Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
First total syntheses of chrestifoline-B and (±)-chrestifoline-C, and improved synthetic routes to bismurrayafoline-A, bismurrayafolinol and chrestifoline-D†‡
Carsten
Börger
,
Arndt W.
Schmidt
and
Hans-Joachim
Knölker
*
Department Chemie, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden, Germany. E-mail: hans-joachim.knoelker@tu-dresden.de
First published on 17th April 2014
Abstract
We describe an efficient synthesis of the methylene-bridged biscarbazole alkaloids bismurrayafoline-A, bismurrayafolinol and chrestifoline B–D using an Ullmann-type coupling at the benzylic position.
A wide range of carbazole alkaloids has been isolated and investigated towards their biological activity.1–3 Much less is known about the pharmaceutical potential of biscarbazoles and only a few synthetic approaches have been described.1,4 We have developed several methods for the synthesis of carbazoles.1,2 Using our palladium-catalysed construction of the carbazole framework,5 we recently described efficient synthetic routes to biscarbazole alkaloids,6–8 for example the oxygen-bridged biscarbazole oxydimurrayafoline and the N-aryl linked murrastifoline-A.7,8 The biscarbazole linkage was constructed by etherification,7 the Buchwald–Hartwig amination,9 or the Ullmann coupling.10 Herein, we report the synthesis of methylene-bridged biscarbazole alkaloids by palladium(0)- and copper(I)-catalysed coupling reactions at the benzylic position at C-3 of the carbazole framework.
The first methylene-bridged biscarbazole alkaloid obtained from natural sources was bismurrayafoline-A (1), isolated in 1983 by Furukawa et al. from the root bark of Murraya euchrestifolia Hayata (Fig. 1).11 Bismurrayafoline-A (1) showed a weak activity against some cancer cell lines.12 In 2001, Bringmann et al. reported the formation of bismurrayafoline-A (1) in up to 19% yield as a by-product of the reduction of mukonine (6d) (7 steps, 9% overall yield of 1).13 We have described a total synthesis of bismurrayafoline-A (1) via an unprecedented rearrangement during an Ullmann coupling (6 steps, 28% overall yield of 1).8 In 1987, Furukawa et al. isolated bismurrayafolinol (2) from the root bark of Murraya euchrestifolia Hayata.14 Bringmann et al. reported the formation of bismurrayafolinol (2) as a by-product in up to 6% yield (7 steps, 3% overall yield of 2) during their synthesis of murrayafoline-A (6a).13 Chrestifoline-D (3) was isolated in 1992 by Furukawa and Wu et al. from the root bark of M. euchrestifolia.15 The first synthetic approach by partial synthesis starting from bismurrayafolinol (2) was reported along with the isolation. Chrestifoline-B (4) and chrestifoline-C (5) were isolated in 1990 by Furukawa et al. from the same natural source.16 Chrestifoline-C (5) has been obtained in an optically active form ([α]D = −5.6, CHCl3),16 but its absolute configuration is not known.
 | ||
| Fig. 1 Bismurrayafoline-A (1), bismurrayafolinol (2), chrestifoline-B (4), chrestifoline-C (5), chrestifoline-D (3), the 1-methoxycarbazole alkaloids 6a–d, girinimbine (7a) and mahanimbine (7b). | ||
The biscarbazoles 1–5 have an identical benzylic subunit which corresponds to murrayafoline-A (6a), but they differ in the benzylic substituent (Fig. 1). For the biscarbazoles 1–3, the benzylic linkage leads to the nitrogen atom of a 1-oxygenated tricyclic carbazole corresponding to murrayafoline-A (6a), koenoline (6b) or murrayanine (6c), respectively. For chrestifoline-B (4) and chrestifoline-C (5), the pyrano[3,2-a]carbazole alkaloids girinimbine (7a) and mahanimbine (7b) serve as benzylic substituents. The mono-carbazole building blocks of the biscarbazoles 1–5 are found in nature as well. The 1-methoxycarbazole alkaloids, murrayafoline-A (6a), koenoline (6b), murrayanine (6c) and mukonine (6d), have been obtained from diverse plants of the Rutaceae family.1 The pyrano[3,2-a]carbazole alkaloids girinimbine (7a) and mahanimbine (7b) were isolated first by Chakraborty et al. from Murraya koenigii Spreng.17,18 Our group previously described synthetic routes to all individual carbazole fragments present in the biscarbazoles 1–5. An efficient iron-mediated synthesis of the 1-methoxycarbazole alkaloids 6a–d was reported early on.7,19 More recently, an optimised palladium-catalysed synthesis of mukonine (6d) was described.7 Several synthetic routes to girinimbine (7a) and an efficient access to mahanimbine (7b) were also developed by our group.20
