Open Access Article
Open Access ArticleLewis acid promoted dual bond formation: facile synthesis of dihydrocoumarins and spiro-tetracyclic dihydrocoumarins†
Pedireddi
Niharika
,
Bokka Venkat
Ramulu
and
Gedu
Satyanarayana
*
Department of Chemistry, Indian Institute of Technology (IIT) Hyderabad, Ordnance Factory Estate Campus, Yeddumailaram – 502 205, Medak District, Andhra Pradesh, India. E-mail: gvsatya@iith.ac.in; Fax: +91 (40) 2301 6032
First published on 25th April 2014
Abstract
Lewis acid (FeCl3) mediated dual bond (C–C and C–O) formation for synthesis of 3,4-dihydrocoumarins is presented. This method has successfully delivered a number of dihydrocoumarins containing dense functionalities on the aromatic ring. Significantly, the present method enabled achieving dihydrocoumarins with tertiary as well as quaternary carbon atoms at the benzylic position. Gratifyingly, the novel spiro-tetracyclic lactones have also been dextrously prepared using this process.
Introduction
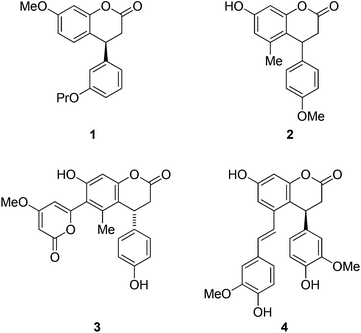
Coumarins are widely prevalent in nature, and show a broad range of biological activities1 such as anti-inflammatory, anti-aging, anti-oxidative and anti-cancer activities.2 The coumarin derivatives, with the core carbon skeleton of 4-aryl-3,4-dihydrocoumarin, are present in several classes of plants (neoflavanoids) exhibiting some interesting biological activities such as antiherpetic activity,3 aldose reductase inhibition,4 protein kinase inhibition,5 and they are also important synthetic intermediates for pharmaceutical compounds. Some tannins possessing this dihydrocoumarin unit are known to have been used in the treatment of infections and diseases.6 Some of the synthetically prepared dihydrocoumarins have gained popularity for their biological advantages. For example, compound 1 is a key intermediate in the synthesis of an endothelin antagonist7 as well as the drug tolterodine,8 which is an antagonist formulated to treat overactive bladder (Fig. 1),9 whereas the dihydrocoumarin 2 acts as a bactericide with the in vitro activity against members of the Tripanosoma family (Fig. 1).10 Some of the naturally occurring dihydrocoumarins like 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and 4
11 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (Fig. 1), obtained from Aloe vera2,12 and Gnetum cleistostachyum,1,13 respectively, show anti-inflammatory and antioxidant activities.14 They are also notably known for their ability to protect low-density lipoproteins from oxidative attack15 and recent studies have also revealed their ability to control the chronic heart and colon-rectal cancer.
11 (Fig. 1), obtained from Aloe vera2,12 and Gnetum cleistostachyum,1,13 respectively, show anti-inflammatory and antioxidant activities.14 They are also notably known for their ability to protect low-density lipoproteins from oxidative attack15 and recent studies have also revealed their ability to control the chronic heart and colon-rectal cancer.
Owing to the diverse advantages of the aryl-dihydrocoumarins, development of various methodologies for their synthesis has received tremendous attention. There have been a reasonable number of reports on the synthesis of these dihydrocoumarins. A few to be mentioned include the transition-metal-mediated catalytic hydrogenation,16 protic acid induced hydroarylation of the cinnamic acids with phenols,17 Lewis acid mediated cyclization between highly activated phenols and arylonitriles,18 the use of oxidants on acids,19 synthesis from ionic liquids, solid state catalysts, the use of molecular iodine as a catalyst, 5-alkylidene Meldrum's acids,20 Baeyer–Villiger oxidation of 1-indanones,21 and microwave assisted synthesis from phenols and cinnamoyl chloride in the presence of the montmorillonite K-10 catalyst.22
In this regard, we recently reported superacid mediated dual C–C bond formation, for the efficient synthesis of indanones.23 Herein we report an efficient and practical method for the facile synthesis of the dihydrocoumarins promoted by a Lewis acid (FeCl3) upon treatment of simple cinnamate esters with phenols. Notably, the approach has some significant advantages compared to those reported earlier. For example, the present method, using a Lewis acid (FeCl3), describes the direct treatment of cinnamate esters with phenols. Particularly, the other striking facet is the facile formation of a stereogenic quaternary carbon atom at the benzylic position and to the best of our knowledge there is no report on dihydrocoumarins with a quaternary carbon atom in such mild acidic transformations. Significantly, this method is applicable to accomplish the novel spiro-tetracyclic lactones which are quite difficult to achieve starting from cyclohexanone derivatives, since self-aromatization is a serious problem either under strong acidic or basic conditions. Moreover, the present study is broadly applied to check the scope and limitations of the method by employing on different cinnamates with varying functionalities on the aromatic rings.
Results and discussion
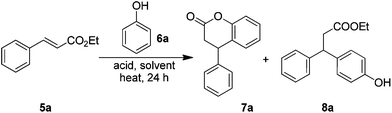
To begin with, the required cinnamate esters 5 were prepared from the corresponding benzaldehydes/acetophenones using the standard Wittig–Horner–Wadsworth–Emmons reaction. In order to determine the best optimized reaction conditions, initially, the simple ethyl cinnamate 5a was chosen as the model to study the reaction with phenol under various conditions as described in Table 1. The initial trials with catalytic amounts of a Lewis acid (FeCl3) did not facilitate the product 7a formation, rather furnished the Michael addition product 8a along with the recovery of the starting material (Table 1, entries 1 to 3). Though the precise mechanism cannot be given at this stage, the regioselective formation of the Michael addition product 8a, under catalytic loading of a Lewis acid would be explained due to selective activation of the enoate double bond by the Lewis acid that may facilitate the para-attack by the phenol. Gratifyingly, increasing the Lewis acid concentration (3 equiv.) at 80 °C in 1,2-dichloroethane resulted in the formation of lactone 7a in fair yields along with the Michael addition product 8a (Table 1, entry 4). An increase of FeCl3 from 3 equiv. to 5 equiv. exclusively gave the final product 7a albeit in moderate yields (Table 1, entry 5). The use of dichloromethane as a solvent was found to be inferior (Table 1, entry 6), while in benzene it better facilitated 8a. The predominant formation of the cyclic product 7a, in a stoichiometric amount of the Lewis acid, may be justified based on simultaneous activation of the enoate double bond, the carbonyl group of the ester as well as the phenol by the Lewis acid that might facilitate the Michael addition/condensation to give the lactone product 7a. The use of AlCl3 neither gave the lactone product nor allowed the recovery of the starting material (Table 1, entry 7). Also, it is concluded that the temperature played an important role, as when the reaction was conducted at ambient temperature, the reaction slowed down (Table 1, entry 8). The use of the other Lewis acid catalysts, like Sc(OTf)3, Cu(OTf)2 and AuCl3, in the catalytic amounts was found to be futile and led to the starting material recovery (Table 1, entries 10 to 12). In the presence of a superacid (TfOH) also the product 7a was formed, however, in moderate yields (Table 1, entry 13).| Entrya | Lewis acid | Solvent (mL) | Temp (°C) | Yieldb (%) | |
|---|---|---|---|---|---|
| 7a | 8a | ||||
| a All reactions were carried out on a 0.5 mmol scale of 5a and 1.5 equiv. of 6a, in solvent DCE (2 mL). b Isolated yields of chromatographically pure products. c Only the starting material was recovered. d Neither the product (7a) nor the starting material was isolated. | |||||
| 1c | FeCl3 (10 mol%) | DCE (2) | 80 | 0 | 0 |
| 2 | FeCl3 (20 mol%) | DCE (2) | 80 | 0 | 10 |
| 3 | FeCl3 (60 mol%) | DCE (2) | 80 | 0 | 30 |
| 4 | FeCl3 (3 equiv.) | DCE (2) | 80 | 60 | 20 |
| 5 | FeCl3 (5 equiv.) | DCE (2) | 80 | 55 | 0 |
| 6 | FeCl3 (3 equiv.) | DCM (2) | rt | 20 | 0 |
| 7d | AlCl3 (3 equiv.) | DCE (2) | 80 | 0 | 0 |
| 8 | FeCl3 (3 equiv.) | DCE (2) | rt | 20 | 0 |
| 9 | FeCl3 (3 equiv.) | Benzene (2) | 80 | 0 | 35 |
| 10c | AuCl3 (10 mol%) | DCE (2) | 80 | 0 | 0 |
| 11c | Sc(OTf)3 (10 mol%) | DCE (2) | 80 | 0 | 0 |
| 12c | Cu(OTf)2 (10 mol%) | DCE (2) | 80 | 0 | 0 |
| 13 | TfOH | DCE (2) | 80 | 50 | 0 |
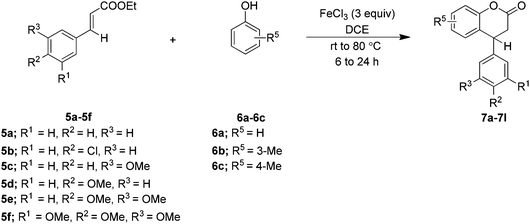
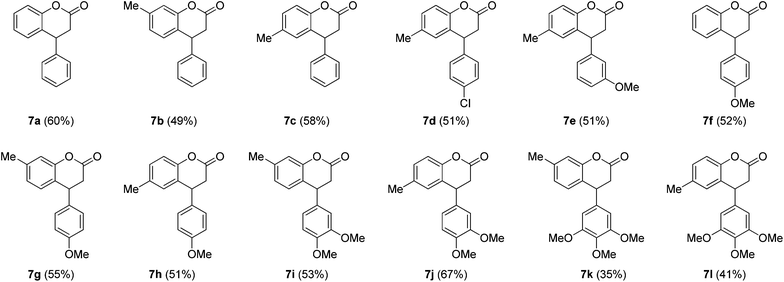
Among all the screened conditions, conditions of entry 4 of Table 1 were found to be the best with regard to the formation of 7a. Therefore, to check the scope and generality of the method, these conditions were applied to different cinnamates 5a–5c containing various functional groups on the aromatic rings with phenols 6a–6c. Gratifyingly, the method was found amenable and gave dihydrocoumarins 7a–7e containing a tertiary carbon atom, as summarized in Table 2. Disappointingly, in the case of electron rich cinnamate esters 5d–5f it could not be amenable under standard conditions, however at the ambient temperature it furnished the clean lactone products 7f–7l (Table 2).
