Open Access Article
Open Access ArticleAlkylene-bridged viologen dendrimers: versatile cell delivery tools with biosensing properties†
Dirk
Bongard
*a,
Wilhelm
Bohr
b,
Marta
Swierczek
c,
Tesfaye Hailu
Degefa
a,
Lorenz
Walder
a and
Roland
Brandt
c
aInstitute of Chemistry of New Materials, University of Osnabrück, Barbarastr. 7, D-49076 Osnabrück, Germany. E-mail: dbongard@uos.de
bInstitute of Analytical and Bioanalytical Chemistry, University of Ulm, Albert-Einstein Allee 11, D-89081 Ulm, Germany
cDepartment of Neurobiology, University of Osnabrück, Barbarastr. 11, D-49076 Osnabrück, Germany
First published on 25th July 2014
Abstract
The synthesis of two types of viologen dendrimers with peripheral carboxyl groups is described. Their interaction with plasmid DNA and CT-DNA and the influence of time evolution and electrolyte on dendriplex formation have been electrochemically investigated. A negative potential shift appearing in the cyclic voltammograms of the dendrimers indicates dendriplex formation on the time scale of 15 to 19 minutes, i.e. similar to those determined empirically for other dendrimer types. The presence or absence of the negative potential shift can be used to check the stability towards sodium chloride and different cell growth media directing to sucrose for cell incubation experiments. The electrolyte content of commercially available cell growth media inhibits the dendriplex formation in solution prior to plasmid addition. Furthermore, a low salt stability of 20 mM sodium chloride for viologen dendriplexes has been confirmed, also recommending the use of lysosomotropic sucrose. The two types of viologen dendrimers have been combined with two plasmids differing in the number of base pairs. Four immortal cell lines have been tested to check the suitability of viologen dendriplexes as gene delivery systems. Probably due to the absence of terminal amino groups and endosomolytic substances only a small transfection efficiency of dendriplexes was achieved at low pH, generally excluding in vivo applications. With the larger pHSV-eGFP plasmid (5743 bp) no transfected cells were observed indicating a preference for shorter plasmids.
Introduction
The overwhelming biological application of dendrimers and dendritic substances is their use as drug delivery systems and as transport vehicles for gene therapy. Many substances lend themselves as candidate system for the non-viral transfection of mammalian cells. Recent examples of new transfection agents include cationic lipids, polymers, dendrimers, aminofullerenes and polycationic-modified cyclodextrins.1–8 In the past, much effort has been devoted to elucidating the mechanism of DNA release from dendriplexes after cell uptake. For polyethylen-imine (PEI) dendrimers the proton sponge mechanism has been established, and the same mechanism is suspected for polyamidoamine dendrimers (PAMAM), explaining their high transfection efficiencies.9 It is known that endocytosis and endosomal inclusion of the dendrimers and dendriplexes are steps in the cellular uptake, but there is a lack of knowledge concerning the DNA release in the cell. Considering the low intracellular diffusion rate of plasmid DNA caused by the molecular crowding within mammalian cells, the viologen dendrimers can help to clarify where the endosomal release occurs – in the perinuclear zone or further away in the cytoplasm.10,11 The size of polymeric or dendritic transfection agents crucially influences their toxicity. Thus, a monomeric cationic compound may form a DNA complex but may exhibit non-tolerable toxicity, whereas the corresponding polymeric compounds generally form the dendriplex more efficiently and show less relative toxicity, rendering them useful as gene and cell delivery systems. It was intriguing for us to investigate if the same can be observed for viologen dendrimers, which exhibit substantial cell toxicity in their monomeric form. Viologens are a class of organic salts containing bipyridinium subunits with two persistent positive charges per viologen (4,4′-bipyridinium) unit. Their most important application is their use in electrochromic displays.12 Here not only do the monomeric N,N′-alkyl and -aryl substituted bipyridinium salts exhibit electrochromic properties, but supramolecular viologen stars also show exceptional intramolecular color addition in their framework.13,14 The strong toxicity of monomeric viologens is well-known (Paraquat™) and there are investigations concerning the cytotoxicity and hematotoxicity of viologen-phosphorus dendrimers, revealing a similar toxicity for the first generation dendrimers compared to the monomeric viologens. Surprisingly, the dendrimer zeroth generation seem to be non-toxic for some cell lines, but specifically toxic against cancer cells.15,16 One author of the current publication (D.B.) previously showed that viologen dendrimers can be used as gene transfection reagents,17 an idea which has been adopted and extended recently by Asaftei and Oupicky.18Principally, cyclic voltammetry requires a redox active species such as a viologen or ferrocene, and viologen dendrimers offer the possibility of investigating the dendriplex formation before incubation using electrochemical methods. Hvastkovs and Buttry observed a negative potential shift in detecting the interaction of dsDNA with tetracationic diviologen compounds.19 The negative potential shift indicates a stabilization of the Vio2+ redox state in the viologens caused by the interaction with the DNA. It is assumed that such small viologens are inserted into the minor groove of the DNA. Here we describe the synthesis of two types of viologen dendrimers and their interaction with two expression vectors, pEGFP-C1 with 4731 bp and the larger pHSV-eGFP with 5734 bp. The redox-active 4,4′-bipyridinium-(viologen)-units within the dendrimers allow an unique electrochemical detection of the dendriplex formation and the feasibility to check the suitability of different cell growth media.