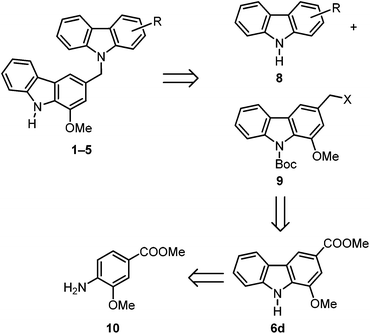
For the synthesis of the biscarbazoles 1–5 we envisaged a retrosynthetic cleavage of the C–N linkage between both carbazole units which leads to the carbazoles 8 and 9 as precursors (Scheme 1). In our approach the nitrogen atom of carbazole 8 serves as a nucleophile. Thus, carbazole 8 is represented by the unprotected naturally occurring carbazoles 6a–c, 7a and 7b. The fragment 9 should have a leaving group at the benzylic position and would be represented by an appropriate koenoline derivative. Compound 9 can be prepared from mukonine (6d) which is readily available using the arylamine 10 as the starting material.7
The Buchwald–Hartwig coupling of bromobenzene and arylamine 10 in the presence of SPhos followed by palladium(II)-catalysed oxidative cyclisation afforded mukonine (6d) in 91% yield over both steps (Scheme 2).7 Boc-protection of 6d and subsequent reduction of the ester group led to the protected koenoline 11. Next, the hydroxy group had to be transformed into a leaving group. However, in light of the high reactivity of the benzylic position of the koenoline derivative 11,7 the resulting compound should have sufficient stability in order to serve as the central intermediate en route to the methylene-bridged biscarbazole alkaloids. Treatment of 11 with p-nitrobenzoyl chloride in the presence of stoichiometric amounts of DMAP afforded quantitatively the p-nitrobenzoate 12. In contrast to the corresponding mesylate,7 the p-nitrobenzoate 12 is much more stable towards benzyl cation formation. Thus, the koenoline derivative 12 with an activated benzylic position is available in five steps and 88% overall yield and was subsequently used as a relay compound for the synthesis of the methylene-bridged biscarbazole alkaloids 1–5.
First, we envisaged a Buchwald–Hartwig coupling reaction of the p-nitrobenzoate 12 with mukonine (6d). Only a few methods have been described so far using transition metal-catalysed coupling reactions at the benzylic position.21 Reaction of 12 and 6d in the presence of catalytic amounts of palladium(II) acetate, rac-BINAP and stoichiometric amounts of caesium carbonate in toluene at reflux provided the desired biscarbazole 13a in 27% yield along with the deprotected biscarbazole 13b in 35% yield (Scheme 2, Table 1). The concomitant partial removal of the Boc group could result either from a pericyclic reaction under thermal conditions22 or from a base-promoted saponification. Coupling of 12 and 6d under Ullmann conditions, using substoichiometric amounts of copper(I) bromide in the presence of pyrrole-2-carboxylic acid,23 led only to the deprotected biscarbazole 13b in 63% yield (see the Experimental procedure). Therefore, the Ullmann coupling reaction is superior for the present synthesis as it provides directly the biscarbazole 13b and thus avoids an additional deprotection step. Reduction of biscarbazole 13b with diisobutylaluminium hydride provided bismurrayafolinol (2) in 96% yield. The spectroscopic data of 2 are in full agreement with those reported for the natural product.§ Reduction of 13b using lithium aluminium hydride afforded bismurrayafolinol (2) in 90% yield but did not lead to bismurrayafoline-A (1). Apparently, N-substituted carbazoles do not form the quinone imine methide intermediate required for the complete reduction to a 3-methylcarbazole,5i,13,24 and thus, further reduction of the 3-(hydroxymethyl)carbazole does not occur. Oxidation of bismurrayafolinol (2) with manganese(IV) oxide25,26 led to chrestifoline-D (3).§
| Reaction conditions | Products, yield (%) |
|---|---|
| 1.3 equiv. 6d, 10 mol% Pd(OAc)2, 10 mol% rac-BINAP, 1.4 equiv. Cs2CO3, toluene, reflux, 4 d. | 13a, 27%; 13b, 35% |
| 1.3 equiv. 6d, 0.2 equiv. CuBr, 0.4 equiv. pyrrole-2-carboxylic acid, 4 equiv. K3PO4, DMSO, 110 °C, 40 h. | 13b, 63% |
Using the Ullmann-type coupling of the p-nitrobenzoate 12 with the appropriate carbazole building blocks 6a, 7a and 7b, the methylene-bridged biscarbazoles 1, 4 and 5 became directly available (Scheme 3).