| a All reactions were carried out on a 0.5 mmol scale of 5 and 1.5 equiv. of 6, in solvent DCE (2 mL). b Isolated yields of chromatographically pure products 7. c For compounds 7a–7e, the reaction was carried out at 80 °C for 24 h. d For compounds 7f–7l, the reaction was carried out at room temperature for 16 h. |
|---|
|
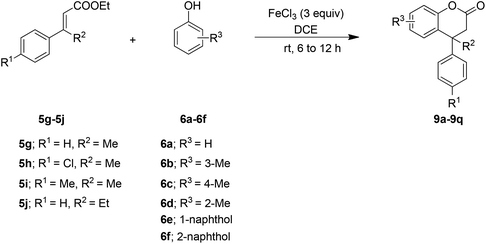
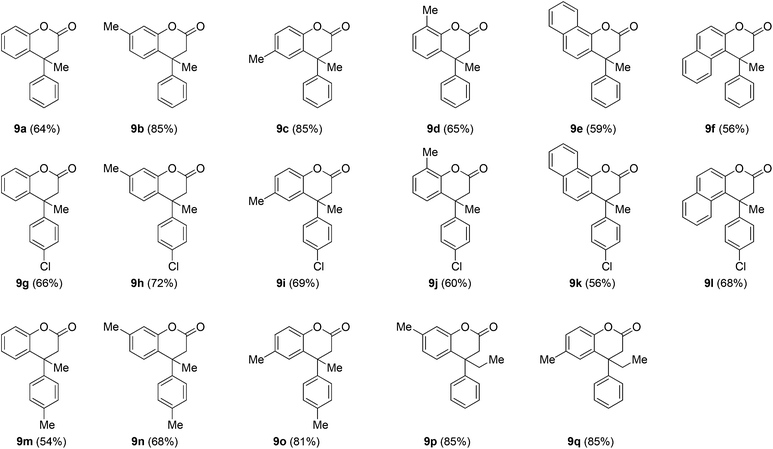
Furthermore, to check the feasibility of the method, β-alkyl (methyl/ethyl) ethyl cinnamates 5g–5j were explored. Interestingly, it was noted that the reaction is temperature and system dependent. For example, when β-methyl cinnamate ester 5g, derived from the corresponding acetophenone, was treated with the phenol 6a using the above optimized reaction conditions at 80 °C (Table 1, entry 4), it was not clean. However, the reaction was quite successful at room temperature and furnished the desired product 9a (Table 3). This would be justified owing to the slightly increased reactivity of 5g that may be due to the presence of the β-methyl substituent which would facilitate the polarization of the enoate double bond by the Lewis acid. After optimizing the reaction conditions for the ester 5g at room temperature, the generality of the reaction was established by employing the reaction between β-alkyl cinnamate esters 5h–5j and the phenols 6a–6f. In general, the method was smooth and furnished the dihydrocoumarins 9b–9q (Table 3).
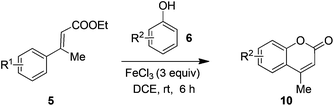
However, when attempting to apply the present method on β-methyl ethyl cinnamates 5k–5l, it did not furnish the expected dihydrocoumarins, rather gave coumarins 10a–10c (Table 4).
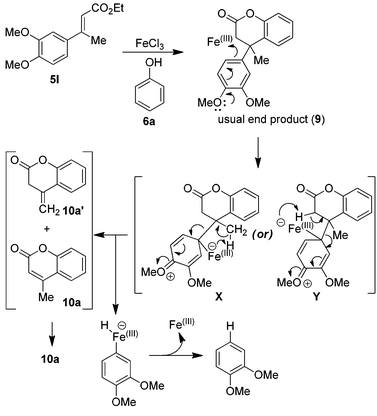
This can be rationalized based on the more reactive nature of the electron rich aromatic ring derived from the cinnamates 5k–5l, with suitably positioned electron donating groups. Though, the precise reaction mechanism cannot be given at this stage, however their formation is believed to be via the formation of the usual dihydrocoumarin product 9 followed by ipso type of aromatic substitution through internal rearrangement/cleavage of either X or Y intermediates, as shown in Scheme 1.
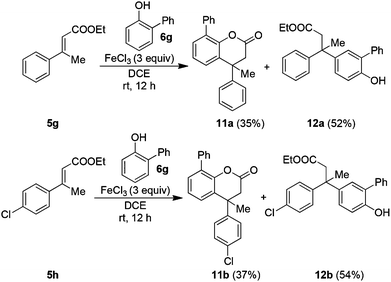
Furthermore, to study the regiochemical preference (i.e. the effect of the ortho-substituent on the Friedel–Crafts alkylation), 2-phenylphenol 6g was used as an external phenol on the cinnamate esters 5g–5h. However, the cyclized products 11a–11b were formed, albeit, in poor yields, while the Michael addition adducts 12a–12b were formed as the by-products, in moderate yields. This can be attributed to the steric crowding of 2-phenylphenol 6g that prefers para-attack on the Michael acceptor ethyl cinnamates 5g–5h (Scheme 2).
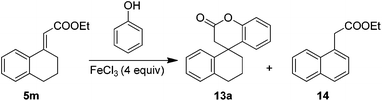
Upon accomplishing the dihydrocoumarins (7, 9 and 11) in a wide generality, we envisioned to use this method in a more applicable facet by performing the reaction with the cinnamate ester 5m obtained from the tetralone. Nevertheless, one could easily realize that such systems derived from six membered ketones would pose a serious problem of self-aromatization either under strong acidic or basic reaction conditions. As expected, the reaction of cinnamate ester 5m under standard conditions at 80 °C was unclear, whereas at room temperature, it gave only the self-aromatized product 14 (Table 5, entries 1 and 2). This made us to realize that the phenol is not sufficient enough to compete with usual self-aromatization. Therefore, performing the reaction with 5 equiv. of the phenol 6a led to the formation of the novel tetracyclic lactone 13a, in 33% yield along with the aromatized product 14 (Table 5, entry 3). On the other hand, interestingly, benzene was identified as the good solvent, hence it gave the product 13a, albeit, in poor yield, even with 1.5 equiv. of phenol 6a (Table 5, entry 4). Gratifyingly, with the increased amount of phenol 6a (5 equiv.), the product 13a was furnished in moderate yields along with the aromatized product 14 (Table 5, entry 5). However, a further increase of phenol 6a quantity (10 equiv.) could not improve the yield of the product drastically (Table 5, entry 6). It is noteworthy that the amount of the Lewis acid is slightly increased to 4 equiv. from 3 equiv. wherever more amount of phenol has been used in order to maintain the reasonable reactivity to promote the reaction, because phenol could also chelate with the Lewis acid and decrease its reactivity.
| Entrya | FeCl3 (equiv.) | Phenol (equiv.) | Solvent (mL) | Temp (°C) | Yieldb (%) | |
|---|---|---|---|---|---|---|
| 13a | 14 | |||||
| a All reactions were carried out on a 0.5 mmol scale of 5m. b Isolated yields of chromatographically pure products. | ||||||
| 1 | 3 | 1.5 | DCE (2) | 80 | 0 | 0 |
| 2 | 3 | 1.5 | DCE (2) | rt | 0 | 38 |
| 3 | 4 | 5 | DCE (2) | rt | 33 | 10 |
| 4 | 3 | 1.5 | Benzene | rt | 15 | 40 |
| 5 | 4 | 5 | Benzene | rt | 40 | 23 |
| 6 | 4 | 10 | Benzene | rt | 42 | 20 |
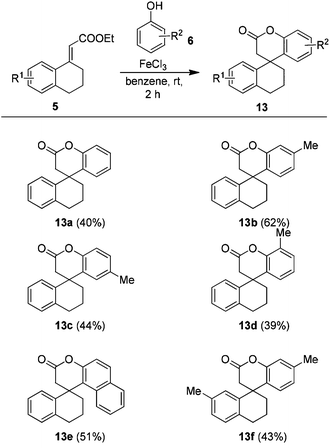
Among the screened conditions, conditions with either 5 or 10 equiv. of phenol 6a in benzene (Table 5, entries 5 and 6) were found to be the best with respect to the formation of 13a. Therefore, these conditions were used to generate different tetracyclic lactones 13. Gratifyingly, the method conveniently furnished the novel tetracyclic lactones 13b–13f, as summarized in Table 6.