Furthermore, the preceding development of ultramicroelectrodes (nanodes) offers the possibility to explore the fate of dendrimers within a single eukaryotic cell.20,21 Some reports exist on the versatile utilization of micrometer- and ultra-micrometer electrodes. The successful amperometric detection of dopamine and other catecholamines in chromaffin cells has been reported by Mosharov et al.22 With the help of micrometer- and submicrometer-sized Pt electrodes the production and release of reactive oxygen and nitrogen species (ROS, RNS) can be amperometrically locally resolved and detected.23 For small viologens it has been shown that there is no interaction with the predominant NADH/NAD+ redox system in the cytosol. Viologen dendrimers reveal the possibility to electrochemically elucidate not only the DNA release of dendriplexes within a mammalian cell, but also the mechanism and location of drug release whether the release occurs in the cytosol or near the cell nucleus (Fig. 1). In this recent study we found that more flexible dendrimers with hexamethylene groups between the viologen units gave dendriplexes with better salt stabilities compared to the methylene bridged dendrimers, and small transfection efficiencies with CHO and PC12 cells were achieved with them.
 | ||
Fig. 1 Schematic depiction of viologen dendrimer detection within a single mammalian cell,  viologen dendrimer in oxidized state, and viologen dendrimer in oxidized state, and  in reduced state. in reduced state. | ||
Results and discussion
Syntheses
The synthetic route to obtain the two types of viologen dendrimers is shown in Schemes 1 and 2. The peripheral unit 2 and the adequate mono-quaternary-4,4′-bipyridinium salts 3, 4 and 5 have been achieved via the Menschutkin reaction of 4,4′-bipyridine in acetonitrile in good yields. After a subsequently performed anion exchange with 3 M aqueous ammonium hexafluorophosphate these compounds were ready for the closed reaction of dendrimers (Scheme 1). Starting from 1,3,5-tris-bromomethyl-benzene both types of viologen dendrimers were obtained in Menschutkin reactions to first form the hydroxymethyl-(p1OH) and hydroxyhexylene precursors (p2OH) in acetonitrile. The direct conversion of 1,3,5-tris-bromo-methylbenzene with 1-(2-hydroxy-ethyl)-4,4′-bipyridinium-hexafluorophosphate (5) (prepared according to the literature24) in acetonitrile resulted in the 0th generation dendrimer G0-OH in a yield of 26%. In the case of the methylene bridged viologen dendrimers the build up proceeded after the activation of precursor p1OH with 5.7 M acetic hydrobromic acid to form the p1Br bromide salt. The following conversion to the hexafluorophosphate salts prepared precursor p1Br for the last reaction step with 1-(2-carboxyethyl)-4,4′-bipyridinium-hexafluorophosphate (2) to afford the according methylene-bridged 1st generation dendrimer G1-COOH in a yield of 39.8%. In the case of the hexylene-bridged dendrimer the hexylene chain spacer was inserted via the Menschutkin reaction of 1,3,5-tris-bromomethylbenzene with 1-(6-hydroxyhexyl)-4,4′-bipyridinium salt (3) to form p2OH. The following activation with hydrobromic acid resulted in precursor p2Br and facilitates the attachment of branching unit (1) to yield p3OH. The last activation step with hydrobromic acid prepared precursor p3Br for the closing with 1-(5-carboxypentyl-4,4′-bipyridinium-hexafluorophosphate (4) to achieve dendrimer G1-Hexyl-COOH in a yield of 41%. | ||
| Scheme 2 Synthesis of the hexylene/methylene bridged viologen dendrimers: all counter ions are PF6−. 2. a.) Every reaction step is followed by an anion exchange with aqueous 3 M NH4PF6. | ||
Cyclic voltammetry
In Fig. 2, the time evolution for the dendriplex formation of G1-COOH and plasmid pC1-eGFP is shown. 20.4 μM G1-COOH yielded a similar negative potential shift and current to those described by Hvastkovs and Buttry for tetracationic diviologens.19 The decrease in current from scan 1 to 5 and the potential shift indicate the interaction between the dendrimer and the DNA (the formation of a dendrimer/DNA complex). The complex formation takes place over an extended period of about 19 minutes, and afterwards no further potential shifting was observed. The formal negative potential shift was −0.105 V, which is comparable to the −0.123 V shift reported for the dsDNA complexed diviologen compound reported by Hvastkovs and Buttry.A similar potential shift and time dependence has been observed for both types of viologen dendrimers. Changing the surface group leads to no significant differences in the electrochemical properties of the dendrimers. By synthesising the more flexible dendrimer we aimed to obtain dendriplexes with better electrolyte stabilities. In Fig. 3, the electrochemical features of G1-Hexyl-COOH/pC1-eGFP under the influence of sodium chloride is shown. We observed the same decrease in current and the potential shift was preserved up to 20 mM sodium chloride (scan 1 and 2, Fig. 3). After that, the current increased and a potential shift to the left side was observed, which is in contrast to the behaviour of the DNA-containing methylene-bridged viologen dendrimers and is probably caused by sodium chloride (scan 3 and 4, Fig. 3).
