.11 (d, J = 4.8 Hz, 3H), 2.88 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 156.4 (C), 156.2 (C), 142.0 (C), 128.6 (CH), 126.8 (CH), 125.9 (CH), 116.3 (C), 113.8 (C), 45.1 (CH2), 40.7 (NMe), 32.1 (NHMe), 31.9 (CH), 13.6 (Me); HRMS (ESI) Calcd for C17H23N5O4Na [M + Na] 384.1648 amu, found 384.1648 amu.
Following the representative procedure, the solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (102 mg, 0.95 mmol) and morpholine 2g (75 mg, 0.95 mmol) in ethanol (3 mL) afforded N, 1-dimethyl-6-morpholino-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 7h. Yield (140 mg, 81%); mp 222 °C; IR Data (ν) 3045, 3010, 2988, 2870, 1666, 1520, 1488, 1396, 1289, 1172, 1080, 720 cm−1; 1H NMR (400 MHz, DMSO-d6) δ 10.25 (s, 1H), 7.31–7.27(m, 2H), 7.22–7.21 (m, 1H), 7.17–7.13 (m, 2H), 5.96 (s, 1H), 3.82–3.35 (s, 6H), 3.33(s, 3H), 3.1 (d, J = 5.4 Hz, 3H), 3.13 (s, 2H), 3.10 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 156.2 (C), 142.9 (C), 128.7 (CH), 128.4 (CH), 126.8 (CH), 125.9 (CH), 116.3 (C), 113.8 (C), 65.3 (CH2), 54.9 (CH2), 41.3 (NMe), 39.5(CH), 32.1 (NHMe); HRMS (ESI) Calcd for C17H21N5O5Na [M + Na] 398.1440 amu, found 398.1441 amu.
Following the representative procedure, the solution of N,1-dimethyl-6-(methylthio)-3,5-dinitro-4-phenyl-1,4-dihydropyridin-2-amine 8a (101 mg, 0.30 mmol) and 1-(6-chloropyridin-3-yl)-N-methylmethanamine 2h (52 mg, 0.30 mmol) in ethanol (3 mL) afforded N2-((6-chloropyridin-3-yl)methyl)-N2,N6,1-trimethyl-3,5-dinitro-4-phenyl-1,4-dihydropyridine-2,6-diamine 9. Yield (120 mg, 98%); mp 231 °C; IR Data (ν) 3023, 2926, 1628, 1593, 1454, 1388, 1344, 1245, 1166, 1111, 1047, 931, 757 cm−1; 1H NMR (400 MHz, DMSO-d6) δ 10.05 (s, 1H), 8.31 (s, 1H), 7.71 (d, J = 7.2 Hz, 1H), 7.40 (dd, J = 8.4 Hz, 1H), 7.29–7.20 (m, 3H), 7.12 (d, J = 7.6 Hz, 2H), 5.99 (s, 1H), 4.54 (d, J = 14.8 Hz, 1H), 4.41 (d, J = 14.8 Hz, 1H), 3.10 (d, J = 7.2 Hz, 6H), 2.80 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 156.5 (C), 156.7 (C), 156.0(C), 150.0 (C), 149.9 (C), 142.2 (C), 140.3 (CH), 130.5 (CH), 128.7 (CH), 126.8 (CH), 125.8 (CH), 124.1 (CH), 116.2 (C), 54.2 (CH2), 53.4 (NMe), 40.8 (NMe), 39.1 (CH), 32.2 (NMe); HRMS (ESI) Calcd for C20H21ClN6O4Na [M + Na] 467.1211 amu, found 467.1211 amu.
Empirical formula, C20H21ClN6O4; formula weight, 444.8715; crystal colour, light yellow; crystal dimensions a = 11.9749(6) Å, b = 15.2082(6) Å, c = 12.0561(8) Å; α = 90, β = 106.909(6), γ = 90; crystal system, monoclinic; V = 2100.7(2) A3; space group P21/c; Z = 4; Dcalcd = 1.406 g cm−3; F(000) = 928.0; R(I ≥ 2σ1) = 0.0423, wR2 = 0.1404. Detailed X-ray crystallographic data was available from the Cambridge Crystallographic Data Centre (for compound 9 CCDC 918142).