Conclusions
In summary, we have developed a simple and practical method for the synthesis of dihydrocoumarins, a ubiquitous system present in many natural products. It was also extended to synthesize the novel spiro-tetracyclic lactones. The strategy is efficient and successful for the synthesis of a number of analogues. Moreover, this method availed the creation of the quaternary centre.Experimental section
General
IR spectra were recorded on a Bruker Tensor 37 (FTIR) spectrophotometer. 1H NMR spectra were recorded on a Bruker Avance 400 (400 MHz) spectrometer at 295 K in CDCl3; chemical shifts (δ ppm) and coupling constants (J in Hz) are reported in a standard fashion with reference to either internal standard tetramethylsilane (TMS) (δH = 0.00 ppm) or CHCl3 (δH = 7.25 ppm). 13C NMR spectra were recorded on a Bruker Avance 400 (100 MHz) spectrometer at RT in CDCl3; chemical shifts (δ in ppm) are reported relative to CHCl3 [δC = 77.00 ppm (central line of triplet)]. In the 13C NMR, the nature of carbons (C, CH, CH2 and CH3) was determined by recording the DEPT-135 spectra, and is given in parentheses and noted as s = singlet (for C), d = doublet (for CH), t = triplet (for CH2) and q = quartet (for CH3). In the 1H-NMR, the following abbreviations were used throughout: s = singlet, d = doublet, t = triplet, q = quartet, qui = quintet, m = multiplet and br. s = broad singlet. The assignment of signals was confirmed from 1H, 13C CPD and DEPT spectra. High-resolution mass spectra (HR-MS) were recorded on an Agilent 6538 UHD Q-TOF using a multimode source. All small scale dry reactions were carried out using the standard syringe-septum technique. Regarding the Horner–Wadsworth–Emmons reaction, TEPA from Avra Synthesis with a purity of 98%, NaH from Sigma–Aldrich (60% immersion in mineral oil), and benzaldehydes/acetophenones from Sisco Research Laboratories having 97–98% purity were used. Solvent THF was dried over sodium metal. Similarly, for cyclization reaction anhydrous FeCl3 from Merck Chemicals and phenols from Sisco Research Laboratories were used. DCE was dried over calcium hydride and used. Reactions were monitored by TLC on silica gel using a mixture of petroleum ether and ethyl acetate as eluents. Reactions were generally run under an argon or nitrogen atmosphere. Solvents were distilled prior to use; petroleum ether with a boiling range of 60 to 80 °C was used. Acme's silica gel (60–120 mesh) was used for column chromatography (approximately 20 g per gram of crude material).General procedure (GP-1) for cyclization
Into an oven dried Schlenk tube under a nitrogen atmosphere were added cinnamate ester 5 (88–133 mg, 0.5 mmol), phenol 6 (70–81 mg, 0.75 mmol) and anhydrous FeCl3 (324.0 mg, 2 mmol) followed by DCE (2 mL). The resulting reaction mixture was stirred at 80 °C for 5a–5c for 24 h (for other esters 5d–5j, the reaction was carried out at room temperature, for 6 to 16 h). Progress of the reaction was monitored by TLC until the reaction was completed. The reaction mixture was quenched by the addition of aqueous NaHCO3 and extracted in ethyl acetate (3 × 20 mL). The combined organic layers were dried (Na2SO4) and concentrated in vacuo. Purification of the residue on a silica gel column using petroleum ether–ethyl acetate as the eluent furnished lactones 7 (35–67%) and 9 (54–90%) as viscous liquids/solids.General procedure (GP-2) for spiro-cyclization
Into an oven dried Schlenk tube under a nitrogen atmosphere were added cinnamate ester 5 (88–133 mg, 0.5 mmol), phenol 6 (235–360 mg, 2.5 mmol) and anhydrous FeCl3 (324.0 mg, 2 mmol) followed by benzene (2.5 mL). The resulting reaction mixture was stirred at rt for 2 h. Progress of the reaction was monitored by TLC until the reaction was completed. The reaction mixture was quenched by the addition of aqueous NaHCO3 and extracted in ethyl acetate (3 × 20 mL). The combined organic layers were dried (Na2SO4) and concentrated in vacuo. Purification of the residue on a silica gel column using petroleum ether–ethyl acetate as the eluent furnished spiro-lactones 13 (39–62%) as viscous liquids/solids.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
12, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 85
4 to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 as the eluent) furnished the lactone 7e (66 mg, 51%) as a viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 1765, 1511, 1493, 1462, 1245, 1199, 1179, 1145, 1033, 820 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (dd, 1H, J = 7.8 and 7.8 Hz, ArH), 7.08 (dd, 1H, J = 8.3 and 2.0 Hz, ArH), 7.01 (d, 1H, J = 7.8 Hz, ArH), 6.81 (dd, 1H, J = 8.3 and 2.0 Hz, ArH), 6.79 (s, 1H, ArH), 6.73 (d, 1H, J = 7.8 Hz, ArH), 6.68 (dd, 1H, J = 2.0 and 2.0 Hz, ArH), 4.25 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.77 (s, 3H, ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.98 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.25 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C
15 as the eluent) furnished the lactone 7e (66 mg, 51%) as a viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 1765, 1511, 1493, 1462, 1245, 1199, 1179, 1145, 1033, 820 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (dd, 1H, J = 7.8 and 7.8 Hz, ArH), 7.08 (dd, 1H, J = 8.3 and 2.0 Hz, ArH), 7.01 (d, 1H, J = 7.8 Hz, ArH), 6.81 (dd, 1H, J = 8.3 and 2.0 Hz, ArH), 6.79 (s, 1H, ArH), 6.73 (d, 1H, J = 7.8 Hz, ArH), 6.68 (dd, 1H, J = 2.0 and 2.0 Hz, ArH), 4.25 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.77 (s, 3H, ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.98 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.25 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.0 (s, ArC), 149.6 (s, ArC), 142.1 (s, ArC), 134.3 (s, ArC), 130.1 (d, ArCH), 129.3 (d, ArCH), 128.6 (d, ArCH), 125.1 (s, ArC), 119.7 (d, ArCH), 116.8 (d, ArCH), 113.6 (d, ArCH), 112.5 (d, ArCH), 55.2 (q, ArOCH3), 40.7 (d, ArCHCH2CO), 37.0 (t, CH2CO), 20.7 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16NaO3]+ = [M + Na]+: 291.0992; found 291.0991.
O), 160.0 (s, ArC), 149.6 (s, ArC), 142.1 (s, ArC), 134.3 (s, ArC), 130.1 (d, ArCH), 129.3 (d, ArCH), 128.6 (d, ArCH), 125.1 (s, ArC), 119.7 (d, ArCH), 116.8 (d, ArCH), 113.6 (d, ArCH), 112.5 (d, ArCH), 55.2 (q, ArOCH3), 40.7 (d, ArCHCH2CO), 37.0 (t, CH2CO), 20.7 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16NaO3]+ = [M + Na]+: 291.0992; found 291.0991.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95
20, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 75
5 to 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 as the eluent) furnished the lactone 7i (79.0 mg, 53%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2852, 1762, 1591, 1516, 1463, 1419, 1253, 1219, 1142, 1025, 817 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 6.93 (s, 1H, ArH), 6.89 (d, 1H, J = 8.3 Hz, ArH), 6.85 (d, 1H, J = 7.8 Hz, ArH), 6.81 (d, 1H, J = 7.8 Hz, ArH), 6.67 (dd, 1H, J = 8.3 and 1.9 Hz, ArH), 6.65 (d, 1H, J = 1.9 Hz, ArH), 4.23 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.85 (s, 3H, ArOCH3), 3.81 (s, 3H, ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.96 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.34 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.0 (s, O–C
25 as the eluent) furnished the lactone 7i (79.0 mg, 53%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2852, 1762, 1591, 1516, 1463, 1419, 1253, 1219, 1142, 1025, 817 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 6.93 (s, 1H, ArH), 6.89 (d, 1H, J = 8.3 Hz, ArH), 6.85 (d, 1H, J = 7.8 Hz, ArH), 6.81 (d, 1H, J = 7.8 Hz, ArH), 6.67 (dd, 1H, J = 8.3 and 1.9 Hz, ArH), 6.65 (d, 1H, J = 1.9 Hz, ArH), 4.23 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.85 (s, 3H, ArOCH3), 3.81 (s, 3H, ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.96 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.34 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.0 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.5 (s, ArC), 149.3 (s, ArC), 148.4 (s, ArC), 139.0 (s, ArC), 132.9 (s, ArC), 127.9 (d, ArCH), 125.4 (d, ArCH), 122.9 (s, ArC), 119.7 (d, ArCH), 117.4 (d, ArCH), 111.5 (d, ArCH), 110.5 (d, ArCH), 55.9 (q, 2C, 2 × ArOCH3), 40.0 (d, ArCHCH2CO), 37.3 (t, CH2CO), 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO4]+ = [M + Na]+: 321.1097; found 321.1098.