 | ||
| Fig. 3 CVs of G1-Hexyl-COOH/pC1-eGFP with 1: 20 μg G1-Hexyl-COOH, and after the addition of 2: 1.4 μg pC1-eGFP ± 0.03; 3: 20 mM sodium chloride and 4: 80 mM sodium chloride. Scan rate: 0.1 V s−1. | ||
The same experiment for G1-COOH resulted in a lower salt stability. For methylene-bridged viologen dendrimers we found a value of 10 mM showing the influence of both ions, i.e. a low salt stability as previously described by Marchioni et al. for methylene-bridged viologen dendrimer complexes with eosin.25 This means that the substitution of the methylene groups by hexyl spacers resulted in a 100% better electrolyte stability.
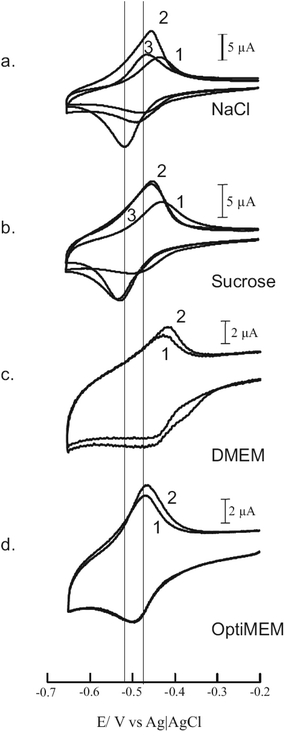
The shift in potential can be used to check the different cell growth media for the electrolyte effect during the incubation of transfection experiments. In standard cell growth media such as DMEM or OptiMEM no negative potential shifts could be observed, which is most likely due to the high content of electrolytes in these media that prevent the dendriplex formation. For this purpose cyclic voltammetry has been used in a qualitative way, with the appearance of a negative potential shift indicating the preservation and a return to the dissociation of the DNA complexes. In Fig. 4, the CVs of G0-OH/calf thymus DNA complexes in DMEM and OptiMEM are compared to physiological sodium chloride and 0.25 M aqueous sucrose. Here the measurements were performed in a three electrode system, in a cell volume of 10 ml and using calf thymus DNA instead of plasmids. The scan rate was 400 mV s−1. The electrode was not polished after the first and followingvoltammetric scans. In contrast to the measurements in Fig. 2 where the current decreased after plasmid addition, here the current increased indicating an adsorption onto the electrode surface. The addition of 150 mM sodium chloride re-established the CV of the pure dendrimer solution indicating the dissociation of the dendrimer/DNA complex (scans 2 to 3, Fig. 4) for the G0-OH/calf thymus DNA system. The cyclic voltammetric measurements with DMEM and OptiMEM were carried out in reversed modi operandi. The dendrimer G0-OH was dissolved in the aqueous cell growth media at pH 8.5 and the calf thymus DNA was added (scan 2, Fig. 4c and d). The experiment with DMEM was performed with 9.5 μM G0-OH and 130 μM CT-DNA, and the analogous OptiMEM trial was carried out with 4 times more dendrimer and nearly double the amount of CT-DNA. Although there was no negative potential shift observed, a small shift of about 10 mV in the positive direction and an increase in current of 1 μA was visible in both cases. This indicates a precipitation onto the electrode surface but was not interpreted as complex formation due to the presence of four times more dendrimer and double the amount of DNA. The potential shift and a stable current were only preserved in 0.25 M sucrose and in this medium dendriplex incubations (transfections) were successfully achieved.
Transfection experiments
Cell incubations (transfections) were achieved with dendrimer G1-Hexyl-COOH for CHO and PC 12 cells, albeit with a very low transfection efficiency. Two expression vectors, pC1-eGFP with 4731 base pairs (bp) and pHSV-eGFP with 5743 bp have been tested with the result that only dendriplexes of the smaller vector (2 μg) combined with G1-Hexyl-COOH (2 μg) resulted in successfully transfected cells (pictures of transfected CHO cells can be seen in the ESI†).The crucial experimental condition was the complete exclusion of all electrolytes during the incubation of the dendriplexes with the cells. With the larger pHSV-eGFP plasmid (5743 bp) no transfected cells were observed, indicating a preference for shorter plasmids. In Fig. 4 it can be seen that the negative potential shift is obtained only in 0.25 M aqueous sucrose, indicating the stability of the dendriplexes, whereas in DMEM and OptiMEM, which are commonly used cell growth media, and sodium chloride at physiological concentration, the dendriplexes dissociated. Therefore, in aqueous sucrose the two cell types were transfected with an efficiency between 0.03 and 1.1% compared to the Superfect transfection agent and an overall transfection efficiency of 0.003% compared to the total number of treated cells.