Thus, reaction of murrayafoline-A (6a)8 with the p-nitrobenzoate 12 provided bismurrayafoline-A (1) in 46% yield.§ The Ullmann-type coupling of the nitrobenzoate 12 with girinimbine (7a)20c afforded chrestifoline-B (4) and the reaction of 12 with mahanimbine (7b)20c provided (±)-chrestifoline-C (5).§ A comparison of the four Ullmann-type couplings described herein reveals that mukonine (6d), which obviously is more stable under the reaction conditions, provided the best yield (63%) for the copper-induced C–N bond formation with compound 12.
In conclusion, five naturally occurring methylene-bridged biscarbazole alkaloids were synthesised using an Ullmann-type coupling at the benzylic carbon atom of the relay compound 12 as the key reaction. Using the present route, the biscarbazole alkaloids 1–5 become available via short and efficient synthetic routes, bismurrayafoline-A (1): 6 steps, 41% overall yield; bismurrayafolinol (2): 7 steps, 53% overall yield; chrestifoline-D (3): 8 steps, 42% overall yield; chrestifoline-B (4): 6 steps, 36% overall yield; and (±)-chrestifoline-C (5): 6 steps, 28% overall yield based on the arylamine 10. Chrestifoline-B (4) and (±)-chrestifoline-C (5) have been obtained by total synthesis for the first time. Studies concerning the bioactivity of the biscarbazole alkaloids 1–5 are in progress.
Experimental procedure
Experimental procedure for the Ullmann-type coupling to the biscarbazole 13b:A solution of the p-nitrobenzoate 12 (66.6 mg, 0.140 mmol), freshly dried potassium phosphate (120 mg, 0.566 mmol), mukonine (6d) (46.4 mg, 0.182 mmol), copper(I) bromide (4.0 mg, 28 μmol), and pyrrole-2-carboxylic acid (6.2 mg, 56 μmol) in DMSO (1 mL) was heated at 110 °C for 40 h. Water (1.5 mL) and a saturated aqueous solution of ammonium chloride (3 mL) were added, the layers were separated and the aqueous layer was extracted three times with ethyl acetate. The combined organic layers were dried over magnesium sulfate and the solvents were removed under reduced pressure. Purification of the residue by flash chromatography on silica gel (gradient elution with petroleum ether–ethyl acetate, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 7
1 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) afforded the biscarbazole 13b (40.9 mg, 63%) as a colourless solid; mp 152.5–153 °C. UV (MeOH): λ = 240 (sh), 252 (sh), 270, 292 (sh), 325 nm; IR (ATR): ν = 3360, 3055, 2992, 2949, 2933, 2836, 1771, 1734, 1716, 1692, 1653, 1625, 1582, 1542, 1503, 1489, 1448, 1401, 1358, 1338, 1306, 1266, 1251, 1200, 1167, 1134, 1103, 1037, 1011, 991, 947, 906, 861, 837, 764, 729, 694, 665, 613 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.81 (s, 3 H), 3.99 (s, 3 H), 4.02 (s, 3 H), 6.05 (s, 2 H), 6.76 (d, J = 0.8 Hz, 1 H), 7.17 (ddd, J = 7.9 Hz, 6.9 Hz, 1.1 Hz, 1 H), 7.27 (ddd, J = 7.8 Hz, 7.0 Hz, 0.8 Hz, 1 H), 7.37 (ddd, J = 8.1 Hz, 7.0 Hz, 1.1 Hz, 1 H), 7.40–7.44 (m, 2 H), 7.497 (d, J = 8.1 Hz, 1 H), 7.500 (s, 1 H), 7.66 (d, J = 1.3 Hz, 1 H), 7.93 (d, J = 7.8 Hz, 1 H), 8.14 (d, J = 7.8 Hz, 1 H), 8.20 (br s, 1 H), 8.54 (d, J = 1.3 Hz, 1 H); 13C NMR and DEPT (125 MHz, CDCl3): δ = 49.76 (CH2), 52.18 (CH3), 55.51 (CH3), 56.08 (CH3), 104.89 (CH), 108.20 (CH), 110.38 (CH), 110.99 (CH), 111.09 (CH), 116.38 (CH), 119.46 (CH), 120.23 (CH), 120.56 (CH), 120.66 (CH), 121.50 (C) 123.51 (C), 123.65 (C), 124.17 (C), 124.55 (C), 125.88 (CH), 126.48 (CH), 129.13 (C), 130.65 (C), 133.19 (C), 139.49 (C), 141.76 (C), 145.87 (C), 146.57 (C), 168.03 (C); ESI-MS (+50 V): m/z = 487 [M + Na]+, (−100 V): m/z = 463 [M − H]−; MS (EI): m/z (%) = 464 (26, M+), 255 (32), 240 (8), 224 (8), 210 (100), 167 (11); HRMS: m/z calcd for C29H24N2O4 (M+): 464.1736; found: 464.1741.