O), 151.5 (s, ArC), 149.3 (s, ArC), 148.4 (s, ArC), 139.0 (s, ArC), 132.9 (s, ArC), 127.9 (d, ArCH), 125.4 (d, ArCH), 122.9 (s, ArC), 119.7 (d, ArCH), 117.4 (d, ArCH), 111.5 (d, ArCH), 110.5 (d, ArCH), 55.9 (q, 2C, 2 × ArOCH3), 40.0 (d, ArCHCH2CO), 37.3 (t, CH2CO), 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO4]+ = [M + Na]+: 321.1097; found 321.1098.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95
20, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 75
5 to 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 as the eluent) furnished the lactone 7j (99.8 mg, 67%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2852, 1762, 1592, 1493, 1420, 1243, 1137, 1025, 813 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.07 (dd, 1H, J = 8.3 and 1.9 Hz, ArH), 7.00 (d, 1H, J = 8.3 Hz, ArH), 6.82 (d, 1H, J = 7.8 Hz, ArH), 6.78 (d, 1H, J = 1.9 Hz, ArH), 6.67 (dd, 1H, J = 7.8 and 1.9 Hz, ArH), 6.66 (s, 1H, ArH), 4.23 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.85 (s, 3H, ArOCH3), 3.82 (s, 3H, ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.96 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.25 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.0 (s, O–C
25 as the eluent) furnished the lactone 7j (99.8 mg, 67%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2852, 1762, 1592, 1493, 1420, 1243, 1137, 1025, 813 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.07 (dd, 1H, J = 8.3 and 1.9 Hz, ArH), 7.00 (d, 1H, J = 8.3 Hz, ArH), 6.82 (d, 1H, J = 7.8 Hz, ArH), 6.78 (d, 1H, J = 1.9 Hz, ArH), 6.67 (dd, 1H, J = 7.8 and 1.9 Hz, ArH), 6.66 (s, 1H, ArH), 4.23 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.85 (s, 3H, ArOCH3), 3.82 (s, 3H, ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.96 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.25 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.0 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.5 (s, ArC), 149.3 (s, ArC), 148.4 (s, ArC), 134.3 (s, ArC), 132.9 (s, ArC), 129.2 (d, ArCH), 128.5 (d, ArCH), 125.6 (s, ArC), 119.7 (d, ArCH), 116.8 (d, ArCH), 111.5 (d, ArCH), 110.5 (d, ArCH), 55.9 (q, 2C, 2 × ArOCH3), 40.4 (d, ArCHCH2CO), 37.3 (t, CH2CO), 20.7 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO4]+ = [M + Na]+: 321.1097; found 321.1100.
O), 149.5 (s, ArC), 149.3 (s, ArC), 148.4 (s, ArC), 134.3 (s, ArC), 132.9 (s, ArC), 129.2 (d, ArCH), 128.5 (d, ArCH), 125.6 (s, ArC), 119.7 (d, ArCH), 116.8 (d, ArCH), 111.5 (d, ArCH), 110.5 (d, ArCH), 55.9 (q, 2C, 2 × ArOCH3), 40.4 (d, ArCHCH2CO), 37.3 (t, CH2CO), 20.7 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO4]+ = [M + Na]+: 321.1097; found 321.1100.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95
15, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 85
5 to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 as the eluent) furnished the lactone 7k (57.4 mg, 35%) as a semi-solid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1767, 1508, 1461, 1235, 1126, 1008, 818 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 6.93 (s, 1H, ArH), 6.90 (d, 1H, J = 7.8 Hz, ArH), 6.88 (d, 1H, J = 7.8 Hz, ArH), 6.34 (s, 2H, ArH), 4.22 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.82 (s, 3H, ArOCH3), 3.79 (s, 6H, 2 × ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.98 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.35 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C
15 as the eluent) furnished the lactone 7k (57.4 mg, 35%) as a semi-solid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1767, 1508, 1461, 1235, 1126, 1008, 818 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 6.93 (s, 1H, ArH), 6.90 (d, 1H, J = 7.8 Hz, ArH), 6.88 (d, 1H, J = 7.8 Hz, ArH), 6.34 (s, 2H, ArH), 4.22 (dd, 1H, J = 7.8 and 6.4 Hz, ArCHCH2CO), 3.82 (s, 3H, ArOCH3), 3.79 (s, 6H, 2 × ArOCH3), 3.03 (dd, 1H, J = 16.1 and 6.4 Hz, ArCHCHaHbCO), 2.98 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.35 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 153.6 (s, 2C, 2 × ArC), 151.5 (s, ArC), 139.2 (s, ArC), 136.2 (s, ArC), 128.0 (d, ArCH), 125.4 (d, ArCH), 122.5 (s, ArC), 117.5 (d, ArCH), 104.5 (d, 2C, 2 × ArCH), 60.8 (q, ArOCH3), 56.1 (q, 2C, 2 × ArOCH3), 40.7 (d, ArCHCH2CO), 37.2 (t, CH2CO), 21.1 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C19H20NaO5]+ = [M + Na]+: 351.1203; found 351.1206.
O), 153.6 (s, 2C, 2 × ArC), 151.5 (s, ArC), 139.2 (s, ArC), 136.2 (s, ArC), 128.0 (d, ArCH), 125.4 (d, ArCH), 122.5 (s, ArC), 117.5 (d, ArCH), 104.5 (d, 2C, 2 × ArCH), 60.8 (q, ArOCH3), 56.1 (q, 2C, 2 × ArOCH3), 40.7 (d, ArCHCH2CO), 37.2 (t, CH2CO), 21.1 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C19H20NaO5]+ = [M + Na]+: 351.1203; found 351.1206.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95
15, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 80
5 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 as the eluent) furnished the lactone 7l (67.2 mg, 41%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2850, 1764, 1591, 1460, 1243, 1124, 1006, 815 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.08 (dd, 1H, J = 8.3 and 1.9 Hz, ArH), 7.01 (d, 1H, J = 8.3 Hz, ArH), 6.81 (d, 1H, J = 1.9 Hz, ArH), 6.34 (s, 2H, 2 × ArH), 4.21 (dd, 1H, J = 7.8 and 6.3 Hz, ArCHCH2CO), 3.83 (s, 3H, ArOCH3), 3.79 (s, 6H, 2 × ArOCH3), 3.04 (dd, 1H, J = 16.1 and 6.3 Hz, ArCHCHaHbCO), 2.96 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.27 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C
15 as the eluent) furnished the lactone 7l (67.2 mg, 41%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2850, 1764, 1591, 1460, 1243, 1124, 1006, 815 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.08 (dd, 1H, J = 8.3 and 1.9 Hz, ArH), 7.01 (d, 1H, J = 8.3 Hz, ArH), 6.81 (d, 1H, J = 1.9 Hz, ArH), 6.34 (s, 2H, 2 × ArH), 4.21 (dd, 1H, J = 7.8 and 6.3 Hz, ArCHCH2CO), 3.83 (s, 3H, ArOCH3), 3.79 (s, 6H, 2 × ArOCH3), 3.04 (dd, 1H, J = 16.1 and 6.3 Hz, ArCHCHaHbCO), 2.96 (dd, 1H, J = 16.1 and 7.8 Hz, ArCHCHaHbCO), 2.27 (s, 3H, ArCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 153.6 (s, 2C, 2 × ArC), 149.5 (s, ArC), 137.3 (s, ArC), 136.2 (s, ArC), 134.4 (s, ArC), 129.4 (d, ArCH), 128.6 (d, ArCH), 125.2 (s, ArC), 116.8 (s, ArC), 104.5 (d, 2C, 2 × ArCH), 60.8 (q, ArOCH3), 56.1 (q, 2C, 2 × ArOCH3), 41.1 (d, ArCHCH2CO), 37.2 (t, CH2CO), 20.7 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C19H20NaO5]+ = [M + Na]+: 351.1203; found 351.1204.
O), 153.6 (s, 2C, 2 × ArC), 149.5 (s, ArC), 137.3 (s, ArC), 136.2 (s, ArC), 134.4 (s, ArC), 129.4 (d, ArCH), 128.6 (d, ArCH), 125.2 (s, ArC), 116.8 (s, ArC), 104.5 (d, 2C, 2 × ArCH), 60.8 (q, ArOCH3), 56.1 (q, 2C, 2 × ArOCH3), 41.1 (d, ArCHCH2CO), 37.2 (t, CH2CO), 20.7 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C19H20NaO5]+ = [M + Na]+: 351.1203; found 351.1204.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9a (76 mg, 64%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 1774, 1586, 1487, 1448, 1283, 1202, 1135, 1051, 910, 758, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.34 (dd, 1H, J = 7.8 and 1.5 Hz, ArH), 7.30 (dd, 2H, J = 7.8 and 1.5 Hz, ArH), 7.27–7.21 (m, 2H, ArH), 7.21–7.16 (m, 3H, ArH), 7.11 (dd, 1H, J = 8.3 and 1.5 Hz, ArH), 3.29 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.84 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.76 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.4 (s, O–C
6 as the eluent) furnished the lactone 9a (76 mg, 64%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 1774, 1586, 1487, 1448, 1283, 1202, 1135, 1051, 910, 758, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.34 (dd, 1H, J = 7.8 and 1.5 Hz, ArH), 7.30 (dd, 2H, J = 7.8 and 1.5 Hz, ArH), 7.27–7.21 (m, 2H, ArH), 7.21–7.16 (m, 3H, ArH), 7.11 (dd, 1H, J = 8.3 and 1.5 Hz, ArH), 3.29 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.84 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.76 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.4 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.2 (s, ArC), 143.9 (s, ArC), 130.8 (s, ArC), 128.7 (d, 3C, 3 × ArCH), 127.1 (d, ArCH), 126.6 (d, ArCH), 126.1 (d, 2C, 2 × ArCH), 124.7 (d, ArCH), 117.3 (d, ArCH), 43.7 (t, CH2CO), 41.1 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C16H15O2]+ = [M + H]+: 239.1067; found: 239.1067.