Acknowledgements
H.S.P.R. thanks UGC, UGC-SAP and DST-FIST for financial assistance. AP thanks Pondicherry University for fellowship. We thank CIF, Pondicherry University and IISc, Bangalore for recording spectra. We thank Shasun Chemicals and Drugs Ltd, Chennai, for generous gift of NMSM.References
- (a) L. F. Tietze, G. Brasche and K. Gericke, in Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed; (b) J. Zhu and H. Bienaymé, in Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed.
- (a) S. Ali and B. A. H. Pourasad, J. Chem. Res., 2014, 38, 62–63 CrossRef; (b) F.-L. Yang, F.-X. Wang, T.-T. Wang, Y.-J. Wang and S.-K. Tian, Chem. Commun., 2014, 50, 2111–2113 RSC; (c) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef; (d) A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed; (e) H. L. Wei, Z. Y. Yan, Y. L. Niu, G. Q. Li and Y. M. Liang, J. Org. Chem., 2007, 72, 8600–8603 CrossRef CAS PubMed; (f) X. H. Duan, X. Y. Liu, L. N. Guo, M. C. Liao, W. M. Liu and Y. M. Liang, J. Org. Chem., 2005, 70, 6980–6983 CrossRef CAS PubMed; (g) B. Jiang, S. J. Tu, P. Kaur, W. Wever and G. G. Li, J. Am. Chem. Soc., 2009, 131, 11660–11661 CrossRef CAS PubMed; (h) B. Jiang, T. Rajale, W. Wever, S. J. Tu and G. G. Li, Chem. – Asian J., 2010, 5, 2318–2335 CrossRef CAS PubMed; (i) C. Cheng, B. Jiang, S. J. Tu and G. G. Li, Green Chem., 2011, 13, 2107–2115 RSC; (j) X. F. Lin, Z. J. Mao, X. X. Dai, P. Lu and Y. G. Wang, Chem. Commun., 2011, 47, 6620–6622 RSC; (k) D. Hong, Y. X. Zhu, Y. Li, X. F. Lin, P. Lu and Y. G. Wang, Org. Lett., 2011, 13, 4668–4671 CrossRef CAS PubMed; (l) Y. X. Zhu, G. W. Yin, D. Hong, P. Lu and Y. G. Wang, Org. Lett., 2011, 13, 1024–1027 CrossRef CAS PubMed.
- (a) A. Hantzsch, Justus Liebigs Ann. Chem., 1882, 215, 1–82 CrossRef; (b) D. M. Stout and A. I. Meyers, Chem. Rev., 1982, 82, 223–243 CrossRef CAS; (c) C. O. Kappe, Tetrahedron, 1993, 49, 6937–7168 CrossRef CAS.
- (a) Y. Sambongi, H. Nitta, K. Ichihashi, M. Futai and I. Ueda, J. Org. Chem., 2002, 67, 3499–3501 CrossRef CAS PubMed; (b) T. Itoh, K. Nagata, A. Kurihara, M. Miyazaki and A. Ohsawa, Tetrahedron Lett., 2002, 43, 3105–3108 CrossRef CAS.
- (a) G. Swarnalatha, G. Prasanthi, N. Sirisha and C. M. Chetty, Int. J. ChemTech Res., 2011, 3, 75–89 CAS; (b) A. Boumendjel, H. Baubichon-Cortay, D. Trompier, T. Perrotton and A. Di Pietro, Med. Res. Rev., 2005, 25, 453–472 CrossRef CAS PubMed; (c) A. Hilgeroth, Med. Chem., 2002, 2, 235–245 CAS; (d) G. Prasanthi, K. V. S. R. G. Prasad and K. Bharathi, Eur. J. Med. Chem., 2014, 73, 97–104 CrossRef CAS PubMed.