3) afforded the biscarbazole 13b (40.9 mg, 63%) as a colourless solid; mp 152.5–153 °C. UV (MeOH): λ = 240 (sh), 252 (sh), 270, 292 (sh), 325 nm; IR (ATR): ν = 3360, 3055, 2992, 2949, 2933, 2836, 1771, 1734, 1716, 1692, 1653, 1625, 1582, 1542, 1503, 1489, 1448, 1401, 1358, 1338, 1306, 1266, 1251, 1200, 1167, 1134, 1103, 1037, 1011, 991, 947, 906, 861, 837, 764, 729, 694, 665, 613 cm−1; 1H NMR (500 MHz, CDCl3): δ = 3.81 (s, 3 H), 3.99 (s, 3 H), 4.02 (s, 3 H), 6.05 (s, 2 H), 6.76 (d, J = 0.8 Hz, 1 H), 7.17 (ddd, J = 7.9 Hz, 6.9 Hz, 1.1 Hz, 1 H), 7.27 (ddd, J = 7.8 Hz, 7.0 Hz, 0.8 Hz, 1 H), 7.37 (ddd, J = 8.1 Hz, 7.0 Hz, 1.1 Hz, 1 H), 7.40–7.44 (m, 2 H), 7.497 (d, J = 8.1 Hz, 1 H), 7.500 (s, 1 H), 7.66 (d, J = 1.3 Hz, 1 H), 7.93 (d, J = 7.8 Hz, 1 H), 8.14 (d, J = 7.8 Hz, 1 H), 8.20 (br s, 1 H), 8.54 (d, J = 1.3 Hz, 1 H); 13C NMR and DEPT (125 MHz, CDCl3): δ = 49.76 (CH2), 52.18 (CH3), 55.51 (CH3), 56.08 (CH3), 104.89 (CH), 108.20 (CH), 110.38 (CH), 110.99 (CH), 111.09 (CH), 116.38 (CH), 119.46 (CH), 120.23 (CH), 120.56 (CH), 120.66 (CH), 121.50 (C) 123.51 (C), 123.65 (C), 124.17 (C), 124.55 (C), 125.88 (CH), 126.48 (CH), 129.13 (C), 130.65 (C), 133.19 (C), 139.49 (C), 141.76 (C), 145.87 (C), 146.57 (C), 168.03 (C); ESI-MS (+50 V): m/z = 487 [M + Na]+, (−100 V): m/z = 463 [M − H]−; MS (EI): m/z (%) = 464 (26, M+), 255 (32), 240 (8), 224 (8), 210 (100), 167 (11); HRMS: m/z calcd for C29H24N2O4 (M+): 464.1736; found: 464.1741.
Notes and references
- (a) H.-J. Knölker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef PubMed; (b) H.-J. Knölker and K. R. Reddy, in The Alkaloids, ed. G. A. Cordell, Academic Press, Amsterdam, 2008, vol. 65, p. 1 Search PubMed; (c) A. W. Schmidt, K. R. Reddy and H.-J. Knölker, Chem. Rev., 2012, 112, 3193 CrossRef CAS PubMed.
- (a) H.-J. Knölker, Top. Curr. Chem., 2005, 244, 115 Search PubMed; (b) I. Bauer and H.-J. Knölker, Top. Curr. Chem., 2012, 309, 203 CrossRef CAS.
- (a) D. P. Chakraborty and S. Roy, in Progress in the Chemistry of Organic Natural Products, ed. W. Herz, H. Grisebach, G. W. Kirby, W. Steglich and C. Tamm, Springer-Verlag, Wien, 1991, vol. 57, p. 71 Search PubMed; (b) D. P. Chakraborty, in The Alkaloids, ed. G. A. Cordell, Academic Press, New York, 1993, vol. 44, p. 257 Search PubMed; (c) C. J. Moody, Synlett, 1994, 681 CrossRef CAS PubMed; (d) D. P. Chakraborty and S. Roy, in Progress in the Chemistry of Organic Natural Products, ed. W. Herz, H. Grisebach, G. W. Kirby, W. Steglich and C. Tamm, Springer-Verlag, Wien, 2003, vol. 85, p. 125 Search PubMed.