O), 151.2 (s, ArC), 143.9 (s, ArC), 130.8 (s, ArC), 128.7 (d, 3C, 3 × ArCH), 127.1 (d, ArCH), 126.6 (d, ArCH), 126.1 (d, 2C, 2 × ArCH), 124.7 (d, ArCH), 117.3 (d, ArCH), 43.7 (t, CH2CO), 41.1 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C16H15O2]+ = [M + H]+: 239.1067; found: 239.1067.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9b (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2972, 2926, 1763, 1623, 1578, 1445, 1413, 1215, 1199, 1164, 1150, 1047, 820, 764, 699 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.29 (ddd, 2H, J = 7.8, 7.3 and 1.9 Hz, ArH), 7.22 (t, 1H, J = 7.3 Hz, ArH), 7.17 (dd, 2H, J = 7.8 and 1.9 Hz, ArH), 7.11 (d, 1H, J = 7.8 Hz, ArH), 6.98 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.92 (d, 1H, J = 1.0 Hz, ArH), 3.26 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.36 (s, 3H, ArCH3), 1.72 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C
6 as the eluent) furnished the lactone 9b (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2972, 2926, 1763, 1623, 1578, 1445, 1413, 1215, 1199, 1164, 1150, 1047, 820, 764, 699 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.29 (ddd, 2H, J = 7.8, 7.3 and 1.9 Hz, ArH), 7.22 (t, 1H, J = 7.3 Hz, ArH), 7.17 (dd, 2H, J = 7.8 and 1.9 Hz, ArH), 7.11 (d, 1H, J = 7.8 Hz, ArH), 6.98 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.92 (d, 1H, J = 1.0 Hz, ArH), 3.26 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.36 (s, 3H, ArCH3), 1.72 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.0 (s, ArC), 144.1 (s, ArC), 139.0 (s, ArC), 128.6 (d, 2C, 2 × ArCH), 127.6 (s, ArC), 127.0 (d, ArCH), 126.3 (d, ArCH), 126.1 (d, 2C, 2 × ArCH), 125.4 (d, ArCH), 117.7 (d, ArCH), 43.9 (t, CH2CO), 40.8 [s, ArC(CH2CO)CH3], 27.6 [q, ArC(CH2CO)CH3], 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16NaO2]+ = [M + Na]+: 275.1043; found 275.1048.
O), 151.0 (s, ArC), 144.1 (s, ArC), 139.0 (s, ArC), 128.6 (d, 2C, 2 × ArCH), 127.6 (s, ArC), 127.0 (d, ArCH), 126.3 (d, ArCH), 126.1 (d, 2C, 2 × ArCH), 125.4 (d, ArCH), 117.7 (d, ArCH), 43.9 (t, CH2CO), 40.8 [s, ArC(CH2CO)CH3], 27.6 [q, ArC(CH2CO)CH3], 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16NaO2]+ = [M + Na]+: 275.1043; found 275.1048.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9c (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2974, 2921, 1763, 1598, 1493, 1266, 1202, 1124, 1049, 913, 824, 732, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.30 (ddd, 2H, J = 7.8, 7.3 and 2.0 Hz, ArH), 7.23 (t, 1H, J = 7.3 Hz, ArH), 7.18 (dd, 2H, J = 7.8 and 2.0 Hz, ArH), 7.11 (dd, 1H, J = 8.3 and 2.0 Hz, ArH), 7.02 (d, 1H, J = 2.0 Hz, ArH), 6.99 (d, 1H, J = 8.3 Hz, ArH), 3.25 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.34 (s, 3H, ArCH3), 1.73 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C
6 as the eluent) furnished the lactone 9c (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2974, 2921, 1763, 1598, 1493, 1266, 1202, 1124, 1049, 913, 824, 732, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.30 (ddd, 2H, J = 7.8, 7.3 and 2.0 Hz, ArH), 7.23 (t, 1H, J = 7.3 Hz, ArH), 7.18 (dd, 2H, J = 7.8 and 2.0 Hz, ArH), 7.11 (dd, 1H, J = 8.3 and 2.0 Hz, ArH), 7.02 (d, 1H, J = 2.0 Hz, ArH), 6.99 (d, 1H, J = 8.3 Hz, ArH), 3.25 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.34 (s, 3H, ArCH3), 1.73 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.1 (s, ArC), 144.0 (s, ArC), 134.3 (s, ArC), 130.3 (s, ArC), 129.2 (d, ArCH), 128.6 (d, 2C, 2 × ArCH), 127.1 (d, ArCH), 126.9 (d, ArCH), 126.1 (d, 2C, 2 × ArCH), 117.0 (d, ArCH), 43.8 (t, CH2CO), 41.0 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3], 20.9 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16NaO2]+ = [M + Na]+: 275.1048; found 275.1042.
O), 149.1 (s, ArC), 144.0 (s, ArC), 134.3 (s, ArC), 130.3 (s, ArC), 129.2 (d, ArCH), 128.6 (d, 2C, 2 × ArCH), 127.1 (d, ArCH), 126.9 (d, ArCH), 126.1 (d, 2C, 2 × ArCH), 117.0 (d, ArCH), 43.8 (t, CH2CO), 41.0 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3], 20.9 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16NaO2]+ = [M + Na]+: 275.1048; found 275.1042.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column chromatography (petroleum ether–ethyl acetate 98
6, UV detection)]. Purification of the residue on a silica gel column chromatography (petroleum ether–ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 94
2 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9d (81.9 mg, 65%) as colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2967, 2921, 1765, 1463, 1445, 1266, 1192, 1101, 915, 751, 699 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.29 (ddd, 2H, J = 8.8, 7.3 and 1.5 Hz, ArH), 7.23 (dd, 1H, J = 8.3 and 1.5 Hz, ArH), 7.21–7.15 (m, 3H, ArH), 7.07 (d, 2H, J = 4.9 Hz, ArH), 3.28 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.81 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.32 (s, 3H, ArCH3), 1.73 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C
6 as the eluent) furnished the lactone 9d (81.9 mg, 65%) as colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2967, 2921, 1765, 1463, 1445, 1266, 1192, 1101, 915, 751, 699 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.29 (ddd, 2H, J = 8.8, 7.3 and 1.5 Hz, ArH), 7.23 (dd, 1H, J = 8.3 and 1.5 Hz, ArH), 7.21–7.15 (m, 3H, ArH), 7.07 (d, 2H, J = 4.9 Hz, ArH), 3.28 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.81 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.32 (s, 3H, ArCH3), 1.73 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.4 (s, ArC), 144.0 (s, ArC), 130.5 (s, ArC), 130.2 (d, ArCH), 128.6 (d, 3C, 3 × ArCH), 127.1 (d, ArCH), 126.6 (s, ArC), 126.1 (d, 2C, 2 × ArCH), 124.1 (d, ArCH), 43.6 (t, CH2CO), 41.1 [s, ArC(CH2CO)CH3], 27.7 [q, ArC(CH2CO)CH3], 15.9 (q, ArCH3) ppm. HR-MS m/z calculated for [C17H17O2]+ = [M + H]+: 253.1223; found: 253.1210.
O), 149.4 (s, ArC), 144.0 (s, ArC), 130.5 (s, ArC), 130.2 (d, ArCH), 128.6 (d, 3C, 3 × ArCH), 127.1 (d, ArCH), 126.6 (s, ArC), 126.1 (d, 2C, 2 × ArCH), 124.1 (d, ArCH), 43.6 (t, CH2CO), 41.1 [s, ArC(CH2CO)CH3], 27.7 [q, ArC(CH2CO)CH3], 15.9 (q, ArCH3) ppm. HR-MS m/z calculated for [C17H17O2]+ = [M + H]+: 253.1223; found: 253.1210.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 98
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 94
2 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9e (84.9 mg, 59%) as a white solid, recrystallized the solid with dichloromethane/hexane, m. p. 144–146 °C. IR (MIR-ATR, 4000–600 cm−1): νmax = 2970, 2928, 1769, 1494, 1468, 1190, 1150, 1068, 816, 750, 700, 625 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 8.29 (dd, 1H, J = 8.8, and 2.0 Hz, ArH), 7.84 (dd, 1H, J = 7.3 and 2.0 Hz, ArH), 7.64 (d, 1H, J = 8.8 Hz, ArH), 7.59–7.51 (m, 2H, ArH), 7.34–7.18 (m, 6H, ArH), 3.34 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.93 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.82 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.3 (s, O–C
6 as the eluent) furnished the lactone 9e (84.9 mg, 59%) as a white solid, recrystallized the solid with dichloromethane/hexane, m. p. 144–146 °C. IR (MIR-ATR, 4000–600 cm−1): νmax = 2970, 2928, 1769, 1494, 1468, 1190, 1150, 1068, 816, 750, 700, 625 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 8.29 (dd, 1H, J = 8.8, and 2.0 Hz, ArH), 7.84 (dd, 1H, J = 7.3 and 2.0 Hz, ArH), 7.64 (d, 1H, J = 8.8 Hz, ArH), 7.59–7.51 (m, 2H, ArH), 7.34–7.18 (m, 6H, ArH), 3.34 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.93 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.82 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.3 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 146.1 (s, ArC), 144.2 (s, ArC), 133.6 (s, ArC), 128.7 (d, 2C, 2 × ArCH), 127.4 (d, ArCH), 127.2 (d, ArCH), 126.8 (d, ArCH), 126.7 (d, ArCH), 126.3 (d, 2C, 2 × ArCH), 125.1 (s, ArC), 124.2 (d, ArCH), 123.7 (s, ArC), 123.6 (d, ArCH), 121.5 (d, ArCH), 44.2 (t, CH2CO), 41.4 [s, ArC(CH2CO)CH3], 27.4 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C20H17O2]+ = [M + H]+: 289.1223; found 289.1221.