However, sucrose combined with the synthetic buffer MES allows for the use of viologen dendrimers as gene delivery system for in vitro applications. Ciftci and Levy have demonstrated that sucrose shows not only a lysosomotropic effect, but also an enhancement in transfection efficiency.26
Conclusion
In summary, the purpose of this work is twofold, and concerns (i) the preparation of new viologen dendrimers structurally tailored for gene transfection and (ii) a new type of electrochemical dendriplex analysis which is based on the electroactivity of certain dendrimer hosts such as the viologen dendrimers presented here. (i) Two types of alkylene-bridged viologen dendrimers have been synthesized with the intention of elucidating an application as in vivo and in vitro gene delivery systems. In analogy to the well-known poly(N-ethyl-4-vinylpyridinium) PEVP polymers we expected a significant transfection efficiency for viologen dendrimers, even without terminal amino groups.27However, due to the absence of terminal amino groups, which are the deciding structural feature in PAMAM- and PEI dendrimers and responsible for the high transfection efficiency, and the absence of endosomolytic substances like chloroquine we found only a very small transfection efficiency for viologen dendriplexes. The disadvantage for PAMAM and PEI dendrimers is a more or less pronounced cytotoxicity based on the pH dependent ratio of protonated to non-protonated primary amino groups, which prevents in vivo application. The persistent pH independent positive charges in the framework of viologen dendrimers endow them with a low cytotoxicity compared to PAMAM and PEI dendrimers whilst providing a sufficient drug and DNA complexation.
The cellular uptake, haemotoxicity and cell viability of structurally cognated viologen-phosphorus dendrimers in erythrocytes has been confirmed.16 The advantage of quaternary amino groups in viologen dendrimers containing bipyridinium units culminate for 0th generation viologen dendrimers in terms of specific toxicity against cancer cells and almost non-toxic behaviour against common immortal cell lines. Surprisingly, only the combination of the smaller plasmid together with the more flexible hexylene-bridged viologen dendrimer showed positive results. Bearing in mind the low electrolyte stability that has been confirmed for eosin/viologen dendrimer complexes by Marchioni et al.25in vivo drug delivery systems may be excluded.
(ii) A new electrochemical method is presented which allows us to discriminate between free viologen dendrimers and the viologen dendrimer condensing DNA chains from peak currents and peak potential shifts in cyclic voltammetry. Most importantly, we found a relatively easy way to study the influence of the NaCl concentration or more generally of the electrolyte concentration on the dendriplex stability by cyclic voltammetry. These results correlate nicely with the observed transfection ability, i.e. reliable transfection conditions can be optimized from the cyclic voltammetry results. If such electrochemical dendriplex monitoring could be combined with ultramicroelectrodes within single mammalian cells, it may be possible to monitor dendriplex dissociation in a spatially resolved manner.
Furthermore, viologen dendrimers are a promising tool for a wide range of bioanalytical and biomedical applications, i.e. related to the methyl-(benzyl) viologen/hydrogenase assay28–32 the electrochemical detection of reduced viologen dendrimer species in single mammalian cells could be possible. Another intriguing problem to be tackled is the preference of groove binding reported for small viologen oligomers with dsDNA19 and the condensation of DNA with viologen dendrimers reported in the current paper.
Experimental section
Syntheses
All of the chemicals used for synthesis were of analytical grade. NMR spectra were recorded with a Bruker-Avance-250 (250 MHz) spectrometer, (δ in ppm). MS spectra were recorded using an Agilent-HP-1100 spectrometer operating in the API-ES mode (m/z (rel%)).1,3,5-Tris-(bromomethyl)-benzene was purchased from Aldrich. The synthesis of 1-(3,5-dihydroxymethyl-phenyl)-4,4′-bipyridinium-hexafluorophosphate (1) is described by Kathiresan et al.33 The synthetic procedures for the hexa-(hydroxymethyl)-(p1OH) and hexa-(bromomethyl)-(p1Br) precursors are described by Heinen.34 The peripheral unit, 1-(2-hydroxyethyl)-4,4′-bipyridinium-hexa-fluorophosphate (5) is described by Lěon.24
General procedure for the counter ion exchange to the chloride salts: 5–30 mg of the hexafluorophosphate salts were dissolved in 1 ml acetonitrile, and dropped into 1 ml 0.5 M tetrabutylammonium-chloride–acetonitrile solution. The precipitates were filtered off and dried in vacuo to obtain 50–77% of the hygroscopic products.
The crude product was dissolved in 3 ml water and dropped into an aqueous solution of 2 ml ammonium-hexafluorophosphate (3 molar). The precipitate was collected and dried to yield 0.68 g (61.3%) of a white powder, m.p. 257 °C (decomp.)
δ H (250 MHz, D2O): 8.95 (d, 3J = 6.6 Hz, 2H, Vio); 8.76 (d, 3J = 6.3 Hz, 2H, Vio); 8.32 (d, 3J = 6.6 Hz, 2H, Vio); 8.06 (d, 3J = 6.4 Hz, 2H, Vio); 4.79 (t, 3J = 6.4 Hz, CH2, 2H); 2.97 (t, 3J = 6.4 Hz, CH2, 2H).