- J. T. David, Biochem. Pharmacol., 2007, 74, 1–9 CrossRef PubMed.
- R. Boer and V. Gekeler, Drugs Future, 1995, 20, 499–509 Search PubMed.
- (a) V. M. Briukhanov, Exp. Clin. Pharmacol., 1994, 57, 47–49 CAS; (b) S. Bahekar and D. Shinde, Acta Pharm. A, 2002, 52, 281–287 CAS.
- (a) N. Edraki, A. R. Mehdipour, M. Khoshneviszadeh and R. Miri, Drug Discovery Today, 2009, 14, 1058–1066 CrossRef CAS PubMed; (b) F. Bossert and W. Vater, Med. Chem. Rev., 1989, 9, 291–324 CAS; (c) G. P. Young, Ann. Emerg. Med., 1984, 13, 712–722 CrossRef CAS.
- (a) S. Chuanwen, C. Yanxia, L. Tianyan, W. Ying, F. Ting, W. Jing and X. Jiahua, Chin. J. Chem., 2012, 30, 1415–1422 CrossRef; (b) W. Zhang, X. Yang, W. Chen, X. Xu, L. Li, H. Zhai and Z. Li, J. Agric. Food Chem., 2010, 58, 2741–2745 CrossRef CAS PubMed; (c) S. Lu, X. Shao, Z. Li, Z. Xu, S. Zhao, Y. Wu and X. Xu, J. Agric. Food Chem., 2012, 60, 322–330 CrossRef CAS PubMed; (d) Z. Ye, L. Shi, X. Shao, X. Xu, Z. Xu and Z. Li, J. Agric. Food Chem., 2013, 61, 312–319 CrossRef CAS PubMed.
- (a) S. G. Ouellet, A. M. Walji and D. W. C. Macmillan, Acc. Chem. Res., 2007, 40, 1327–1339 CrossRef CAS PubMed; (b) S. Hoffmann, M. Nicoletti and B. List, J. Am. Chem. Soc., 2006, 128, 13074–13075 CrossRef CAS PubMed; (c) G. Li and J. C. Antilla, Org. Lett., 2009, 11, 1075–1078 CrossRef CAS PubMed; (d) R. He, P. H. Toy and Y. Lama, Adv. Synth. Catal., 2008, 350, 54–60 CrossRef CAS.
- (a) A. B. Charette, S. Mathieu and J. Martel, Org. Lett., 2005, 7, 5401–5404 CrossRef CAS PubMed; (b) L. Z. Chai, Y. K. Zhao, Q. J. Sheng and Z.-Q. Liu, Tetrahedron Lett., 2006, 47, 9283–9285 CrossRef CAS PubMed; (c) J. Chen and A. J. McNeil, J. Am. Chem. Soc., 2008, 130, 16496–16497 CrossRef CAS PubMed; (d) R. Lavilla, M. C. Bernabeu, I. Carranco and J. L. Diaz, Org. Lett., 2003, 5, 717–720 CrossRef CAS PubMed; (e) I. Carranco, J. L. Diaz, O. Jimenez, M. Vendrell, F. Albericio, M. Royo and R. Lavilla, J. Comb. Chem., 2005, 7, 33–41 CrossRef CAS PubMed; (f) E. Vicente-Garcia, F. Catti, R. Ramon and R. Lavilla, Org. Lett., 2010, 12, 860–863 CrossRef CAS PubMed; (g) S. Maiti, V. Sridharan and J. C. Menéndez, J. Comb. Chem., 2010, 12, 713–722 CrossRef CAS PubMed.