O), 146.1 (s, ArC), 144.2 (s, ArC), 133.6 (s, ArC), 128.7 (d, 2C, 2 × ArCH), 127.4 (d, ArCH), 127.2 (d, ArCH), 126.8 (d, ArCH), 126.7 (d, ArCH), 126.3 (d, 2C, 2 × ArCH), 125.1 (s, ArC), 124.2 (d, ArCH), 123.7 (s, ArC), 123.6 (d, ArCH), 121.5 (d, ArCH), 44.2 (t, CH2CO), 41.4 [s, ArC(CH2CO)CH3], 27.4 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C20H17O2]+ = [M + H]+: 289.1223; found 289.1221.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9f (81 mg, 56%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2920, 2851, 1775, 1599, 1512, 1494, 1459, 1336, 1210, 1163, 1019, 982, 912, 814, 733, 701 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.79 (dd, 2H, J = 8.8 and 7.3 Hz, ArH), 7.40–7.19 (m, 7H, ArH), 7.13 (d, 1H, J = 8.3 Hz, ArH), 7.08 (ddd, 1H, J = 8.8, 7.8 and 1.4 Hz, ArH), 3.07 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.86 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.94 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 166.5 (s, O–C
6 as the eluent) furnished the lactone 9f (81 mg, 56%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2920, 2851, 1775, 1599, 1512, 1494, 1459, 1336, 1210, 1163, 1019, 982, 912, 814, 733, 701 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.79 (dd, 2H, J = 8.8 and 7.3 Hz, ArH), 7.40–7.19 (m, 7H, ArH), 7.13 (d, 1H, J = 8.3 Hz, ArH), 7.08 (ddd, 1H, J = 8.8, 7.8 and 1.4 Hz, ArH), 3.07 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.86 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.94 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 166.5 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.9 (s, ArC), 147.0 (s, ArC), 131.9 (s, ArC), 130.4 (s, ArC), 130.1 (s, ArC), 129.0 (d, 2C, 2 × ArCH), 128.8 (d, ArCH), 127.0 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 125.9 (d, ArCH), 125.8 (d, ArCH), 124.4 (d, ArCH), 122.8 (s, ArC), 117.8 (d, ArCH), 48.0 (t, CH2CO), 42.9 [s, ArC(CH2CO)CH3], 24.7 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C20H16NaO2]+ = [M + Na]+: 311.1048; found 311.1042.
O), 149.9 (s, ArC), 147.0 (s, ArC), 131.9 (s, ArC), 130.4 (s, ArC), 130.1 (s, ArC), 129.0 (d, 2C, 2 × ArCH), 128.8 (d, ArCH), 127.0 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 125.9 (d, ArCH), 125.8 (d, ArCH), 124.4 (d, ArCH), 122.8 (s, ArC), 117.8 (d, ArCH), 48.0 (t, CH2CO), 42.9 [s, ArC(CH2CO)CH3], 24.7 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C20H16NaO2]+ = [M + Na]+: 311.1048; found 311.1042.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9g (90 mg, 66%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2973, 2925, 1763, 1586, 1487, 1449, 1279, 1232, 1198, 1134, 1095, 1012, 910, 828, 756, 683 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.32 (ddd, 1H, J = 8.3, 7.3 and 2.0 Hz, ArH), 7.25 (d, 2H, J = 8.3 Hz, ArH), 7.22 (dd, 1H, J = 7.8 and 2.0 Hz, ArH), 7.18 (ddd, 1H, J = 8.3, 7.8 and 2.0 Hz, ArH), 7.10 (dd, 1H, J = 7.3 and 2.0 Hz, ArH), 7.09 (d, 2H, J = 8.3 Hz, ArH), 3.22 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.82 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.72 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.2 (s, O–C
6 as the eluent) furnished the lactone 9g (90 mg, 66%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2973, 2925, 1763, 1586, 1487, 1449, 1279, 1232, 1198, 1134, 1095, 1012, 910, 828, 756, 683 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.32 (ddd, 1H, J = 8.3, 7.3 and 2.0 Hz, ArH), 7.25 (d, 2H, J = 8.3 Hz, ArH), 7.22 (dd, 1H, J = 7.8 and 2.0 Hz, ArH), 7.18 (ddd, 1H, J = 8.3, 7.8 and 2.0 Hz, ArH), 7.10 (dd, 1H, J = 7.3 and 2.0 Hz, ArH), 7.09 (d, 2H, J = 8.3 Hz, ArH), 3.22 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.82 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.72 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.2 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.1 (s, ArC), 142.5 (s, ArC), 133.1 (s, ArC), 130.2 (s, ArC), 129.0 (d, ArCH), 128.8 (d, 2C, 2 × ArCH), 127.6 (d, 2C, 2 × ArCH), 126.4 (d, ArCH), 124.8 (d, ArCH), 117.4 (d, ArCH), 43.6 (t, CH2CO), 40.8 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C16H13ClNaO2]+ = [M + Na]+: 295.0502; found 295.0500.
O), 151.1 (s, ArC), 142.5 (s, ArC), 133.1 (s, ArC), 130.2 (s, ArC), 129.0 (d, ArCH), 128.8 (d, 2C, 2 × ArCH), 127.6 (d, 2C, 2 × ArCH), 126.4 (d, ArCH), 124.8 (d, ArCH), 117.4 (d, ArCH), 43.6 (t, CH2CO), 40.8 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C16H13ClNaO2]+ = [M + Na]+: 295.0502; found 295.0500.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9h (103 mg, 72%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2964, 2925, 1766, 1579, 1494, 1413, 1257, 1213, 1162, 1096, 1048, 1012, 820, 736 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.24 (d, 2H, J = 8.8 Hz, ArH), 7.09 (d, 2H, J = 8.8 Hz, ArH), 7.08 (d, 1H, J = 7.8 Hz, ArH), 6.98 (d, 1H, J = 7.8 Hz, ArH), 6.91 (s, 1H, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.79 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.36 (s, 3H, ArCH3), 1.69 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.4 (s, O–C
6 as the eluent) furnished the lactone 9h (103 mg, 72%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2964, 2925, 1766, 1579, 1494, 1413, 1257, 1213, 1162, 1096, 1048, 1012, 820, 736 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.24 (d, 2H, J = 8.8 Hz, ArH), 7.09 (d, 2H, J = 8.8 Hz, ArH), 7.08 (d, 1H, J = 7.8 Hz, ArH), 6.98 (d, 1H, J = 7.8 Hz, ArH), 6.91 (s, 1H, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.79 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.36 (s, 3H, ArCH3), 1.69 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.4 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.0 (s, ArC), 142.8 (s, ArC), 139.4 (s, ArC), 133.0 (s, ArC), 128.8 (d, 2C, 2 × ArCH), 127.6 (d, 2C, 2 × ArCH), 127.1 (s, ArC), 126.1 (d, ArCH), 125.6 (d, ArCH), 117.8 (d, ArCH), 43.8 (t, CH2CO), 40.6 [s, ArC(CH2CO)CH3], 27.6 [q, ArC(CH2CO)CH3], 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H15ClNaO2]+ = [M + Na]+: 309.0658; found 309.0656.
O), 151.0 (s, ArC), 142.8 (s, ArC), 139.4 (s, ArC), 133.0 (s, ArC), 128.8 (d, 2C, 2 × ArCH), 127.6 (d, 2C, 2 × ArCH), 127.1 (s, ArC), 126.1 (d, ArCH), 125.6 (d, ArCH), 117.8 (d, ArCH), 43.8 (t, CH2CO), 40.6 [s, ArC(CH2CO)CH3], 27.6 [q, ArC(CH2CO)CH3], 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H15ClNaO2]+ = [M + Na]+: 309.0658; found 309.0656.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9i (99 mg, 69%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2972, 2924, 1761, 1592, 1491, 1413, 1278, 1199, 1124, 1095, 1012, 914, 824, 717, 671 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (d, 2H, J = 8.8 Hz, ArH), 7.12 (dd, 1H, J = 8.3 and 1.4 Hz, ArH), 7.10 (d, 2H, J = 8.8 Hz, ArH), 7.01 (d, 1H, J = 1.4 Hz, ArH), 6.99 (d, 1H, J = 8.3 Hz, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.35 (s, 3H, ArCH3), 1.72 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.4 (s, O–C
6 as the eluent) furnished the lactone 9i (99 mg, 69%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2972, 2924, 1761, 1592, 1491, 1413, 1278, 1199, 1124, 1095, 1012, 914, 824, 717, 671 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (d, 2H, J = 8.8 Hz, ArH), 7.12 (dd, 1H, J = 8.3 and 1.4 Hz, ArH), 7.10 (d, 2H, J = 8.8 Hz, ArH), 7.01 (d, 1H, J = 1.4 Hz, ArH), 6.99 (d, 1H, J = 8.3 Hz, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.35 (s, 3H, ArCH3), 1.72 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.4 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.1 (s, ArC), 142.6 (s, ArC), 134.5 (s, ArC), 133.1 (s, ArC), 129.8 (s, ArC), 129.5 (d, ArCH), 128.8 (d, 2C, 2 × ArCH), 127.7 (d, 2C, 2 × ArCH), 126.7 (d, ArCH), 117.2 (d, ArCH), 43.8 (t, CH2CO), 40.8 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3], 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H15ClNaO2]+ = [M + Na]+: 309.0658; found 309.0650.