δ C (63 MHz, D2O): 173.7 (1C, COOH); 151.0 (1C, Cq, Vio); 144.9 (2C, CH, Vio); 143.8 (2C, CH, Vio); 143.3 (1C, Cq, Vio); 124.7 (2C, CH, Vio); 122.53 (2C, CH, Vio); 56.2 (1C, CH2); 34.6 (1C, CH2).
API-ES MS: m/z: 229.1 (100%); 229.9 (10%).
A subsequently performed anion exchange with 2 ml aqueous 3 M ammonium-hexafluorophosphate gave 0.83 g (39.8%) of a greyish-white powder.
δ H (250 MHz, CD3CN): 8.88 (d, 3J = 7.5 Hz, 2H, Vio); 8.82 (d, 3J = 5.0 Hz, 2H, Vio); 8.36 (d, 3J = 7.5 Hz, 2H, Vio); 7.94 (d, 3J = 5.0 Hz, 2H, Vio); 4.59 (t, 3J = 7.5 Hz, 2H, CH2); 3.52 (t, 3J = 5.0 Hz, 2H, CH2); 2.77 (s, 2H, CH2); 1.49 (t, 3J = 7.5 Hz, 4H, CH2).
δ C (63 MHz, CD3CN): 153.37 (Cq, 1C, Vio); 149.45 (Cq, 1C, Vio); 145.01 (CH, 2C, Vio), 143.44 (CH, 2C, Vio); 126.28 (CH, 2C, Vio); 123.24 (CH, 2C, Vio); 61.96 (CH2, 1C); 61.69 (CH2, 1C); 32.48 (CH2, 1C); 31.24 (CH2, 1C); 25.76 (CH2, 1C); 25.33 (CH2, 1C).
API-ES MS: m/z: 256.96 (100%), 257.9.
δ H (250 MHz, CD3CN): 8.76 (d, 3J = 4.93 Hz, 2H, Vio); 8.68 (d, 3J = 6.11 Hz, 2H, Vio); 8.22 (d, 3J = 5.73 Hz, 2H, Vio); 7.70 (d, 3J = 4.91 Hz, 2H, Vio); 4.46 (t, 3J = 7.34 Hz, 2H, CH2); 2.22 (t, 3J = 7.08 Hz, 2H, CH2); 1.93 (q, 3J = 7.88 Hz, 2H, CH2); 1.55 (q, 3J = 7.38 Hz, 2H, CH2); 1.35 (t, 3J = 7.4 Hz, 2H, CH2).
δ C (63 MHz, CD3CN): 174.04 (1C, COOH); 154.03 (1C, Cq, Vio); 151.04 (2C, CH, Vio); 144.91 (2C, CH, Vio); 141.39 (1C, Cq, Vio); 126.04 (2C, CH, Vio); 121.91 (2C, CH, Vio); 61.22 (1C, CH2); 32.72 (1C, CH2); 30.51 (1C, CH2); 24.93 (1C, CH2); 23.72 (1C, CH2).
API-ES MS: m/z: 271.2 (100%); 272.2 (20%).
δ H (250 MHz, CD3CN): 8.94 (dd, 3J = 6.4 Hz, 2J = 2.5 Hz, 12H, Vio); 8.42 (t, 3J = 6.9 Hz, 12H, Vio); 7.69 (s, 3H, aromat. H); 5.86 (s, 6H, CH2); 4.72 (t, 3J = 4.6 Hz, 6H, CH2); 4.02 (t, 3J = 4.6 Hz, 6H, CH2); 3.49 (t, 3J = 5.3 Hz, 3H, OH).
δ C (63 MHz, CD3CN): 151.14 (3C, Cq, Vio); 150.38 (3C, Cq, Vio); 146.51 (6C, CH, Vio); 146.20 (6C, CH, Vio); 135.32 (3C, Cq, aromat.); 132.27 (3C, CH, aromat.); 127.88 (6C, CH, Vio); 127.23 (6C, CH, Vio); 64.44 (3C, CH2); 64.03 (3C, CH2); 60.62 (3C, CH2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solution was dropped into an mixture of 1 ml aqueous 3 molar ammonium-hexafluorophosphate and 4 ml water. The white precipitate was filtered off and dried to yield 0.031 g of the product. The filtrate of the reaction mixture was evaporated to dryness and the residue was treated with the same procedure to obtain 0.029 g of the product. Overall yield: 0.060 g (1.22 × 10−5 mol), 39.8%. MW (C144H138F108N18O12P18): 4903.14 g mol−1.
1). The solution was dropped into an mixture of 1 ml aqueous 3 molar ammonium-hexafluorophosphate and 4 ml water. The white precipitate was filtered off and dried to yield 0.031 g of the product. The filtrate of the reaction mixture was evaporated to dryness and the residue was treated with the same procedure to obtain 0.029 g of the product. Overall yield: 0.060 g (1.22 × 10−5 mol), 39.8%. MW (C144H138F108N18O12P18): 4903.14 g mol−1.