- (a) H. Furukawa, Trends Heterocycl. Chem., 1993, 3, 185 Search PubMed; (b) S. Tasler and G. Bringmann, Chem. Rec., 2002, 2, 113 CrossRef PubMed.
- Reviews: (a) H.-J. Knölker, Curr. Org. Synth., 2004, 1, 309 CrossRef; (b) H.-J. Knölker, Chem. Lett., 2009, 38, 8 CrossRef; applications: (c) H.-J. Knölker and N. O'Sullivan, Tetrahedron, 1994, 50, 10893 CrossRef; (d) H.-J. Knölker and K. R. Reddy, Heterocycles, 2003, 60, 1049 CrossRef; (e) R. Forke, A. Jäger and H.-J. Knölker, Org. Biomol. Chem., 2008, 6, 2481 RSC; (f) R. Forke, M. P. Krahl, F. Däbritz, A. Jäger and H.-J. Knölker, Synlett, 2008, 1870 CAS; (g) T. Gensch, M. Rönnefahrt, R. Czerwonka, A. Jäger, O. Kataeva, I. Bauer and H.-J. Knölker, Chem. – Eur. J., 2012, 18, 770 CrossRef CAS PubMed; (h) L. Huet, R. Forke, A. Jäger and H.-J. Knölker, Synlett, 2012, 1230 CAS; (i) C. Börger and H.-J. Knölker, Tetrahedron, 2012, 68, 6727 CrossRef PubMed.
- (a) V. P. Kumar, K. K. Gruner, O. Kataeva and H.-J. Knölker, Angew. Chem., 2013, 125, 11279 ( Angew. Chem. Int. Ed. , 2013 , 52 , 11073 ) CrossRef; (b) C. Börger, A. W. Schmidt and H.-J. Knölker, Synlett, 2014 DOI:10.1055/s-0033-1338621.
- C. Börger, M. P. Krahl, M. Gruner, O. Kataeva and H.-J. Knölker, Org. Biomol. Chem., 2012, 10, 5189 Search PubMed.
- C. Börger, O. Kataeva and H.-J. Knölker, Org. Biomol. Chem., 2012, 10, 7269 Search PubMed.
- (a) A. S. Guram, R. A. Rennels and S. L. Buchwald, Angew. Chem., 1995, 107, 1456 ( Angew. Chem. Int. Ed. Engl. , 1995 , 34 , 1348 ) CrossRef; (b) J. Louie and J. F. Hartwig, Tetrahedron Lett., 1995, 36, 3609 CrossRef CAS; (c) S. Shekhar, P. Ryberg, J. F. Hartwig, J. S. Mathew, D. G. Blackmond, E. R. Strieter and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 3584 CrossRef CAS PubMed; (d) B. Schlummer and U. Scholz, Adv. Synth. Catal., 2004, 346, 1599 CrossRef CAS.
- (a) F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2382 CrossRef; (b) F. Ullmann, Ber. Dtsch. Chem. Ges., 1904, 37, 853 CrossRef; (c) I. Goldberg, Ber. Dtsch. Chem. Ges., 1906, 39, 1691 CrossRef; (d) S. V. Ley and A. W. Thomas, Angew. Chem., 2003, 115, 5558 ( Angew. Chem. Int. Ed. , 2003 , 42 , 5400 ) CrossRef.
- H. Furukawa, T.-S. Wu and T. Ohta, Chem. Pharm. Bull., 1983, 31, 4202 CrossRef CAS.
- M. Itoigawa, Y. Kashiwada, C. Ito, H. Furukawa, Y. Tachibana, K. F. Bastow and K.-H. Lee, J. Nat. Prod., 2000, 63, 893 CrossRef CAS PubMed.
- G. Bringmann and S. Tasler, Tetrahedron, 2001, 57, 2337 CrossRef CAS.
- C. Ito, T.-S. Wu and H. Furukawa, Chem. Pharm. Bull., 1987, 35, 450 CrossRef CAS.
- C. Ito, N. Okahana, T.-S. Wu, M.-L. Wang, J.-S. Lai, C.-S. Kuoh and H. Furukawa, Chem. Pharm. Bull., 1992, 40, 230 CrossRef CAS.