O), 149.1 (s, ArC), 142.6 (s, ArC), 134.5 (s, ArC), 133.1 (s, ArC), 129.8 (s, ArC), 129.5 (d, ArCH), 128.8 (d, 2C, 2 × ArCH), 127.7 (d, 2C, 2 × ArCH), 126.7 (d, ArCH), 117.2 (d, ArCH), 43.8 (t, CH2CO), 40.8 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3], 21.0 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H15ClNaO2]+ = [M + Na]+: 309.0658; found 309.0650.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9j (85.8 mg, 60%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2965, 2923, 1770, 1493, 1465, 1195, 1098, 1012, 828, 787, 754 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (dd, 2H, J = 8.8, and 2.4 Hz, ArH), 7.18 (dd, 1H, J = 7.3 and 2.4 Hz, ArH), 7.10 (dd, 2H, J = 8.8 and 2.4 Hz, ArH), 7.06 (dd, 2H, J = 8.8 and 2.4 Hz, ArH), 3.22 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.7 Hz, CHaHbCO), 2.31 (s, 3H, ArCH3), 1.70 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.3 (s, O–C
6 as the eluent) furnished the lactone 9j (85.8 mg, 60%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2965, 2923, 1770, 1493, 1465, 1195, 1098, 1012, 828, 787, 754 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (dd, 2H, J = 8.8, and 2.4 Hz, ArH), 7.18 (dd, 1H, J = 7.3 and 2.4 Hz, ArH), 7.10 (dd, 2H, J = 8.8 and 2.4 Hz, ArH), 7.06 (dd, 2H, J = 8.8 and 2.4 Hz, ArH), 3.22 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.80 (d, 1H, J = 15.7 Hz, CHaHbCO), 2.31 (s, 3H, ArCH3), 1.70 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.3 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.4 (s, ArC), 142.7 (s, ArC), 133.0 (s, ArC), 130.5 (d, ArCH), 130.0 (s, ArC), 128.6 (d, 2C, 2 × ArCH), 127.7 (d, 2C, 2 × ArCH), 126.8 (s, ArC), 124.3 (d, ArCH), 123.9 (d, ArCH), 43.5 (t, CH2CO), 40.9 [s, ArC(CH2CO)CH3], 27.7 [q, ArC(CH2CO)CH3], 15.9 (q, ArCH3) ppm. HR-MS (APCI+) m/z calculated for [C17H16ClO2]+ = [M + H]+: 287.0833; found: 287.0824.
O), 149.4 (s, ArC), 142.7 (s, ArC), 133.0 (s, ArC), 130.5 (d, ArCH), 130.0 (s, ArC), 128.6 (d, 2C, 2 × ArCH), 127.7 (d, 2C, 2 × ArCH), 126.8 (s, ArC), 124.3 (d, ArCH), 123.9 (d, ArCH), 43.5 (t, CH2CO), 40.9 [s, ArC(CH2CO)CH3], 27.7 [q, ArC(CH2CO)CH3], 15.9 (q, ArCH3) ppm. HR-MS (APCI+) m/z calculated for [C17H16ClO2]+ = [M + H]+: 287.0833; found: 287.0824.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 94
2 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9k (77.8 mg, 56%) as a white solid, and the solid was recrystallized with dichloromethane/hexane, m. p. 152–154 °C. IR (MIR-ATR, 4000–600 cm−1): νmax = 2958, 2921, 2851, 1767, 1493, 1463, 1374, 1247, 1191, 1151, 1068, 1012, 816, 751, 699, 660 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 8.28 (dd, 1H, J = 6.8 and 2.4 Hz, ArH), 7.84 (dd, 1H, J = 6.8 and 2.4 Hz, ArH), 7.66 (d, 1H, J = 8.8 Hz, ArH), 7.60–7.52 (m, 2H, ArH), 7.25 (dd, 3H, J = 8.8 and 2.0 Hz, ArH), 7.15 (dd, 2H, J = 8.8 and 2.0 Hz, ArH), 3.29 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.94 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.81 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.0 (s, O–C
6 as the eluent) furnished the lactone 9k (77.8 mg, 56%) as a white solid, and the solid was recrystallized with dichloromethane/hexane, m. p. 152–154 °C. IR (MIR-ATR, 4000–600 cm−1): νmax = 2958, 2921, 2851, 1767, 1493, 1463, 1374, 1247, 1191, 1151, 1068, 1012, 816, 751, 699, 660 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 8.28 (dd, 1H, J = 6.8 and 2.4 Hz, ArH), 7.84 (dd, 1H, J = 6.8 and 2.4 Hz, ArH), 7.66 (d, 1H, J = 8.8 Hz, ArH), 7.60–7.52 (m, 2H, ArH), 7.25 (dd, 3H, J = 8.8 and 2.0 Hz, ArH), 7.15 (dd, 2H, J = 8.8 and 2.0 Hz, ArH), 3.29 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.94 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.81 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.0 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 146.2 (s, ArC), 142.8 (s, ArC), 133.7 (s, ArC), 133.2 (s, ArC), 128.9 (d, 2C, 2 × ArCH), 127.8 (d, 2C, 2 × ArCH), 127.5 (d, ArCH), 127.0 (d, ArCH), 126.9 (d, ArCH), 124.5 (s, ArC), 124.5 (d, ArCH), 123.7 (s, ArC), 123.2 (d, ArCH), 121.6 (d, ArCH), 44.2 (t, CH2CO), 41.1 [s, ArC(CH2CO)CH3], 27.4 [q, ArC(CH2CO)CH3] ppm. HR-MS (APCI+) m/z calculated for [C20H16O2Cl]+ = [M + H]+: 323.0833; found: 323.0822.
O), 146.2 (s, ArC), 142.8 (s, ArC), 133.7 (s, ArC), 133.2 (s, ArC), 128.9 (d, 2C, 2 × ArCH), 127.8 (d, 2C, 2 × ArCH), 127.5 (d, ArCH), 127.0 (d, ArCH), 126.9 (d, ArCH), 124.5 (s, ArC), 124.5 (d, ArCH), 123.7 (s, ArC), 123.2 (d, ArCH), 121.6 (d, ArCH), 44.2 (t, CH2CO), 41.1 [s, ArC(CH2CO)CH3], 27.4 [q, ArC(CH2CO)CH3] ppm. HR-MS (APCI+) m/z calculated for [C20H16O2Cl]+ = [M + H]+: 323.0833; found: 323.0822.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9l (110 mg, 68%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2964, 2925, 1777, 1600, 1513, 1492, 1458, 1336, 1212, 1096, 1012, 913, 814, 748 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.80 (dd, 2H, J = 8.8 and 7.3 Hz, ArH), 7.36–7.30 (m, 2H, ArH), 7.28 (d, 2H, J = 8.3 Hz, ArH), 7.20 (d, 2H, J = 8.3 Hz, ArH), 7.14 (ddd, 1H, J = 8.8, 8.8 and 1.5 Hz, ArH), 7.11 (d, 1H, J = 8.8 Hz, ArH), 3.00 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.84 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.93 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 166.2 (s, O–C
6 as the eluent) furnished the lactone 9l (110 mg, 68%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2964, 2925, 1777, 1600, 1513, 1492, 1458, 1336, 1212, 1096, 1012, 913, 814, 748 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.80 (dd, 2H, J = 8.8 and 7.3 Hz, ArH), 7.36–7.30 (m, 2H, ArH), 7.28 (d, 2H, J = 8.3 Hz, ArH), 7.20 (d, 2H, J = 8.3 Hz, ArH), 7.14 (ddd, 1H, J = 8.8, 8.8 and 1.5 Hz, ArH), 7.11 (d, 1H, J = 8.8 Hz, ArH), 3.00 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.84 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.93 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 166.2 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.8 (s, ArC), 145.5 (s, ArC), 132.8 (s, ArC), 131.9 (s, ArC), 130.3 (d, ArCH), 130.1 (s, ArC), 129.2 (d, 2C, 2 × ArCH), 129.0 (d, ArCH), 127.4 (d, 2C, 2 × ArCH), 126.0 (d, ArCH), 125.7 (d, ArCH), 124.6 (d, ArCH), 122.0 (s, ArC), 117.8 (d, ArCH), 47.8 (t, CH2CO), 42.6 [s, ArC(CH2CO)CH3], 24.7 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C20H15ClNaO2]+ = [M + Na]+: 345.0658; found 345.0655.