δ H (250 MHz, CD3CN): 8.88–8.83 (m, CH, arom., Vio, 36H); 8.41–8.30 (m, CH, arom., Vio, 36 H); 7.60 (s, CH, arom., 9H); 7.55 (s, CH, arom., 3H); 5.83 (s, CH2, 12H); 5.74 (s, CH2, 12H); 4.75–4.69 (b, CH2, 12H); 2.99 (b, CH2, 12H).
δ C (63 MHz, CD3CN): δ(ppm): 150.98 (6C, Cq, Vio); 150.36 (6C, Cq, Vio); 146.81 (12C, CH, Vio); 146.15 (24C, CH, Vio); 135.50 (6C, Cq, arom.); 135.44 (6C, Cq, arom.); 132.43 (9C, CH, arom.); 127.81 (24C, CH, Vio); 127.09 (12C, CH, Vio), 64.06 (12C, CH2); 58.42 (6C, CH2); 35.11 (6C, CH2).
δ H (250 MHz, CD3CN): 8.95 (t, 3J = 5.81 Hz, 12H, Vio); 8.43 (d, 3J = 7.08 Hz, 12H, Vio); 7.69 (s, CH, arom., 3H); 5.86 (s, CH2, 6H); 4.65 (t, 3J = 7.43 Hz, CH2, 6H); 3.52 (t, 3J = 7.3 Hz, CH2, 6H); 3.39 (t, 3J = 5.6 Hz, 3H, OH); 1.97 (q, 3J = 2.46 Hz, CH2, 6H); 1.44 (q, 3J = 2.53 Hz, CH2, 18H).
δ C (63 MHz, d6-DMSO): 150.26 (Cq, 3C, Vio); 149.12 (Cq, 3C, Vio); 146.88 (CH, 6C, Vio), 146.65 (CH, 6C, Vio); 136.31 (Cq, arom., 3C); 131.48 (CH, arom., 3C); 127.72 (CH, 6C, Vio); 127.47 (CH, 6C, Vio); 63.55 (CH2, 3C); 61.86 (CH2, 3C); 61.30 (CH2, 3C); 33.01 (CH2, 3C); 31.73 (CH2, 3C); 26.20 (CH2, 3C); 25.81 (CH2, 3C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solution was dropped into 2.5 ml 3 molar ammonium-hexafluorophosphate. The precipitate was collected and dried under high vacuum to yield 0.135 g (6.9 × 10−5 mol), 88.5%. MW (C57H69Br3F36N6P6): 1947.71 g mol−1.
1). The solution was dropped into 2.5 ml 3 molar ammonium-hexafluorophosphate. The precipitate was collected and dried under high vacuum to yield 0.135 g (6.9 × 10−5 mol), 88.5%. MW (C57H69Br3F36N6P6): 1947.71 g mol−1.
δ H (250 MHz, CD3CN): 8.95 (t, 3J = 7.3 Hz, 12H, Vio); 8.40 (d, 3J = 6.9 Hz, 12H, Vio); 7.70 (s, 3H, CH, arom.); 5.87 (s, 6H, CH2); 4.64 (t, 3J = 7.5 Hz, 6H, CH2); 3.52 (t, 3J = 6.7 Hz, 6H, CH2); 2.25 (q, 12H, CH2); 1.47 (q, 12H, CH2).
δ C (63 MHz, d6-DMSO): 150.19 (Cq, 3C, Vio); 149.27 (Cq, 3C, Vio); 146.90 (CH, arom., 6C, Vio); 146.61 (CH, arom., 6C, Vio); 136.37 (Cq, arom., 3C); 131.60 (Cq, arom., 3C); 127.80 (CH, 6C, Vio); 127.50 (CH, 6C, Vio); 63.51 (CH2, 3C); 61.76 (CH2, 3C); 35.85 (CH2, 3C); 32.71(CH2, 3C); 31.42 (CH2, 3C); 27.72 (CH2, 3C); 25.37 (CH2, 3C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and dropped into a solution of 3 ml aqueous 3 molar ammonium-hexafluorophosphate. The precipitate was filtered off and dried under high vacuum to yield 0.092 g, (2.6 × 10−5 mol), 97% of a brown powder. MW (C114H126F72N12O6P12): 3499.89 g mol−1.
1) and dropped into a solution of 3 ml aqueous 3 molar ammonium-hexafluorophosphate. The precipitate was filtered off and dried under high vacuum to yield 0.092 g, (2.6 × 10−5 mol), 97% of a brown powder. MW (C114H126F72N12O6P12): 3499.89 g mol−1.
δ H (250 MHz, CD3CN): 8.95 (d, 3J = 5.9 Hz, 24H, Vio); 8.42 (d, 3J = 6.1 Hz, 24H, Vio); 7.69 (s, 3H, CH arom.); 7.57 (s, 3H, CH arom.); 7.41 (s, 6H, CH arom.); 5.85 (s, 12H, CH2); 4.64 (s, 24H, CH2); 3.49 (t, 6H, OH, 3J = 3.6 Hz); 1.97 (m, 12H, CH2); 1.49 (t, 12H, 3J = 6.0 Hz).