- C. Ito, T.-S. Wu and H. Furukawa, Chem. Pharm. Bull., 1990, 38, 1143 CrossRef CAS.
- D. P. Chakraborty, B. K. Barman and P. K. Bose, Sci. Cult., 1964, 30, 445 CAS.
- D. P. Chakraborty, K. C. Das and P. K. Bose, Sci. Cult., 1966, 32, 83 Search PubMed.
- (a) H.-J. Knölker and M. Bauermeister, J. Chem. Soc., Chem. Commun., 1990, 664 RSC; (b) H.-J. Knölker and M. Bauermeister, Tetrahedron, 1993, 49, 11221 CrossRef; (c) H.-J. Knölker and M. Wolpert, Tetrahedron Lett., 1997, 38, 533 CrossRef; (d) H.-J. Knölker and M. Wolpert, Tetrahedron, 2003, 59, 5317 CrossRef.
- (a) K. K. Gruner and H.-J. Knölker, Org. Biomol. Chem., 2008, 6, 3902 RSC; (b) K. K. Gruner, T. Hopfmann, K. Matsumoto, A. Jäger, T. Katsuki and H.-J. Knölker, Org. Biomol. Chem., 2011, 9, 2057 RSC; (c) R. Hesse, K. K. Gruner, O. Kataeva, A. W. Schmidt and H.-J. Knölker, Chem. – Eur. J., 2013, 19, 14098 CrossRef CAS PubMed.
- See for example: (a) R. A. Earley and M. J. Gallagher, J. Chem. Soc. C, 1970, 158 RSC; (b) U. Nettekoven and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 1166 CrossRef CAS PubMed; (c) S. L. Marquard, D. C. Rosenfeld and J. F. Hartwig, Angew. Chem., 2010, 122, 805 ( Angew. Chem. Int. Ed. , 2010 , 49 , 793 ) CrossRef.
- (a) V. H. Rawal and M. P. Cava, Tetrahedron Lett., 1985, 26, 6141 CrossRef CAS; (b) K. E. Knott, S. Auschill, A. Jäger and H.-J. Knölker, Chem. Commun., 2009, 1467 RSC.
- R. A. Altman, K. W. Anderson and S. L. Buchwald, J. Org. Chem., 2008, 73, 5167 CrossRef CAS PubMed.
- D. P. Chakraborty, S. Roy and A. K. Dutta, J. Indian Chem. Soc., 1987, 64, 215 CAS.
- (a) A. J. Fatiadi, Synthesis, 1976, 65 CrossRef CAS; (b) H.-J. Knölker, J. Prakt. Chem., 1995, 337, 75 CrossRef.
- Manganese dioxide (precipitated, active) from Merck (art. 805958).
Footnotes |
| † Part 117 of Transition Metals in Organic Synthesis; for Part 116, see ref. 6b. |
| ‡ Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of the biscarbazole alkaloids 1–5. See DOI: 10.1039/c4ob00609g |
| § Bismurrayafoline-A (1): Light brownish solid, mp 209–210 °C; UV (MeOH): λ = 224, 243, 251, 262 (sh), 282, 292, 323 (sh), 338, 352 (sh) nm; MS (EI): m/z (%) = 420 (M+, 40), 210 (100). Bismurrayafolinol (2): Colourless solid, mp 120–121.5 °C; UV (MeOH): λ = 225, 243, 252, 261 (sh), 281, 292, 331, 343 (sh) nm; MS (EI): m/z (%) = 436 (M+, 12), 210 (100). Chrestifoline-D (3): Colourless solid, mp 163.5–164 °C; UV (MeOH): λ = 222, 242, 251, 275, 291, 338, 346 (sh) nm; MS (EI): m/z (%) = 434 (M+, 22), 210 (100). Chrestifoline-B (4): Light brownish solid, mp 106–107 °C; UV (MeOH): λ = 227 (sh), 239, 251 (sh), 258 (sh), 290, 325, 338, 358 (sh) nm; ESI-MS (−10 V): m/z = 471 [M − H]−. (±)-Chrestifoline-C (5): Light brownish solid, mp 90 °C; UV (MeOH): λ = 228 (sh), 242, 251 (sh), 282 (sh), 290, 327, 340, 360 nm; ESI-MS (+10 V): m/z = 541 [M + H]+. For the 1H and 13C NMR spectra of the biscarbazoles 1–5, see: ESI.‡ |
| This journal is © The Royal Society of Chemistry 2014 |