Palladium mediated intramolecular multiple C–X/C–H cross coupling and C–H activation: synthesis of carbazole alkaloids calothrixin B and murrayaquinone A†
Abstract
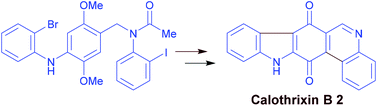
Straightforward palladium mediated syntheses of calothrixin B and murrayaquinone A are described. Regioselective palladium mediated intramolecular multiple C–X/C–H cross coupling reaction on N-(4-((2-bromophenyl)amino)-2,5-dimethoxybenzyl)-N-(2-iodophenyl)acetamide followed by CAN oxidation afforded calothrixin B in excellent yield in two steps. A linear synthesis has also been developed for calothrixin B. Utilizing C–H functionalization as well as palladium mediated intramolecular C–X/C–H cross coupling reaction, murrayaquinone A synthesis was achieved. Overall, these synthetic methodologies provide an expedient entry to these biologically active alkaloids in a short reaction sequence.

 Please wait while we load your content...
Please wait while we load your content...