δ C (63 MHz, CD3CN): 150.51 (Cq, 3C, Vio); 150.3 (Cq, 3C, Vio); 150.16 (Cq, 3C, Vio); 150.10 (Cq, 3C, Vio); 146.23 (CH, 6C, Vio); 146.11 (CH, 6C, Vio); 145.95 (CH, 6C, Vio); 144.24 (CH, 6C, Vio); 135.30 (Cq, 6C, arom.); 134.13 (Cq, 3C, arom.); 132.20 (CH, 6C, arom.); 131.90 (CH, 3C, arom.); 129.05 (CH, 6C, arom.); 127.85 (CH, 6C, Vio); 127.66 (CH, 6C, Vio); 126.66 (CH, 6C, Vio); 126.37 (CH, 6C, Vio); 64.56 (CH2, 3C); 64.03 (CH2, 3C); 63.46 (CH2, 6C); 62.92 (CH2, 3C); 62.29 (CH2, 3C); 30.97 (CH2, 6C); 25.31 (CH2, 6C).
After drying the precipitate a brown powder was obtained, yield 0.055 g (1.42 × 10−5 mol), 89%. MW (C114H120Br6F72N12P12): 3877.28 g mol−1.
δ H (250 MHz, CD3CN): 8.96 (d, 3J = 7.1 Hz, 24H, Vio); 8.43 (d, 3J = 6.5 Hz, 24H, Vio); 8.11 (d, 3J = 6.8 Hz, Vio); 7.84 (s, 3H, CH arom.) 7.70 (s, 6H, CH arom.); 7.52 (s, 3H, CH arom.); 5.86 (s, 12H, CH2); 4.67 (s, 12H, CH2); 4.64 (s, 12H, CH2); 2.04 (m, 12H, CH2); 1.44 (b, 12H, CH2).
δ H (250 MHz, CD3CN): 8.93 (d, 3J = 5.9 Hz, Vio, 48H); 8.42 (t, 3J = 5.8 Hz, Vio, 48H); 7.69 (s, CH arom., 12H); 5.86 (s, CH2, 24H); 4.65 (t, 3J = 7.1 Hz, CH2, 24H); 3.48 (q, 6H, COOH); 2.34 (t, CH2, 12H); 1.95 (m, 24H, CH2); 1.67 (t, 3J = 6.7 Hz, CH2, 12H); 1.44 (t, 3J = 6.7 Hz, CH2, 24H).
δ C (63 MHz, D2O, Cl−): 151.19 (Cq, Vio, 12C); 146.03 (CH, Vio, 36 C); 135.43 (Cq, arom., 12C); 131.99 (CH, arom., 12C); 127.75 (CH, Vio, 24C); 127.38 (CH, Vio, 12C); 63.95 (CH2, 12C); 62.34 (CH2, 6C); 49.19 (CH2, 6C); 30.78 (CH2, 6C); 30.52 (CH2, 12C); 25.06 (CH2, 12C).
Cyclic voltammetry
The cyclic voltammetric measurements give an insight into the complex formation and offer a method to check the electrolyte stability of the dendriplexes in the presence of the cell growth media. The cyclic voltammetric measurements in Fig. 2 and 3 were carried out with a three-electrode system under argon, using a glassy carbon working electrode (0.018 cm2) in a volume of 500 microliters against a 0.1 M Ag/AgCl-reference. The 10 mM TE-buffer was adjusted to pH 7.5. The working electrode was polished for every measurement. This excludes current contribution from accumulated precipitations but does not prevent adsorption during a single scan. Unless mentioned otherwise, the scan rate was 0.1 V s−1. The measurements in Fig. 4 were performed in 10 ml TE buffer (10 mM) with calf thymus DNA (CT-DNA) using a glassy carbon working electrode (0.07 cm2) at a scan rate of 400 mV s−1.Cell culture and transfection experiments
Acknowledgements
We thank Angelika Hilderink for expert technical assistance and Stefan Walter for the measurement of MS spectra. This work was supported by a fellowship of the Interdisciplinary graduate college 612 of the Deutsche Forschungsgemeinschaft (D.B.).Notes and references
- B. Wang, J. Zhou, S. Cui, B. Yang, Y. Zhao, B. Zhao, Y. Duan and S. Zhang, Afr. J. Biotechnol., 2012, 11(11), 2763–2773 CAS.
- D. E. Fein, M. P. Limberis, S. F. Maloney, J. M. Heath, J. M. Wilson and S. L. Diamond, Mol. Ther., 2009, 17(12), 2078–2087 CrossRef CAS PubMed.
- R. Bakry, R. M. Vallant, M. Najam-ul-Haq, M. Rainer, Z. Szabo, C. W. Huck and G. K. Bonn, Int. J. Nanomed., 2007, 2(4), 639–649 CAS.
- C. O. Mellet, J. M. Benito and J. M. Gracia Fernandez, Chem. – Eur. J., 2010, 16, 6728–6742 CrossRef PubMed.