- (a) J.-P. Wan and Y. Liu, RSC Adv., 2012, 2, 9763–9777 RSC; (b) S. Ghosh, F. Saikh, J. Das and A. K. Pramanik, Tetrahedron Lett., 2013, 54, 58–62 CrossRef CAS PubMed; (c) S. S. Kahandal, S. R. Kale, M. B. Gawande and R. V. Jayaram, Catal. Sci. Technol., 2014, 4, 672–680 RSC; (d) T. D. Ananda Kumar, P. Mohana, C. V. S. Subrahmanyam and K. Satyanarayana, Synth. Commun., 2014, 44, 574–582 CrossRef; (e) M. Nasr-Esfahani, S. J. Hoseini, M. Montazerozohori, R. Mehrabi and H. Nasrabadi, J. Mol. Catal. A: Chem., 2014, 382, 99–105 CrossRef CAS PubMed; (f) C. G. Evans and J. E. Gestwicki, Org. Lett., 2009, 11, 2957–2959 CrossRef CAS PubMed.
- (a) H. S. P. Rao and K. Geetha, Tetrahedron Lett., 2009, 50, 3836–3839 CrossRef CAS PubMed; (b) H. S. P. Rao, K. Geetha and M. Kamalraj, RSC Adv., 2011, 1, 1050–1059 RSC; (c) H. S. P. Rao, K. Geetha and M. Kamalraj, Tetrahedron, 2011, 67, 8146–8154 CrossRef PubMed; (d) A. Parthiban, J. Muthukumaran, A. Moushumi Priya, S. Jayachandran, R. Krishna and H. S. P. Rao, Med. Chem. Res., 2013, 23, 642–659 CrossRef.
- (a) P. Metzner and A. Thuillier, in Sulfur Reagents in Organic Synthesis, Academic Press, New York, 1994 Search PubMed; (b) M. Kolb, Synthesis, 1990, 171–190 CrossRef CAS; (c) Y. Tominaga and Y. Matsuda, J. Heterocycl. Chem., 1985, 22, 937–949 CrossRef CAS; (d) H. Ila, H. Junjappa and P. K. Mohanta, in Progress in Heterocyclic Chemistry, ed. G. W. Gribble and T. L. Gilchrist, Pergamon Press, Oxford, 2001, vol. 13, pp. 1–24 Search PubMed.
- Y. Nakaike, N. Nishiwaki, M. Ariga and Y. Tobe, J. Org. Chem., 2014, 79, 2163–2169 CrossRef CAS PubMed.
- (a) S. E. Denmark and J. A. Sternberg, J. Am. Chem. Soc., 1986, 108, 8277–8279 CrossRef CAS; (b) L. F. Tietze and A. Schuffenhauer, Eur. J. Org. Chem., 1998, 1629–1637 CrossRef CAS; (c) D. Cheng, J. Zhou, E. Saiah and G. Beaton, Org. Lett., 2002, 4, 4411–4414 CrossRef CAS PubMed.
- C. F. Bernasconi and Z. Rappoport, Acc. Chem. Res., 2009, 42, 993–1003 CrossRef CAS PubMed.
- J. Peter and N. Ralf, Pestic. Sci., 2008, 64, 1084–1098 CrossRef PubMed.
- Y. Kashiwada, Agrochem. Jpn., 1996, 68, 18–19 Search PubMed.
- T. Iwasa, N. Motoyama, J. T. Ambrose and M. R. Roe, Crop Prot., 2004, 23, 371–378 CrossRef CAS PubMed.
- (a) A. Elbert and R. Nauen, Pest Manage. Sci., 2000, 56, 60–64 CrossRef CAS; (b) K. D. Ninsin, Pest Manage. Sci., 2004, 60, 839–841 CrossRef CAS PubMed; (c) D. M. Sanchez, R. M. Hollingworth, E. J. Grafius and D. D. Moyer, Pest Manage. Sci., 2006, 62, 30–37 CrossRef PubMed; (d) K. G. Gorman, G. Devine, J. Bennison, P. Coussons, N. Punchard and I. Denholm, Pest Manage. Sci., 2007, 63, 555–558 CrossRef CAS PubMed.
- S. Chuanwen, C. Yanxia, L. Tianyan, W. Ying, F. Ting, W. Jing and X. Jiahua, Chin. J. Chem., 2012, 30, 1415–1422 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for all new compounds. CCDC 992572 and 918142. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ob00628c |
| This journal is © The Royal Society of Chemistry 2014 |