- R. Maeda-Mamiya, E. Noiri, H. Isobe, W. Nakanishi, K. Okamoto, K. Doi, T. Sugaya, T. Izumi, T. Homma and E. Nakamura, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(12), 5339–5344 CrossRef CAS PubMed.
- E. Nakamura and H. Isobe, Chem. Rec., 2010, 10(5), 260–270 CrossRef CAS PubMed.
- R. Challa, A. Ahuja, J. Ali and R. H. Khar, AAPS PharmSciTech, 2005, 6(2), E329–E357 CrossRef PubMed.
- V. J. Stella and Q. He, Toxicol. Pathol., 2008, 36, 30–42 CrossRef CAS PubMed.
- O. Boussif, F. Lezoualch, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J.-P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297–7301 CrossRef CAS.
- A. Elouahabi and J.-M. Ruysschaert, Mol. Ther., 2005, 11, 336–347 CrossRef CAS PubMed.
- T. W. J. Steele and W. T. Shier, Pharm. Res., 2010, 11, 336–347 Search PubMed.
- M. Moller, S. Asaftei, D. Corr, M. Ryan and L. Walder, Adv. Mater., 2004, 16(17), 1558–1562 CrossRef.
- D. Bongard, M. Moller, S. N. Rao, D. Corr and L. Walder, Helv. Chim. Acta, 2005, 88, 3200 CrossRef CAS.
- V.-A. Constantin, D. Bongard and L. Walder, Eur. J. Org. Chem., 2012, 913–921 CrossRef CAS.
- N. Katir, J. P. Majoral, A. El Kadib, A.-M. Caminade and M. Bousmina, Eur. J. Org. Chem., 2012, 269–273 CrossRef CAS.
- K. Ciepluch, N. Katir, A. E. Kadib, A. Felczak, K. Zawadzka, M. Weber, B. Klajnert, K. Lisowska, A.-M. Caminade, M. Bousmina, M. Bryszewska and J. P. Majoral, Mol. Pharm., 2012, 9, 448–457 CrossRef CAS PubMed.
- D. Bongard, PhD thesis, University of Osnabrueck, 2008.
- J. Li, A.-M. Lepadatu, Y. Zhu, M. Ciobanu, Y. Wang, S. C. Asaftei and D. Oupicky, Bioconjugate Chem., 2014, 25, 907–917 CrossRef CAS PubMed.
- E. G. Hvastkovs and D. A. Buttry, Langmuir, 2006, 22, 10821–10829 CrossRef CAS PubMed.
- R. M. Wigthman, Science, 2006, 311, 1570–1574 CrossRef PubMed.
- P. Sun, F. O. Laforge, T. P. Abeyweera, S. A. Rotenberg, J. Carpino and M. V. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 443–448 CrossRef CAS PubMed.
- E. V. Mosharov, L.-W. Gong, B. Khanna, D. Sulzer and M. Lindau, J. Neurosci., 2003, 23(13), 5835–5845 CAS.
- Y. Wang, M. Noël, J. Velmurugan, W. Nogala, M. V. Mirkin, C. Lu, M. G. Collignon, F. Lemaître and C. Amatore, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(29), 11534–11539 CrossRef CAS PubMed.
- P. Lěon, C. Garbay-Jaureguiberry, B. Lambert, J. B. Le Pecq and B. P. Roques, J. Med. Chem., 1988, 31, 1021–1026 CrossRef.
- F. Marchioni, M. Venturi, A. Credi, V. Balzani, M. Belohradsky, A. M. Elizarov, H.-R. Tseng and J. F. Stoddart, J. Am. Chem. Soc., 2003, 126, 568–573 CrossRef PubMed.
- K. Ciftci and R. J. Levy, Int. J. Pharm., 2001, 218, 81–92 CrossRef CAS.
- A. V. Kabanov, S. V. Vinogradov, Y. G. Suzdaltseva and V. Y. Alakhov, Bioconjugate Chem., 1995, 6, 639–643 CrossRef CAS.
- M. S. Naik and D. J. D. Nicholas, Biochim. Biophys. Acta, 1967, 131, 204–207 CrossRef CAS.
- Y. Nagai, R. F. Elleway and D. J. D. Nicholas, Biochim. Biophys. Acta, 1968, 153, 766–776 CrossRef CAS.
- W. Wallace and D. J. D. Nicholas, Biochem. J., 1968, 109, 763–773 CAS.
- L. Yu and M. J. Wolin, J. Bacteriol., 1969, 98, 51–55 CAS.
- T. Yagi, Proc. Natl. Acad. Sci. U. S. A., 1976, 73, 2947–2949 CrossRef CAS.
- M. Kathiresan, L. Walder, F. Ye and H. Reuter, Tetrahedron Lett., 2010, 51(16), 2188–2192 CrossRef CAS PubMed.
- S. Heinen and L. Walder, Angew. Chem., Int. Ed., 2000, 39, 806–810 CrossRef CAS; S. Heinen, PhD Thesis, 1998.
- J. Leschik, A. Welzel, C. Weissmann, A. Eckert and R. Brandt, J. Neurochem., 2007, 101, 1303–1315 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ob00560k |
| This journal is © The Royal Society of Chemistry 2014 |