O), 149.8 (s, ArC), 145.5 (s, ArC), 132.8 (s, ArC), 131.9 (s, ArC), 130.3 (d, ArCH), 130.1 (s, ArC), 129.2 (d, 2C, 2 × ArCH), 129.0 (d, ArCH), 127.4 (d, 2C, 2 × ArCH), 126.0 (d, ArCH), 125.7 (d, ArCH), 124.6 (d, ArCH), 122.0 (s, ArC), 117.8 (d, ArCH), 47.8 (t, CH2CO), 42.6 [s, ArC(CH2CO)CH3], 24.7 [q, ArC(CH2CO)CH3] ppm. HR-MS (ESI+) m/z calculated for [C20H15ClNaO2]+ = [M + Na]+: 345.0658; found 345.0655.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 100
5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 94
0 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9m (68.1 mg, 54%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 1761, 1596, 1494, 1418, 1281, 1250, 1201, 1136, 1125, 1052, 915, 813 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.31 (ddd, 1H, J = 7.8, 7.8 and 1.5 Hz, ArH), 7.21 (dd, 1H, J = 7.8 and 1.5 Hz, ArH), 7.16 (ddd, 1H, J = 7.8, 7.8 and 1.5 Hz, ArH), 7.13–7.07 (m, 3H, ArH), 7.05 (d, 2H, J = 8.3 Hz, ArH), 3.26 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.80 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.30 (s, 3H, ArCH3), 1.72 [s, 3H, ArC(CH3)CH2CO] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C
6 as the eluent) furnished the lactone 9m (68.1 mg, 54%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 1761, 1596, 1494, 1418, 1281, 1250, 1201, 1136, 1125, 1052, 915, 813 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.31 (ddd, 1H, J = 7.8, 7.8 and 1.5 Hz, ArH), 7.21 (dd, 1H, J = 7.8 and 1.5 Hz, ArH), 7.16 (ddd, 1H, J = 7.8, 7.8 and 1.5 Hz, ArH), 7.13–7.07 (m, 3H, ArH), 7.05 (d, 2H, J = 8.3 Hz, ArH), 3.26 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.80 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.30 (s, 3H, ArCH3), 1.72 [s, 3H, ArC(CH3)CH2CO] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.7 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.1 (s, ArC), 140.9 (s, ArC), 136.8 (s, ArC), 131.0 (s, ArC), 129.4 (d, 2C, 2 × ArCH), 128.6 (d, ArCH), 126.6 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 124.7 (d, ArCH), 117.3 (d, ArCH), 43.8 (t, CH2CO), 40.8 [s, ArC(CH3)CH2CO], 27.5 [q, ArC(CH3)CH2CO], 20.8 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H17O2]+ = [M]+: 253.1223; found 253.1223.
O), 151.1 (s, ArC), 140.9 (s, ArC), 136.8 (s, ArC), 131.0 (s, ArC), 129.4 (d, 2C, 2 × ArCH), 128.6 (d, ArCH), 126.6 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 124.7 (d, ArCH), 117.3 (d, ArCH), 43.8 (t, CH2CO), 40.8 [s, ArC(CH3)CH2CO], 27.5 [q, ArC(CH3)CH2CO], 20.8 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H17O2]+ = [M]+: 253.1223; found 253.1223.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 100
5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 94
0 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9n (90.4 mg, 68%) as a yellow viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 1766, 1578, 1506, 1449, 1414, 1253, 1200, 1164, 1124, 1048, 815 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.11 (d, 1H, J = 7.8 Hz, ArH), 7.10 (d, 2H, J = 8.3 Hz, ArH), 7.05 (d, 2H, J = 8.3 Hz, ArH), 6.98 (d, 1H, J = 7.8 Hz, ArH), 6.91 (s, 1H, ArH), 3.24 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.78 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.36 (s, 3H, ArCH3), 2.30 (s, 3H, ArCH3), 1.70 [s, 3H, ArC(CH3)CH2CO] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C
6 as the eluent) furnished the lactone 9n (90.4 mg, 68%) as a yellow viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 1766, 1578, 1506, 1449, 1414, 1253, 1200, 1164, 1124, 1048, 815 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.11 (d, 1H, J = 7.8 Hz, ArH), 7.10 (d, 2H, J = 8.3 Hz, ArH), 7.05 (d, 2H, J = 8.3 Hz, ArH), 6.98 (d, 1H, J = 7.8 Hz, ArH), 6.91 (s, 1H, ArH), 3.24 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.78 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.36 (s, 3H, ArCH3), 2.30 (s, 3H, ArCH3), 1.70 [s, 3H, ArC(CH3)CH2CO] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.9 (s, ArC), 141.1 (s, ArC), 138.8 (s, ArC), 136.6 (s, ArC), 129.3 (d, 2C, 2 × ArCH), 127.8 (s, ArC), 126.2 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 125.3 (d, ArCH), 117.6 (d, ArCH), 43.9 (t, CH2CO), 40.4 [s, ArC(CH3)CH2CO], 27.5 [q, ArC(CH3)CH2CO], 20.9 (q, ArCH3), 20.8 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1197.
O), 150.9 (s, ArC), 141.1 (s, ArC), 138.8 (s, ArC), 136.6 (s, ArC), 129.3 (d, 2C, 2 × ArCH), 127.8 (s, ArC), 126.2 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 125.3 (d, ArCH), 117.6 (d, ArCH), 43.9 (t, CH2CO), 40.4 [s, ArC(CH3)CH2CO], 27.5 [q, ArC(CH3)CH2CO], 20.9 (q, ArCH3), 20.8 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1197.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column chromatography (petroleum ether–ethyl acetate 100
5, UV detection)]. Purification of the residue on a silica gel column chromatography (petroleum ether–ethyl acetate 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 94
0 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9o (107.7 mg, 81%) as a yellow viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 1763, 1594, 1493, 1416, 1279, 1199, 1134, 1123, 1051, 913, 814 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.15–7.08 (m, 3H, ArH), 7.06 (d, 2H, J = 8.3 Hz, ArH), 7.01 (d, 1H, J = 1.5 Hz, ArH), 6.98 (d, 1H, J = 8.3 Hz, ArH), 3.33 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.78 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.33 (s, 3H, ArCH3), 2.30 (s, 3H, ArCH3), 1.71 [s, 3H, ArC(CH3)CH2CO] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.9 (s, O–C
6 as the eluent) furnished the lactone 9o (107.7 mg, 81%) as a yellow viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 1763, 1594, 1493, 1416, 1279, 1199, 1134, 1123, 1051, 913, 814 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.15–7.08 (m, 3H, ArH), 7.06 (d, 2H, J = 8.3 Hz, ArH), 7.01 (d, 1H, J = 1.5 Hz, ArH), 6.98 (d, 1H, J = 8.3 Hz, ArH), 3.33 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.78 [d, 1H, J = 15.6 Hz, ArC(CH3)CHaHbCO], 2.33 (s, 3H, ArCH3), 2.30 (s, 3H, ArCH3), 1.71 [s, 3H, ArC(CH3)CH2CO] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.9 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.1 (s, ArC), 141.0 (s, ArC), 136.7 (s, ArC), 134.2 (s, ArC), 130.6 (s, ArC), 129.3 (d, 2C, 2 × ArCH), 129.1 (d, ArCH), 126.9 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 117.0 (d, ArCH), 43.9 (t, CH2CO), 40.7 [s, ArC(CH3)CH2CO], 27.5 [q, ArC(CH3)CH2CO], 20.9 (q, ArCH3), 20.8 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1199.
O), 149.1 (s, ArC), 141.0 (s, ArC), 136.7 (s, ArC), 134.2 (s, ArC), 130.6 (s, ArC), 129.3 (d, 2C, 2 × ArCH), 129.1 (d, ArCH), 126.9 (d, ArCH), 126.0 (d, 2C, 2 × ArCH), 117.0 (d, ArCH), 43.9 (t, CH2CO), 40.7 [s, ArC(CH3)CH2CO], 27.5 [q, ArC(CH3)CH2CO], 20.9 (q, ArCH3), 20.8 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1199.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9p (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2970, 2924, 1762, 1623, 1577, 1446, 1412, 1208, 1192, 1161, 1150, 1061, 814, 759, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.27 (dd, 2H, J = 7.3 and 7.3 Hz, ArH), 7.22–7.10 (m, 4H, ArH), 6.99 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.88 (d, 1H, J = 1.0 Hz, ArH), 3.25 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.81 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.34 (s, 3H, Ar–CH3), 2.25–2.00 (m, 2H, CH2CH3), 0.88 (t, 3H, J = 7.3 Hz, CH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.3 (s, O–C
6 as the eluent) furnished the lactone 9p (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2970, 2924, 1762, 1623, 1577, 1446, 1412, 1208, 1192, 1161, 1150, 1061, 814, 759, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.27 (dd, 2H, J = 7.3 and 7.3 Hz, ArH), 7.22–7.10 (m, 4H, ArH), 6.99 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.88 (d, 1H, J = 1.0 Hz, ArH), 3.25 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.81 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.34 (s, 3H, Ar–CH3), 2.25–2.00 (m, 2H, CH2CH3), 0.88 (t, 3H, J = 7.3 Hz, CH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.3 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.4 (s, ArC), 143.1 (s, ArC), 138.8 (s, ArC), 128.5 (d, 2C, 2 × ArCH), 126.8 (d, ArCH), 126.7 (d, ArCH), 126.6 (d, 2C, 2 × ArCH), 126.0 (s, ArC), 125.0 (d, ArCH), 117.9 (d, ArCH), 44.5 [s, ArC(CH2CO)Et], 39.8 (t, CH2CO), 32.1 (t, CH2CH3), 20.9 (q, ArCH3), 8.8 (q, CH2CH3), ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1200.
O), 151.4 (s, ArC), 143.1 (s, ArC), 138.8 (s, ArC), 128.5 (d, 2C, 2 × ArCH), 126.8 (d, ArCH), 126.7 (d, ArCH), 126.6 (d, 2C, 2 × ArCH), 126.0 (s, ArC), 125.0 (d, ArCH), 117.9 (d, ArCH), 44.5 [s, ArC(CH2CO)Et], 39.8 (t, CH2CO), 32.1 (t, CH2CH3), 20.9 (q, ArCH3), 8.8 (q, CH2CH3), ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1200.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 9q (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2970, 2924, 1764, 1598, 1491, 1259, 1197, 1124, 1060, 922, 823, 734, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.29 (dd, 2H, J = 7.8 and 7.3 Hz, ArH), 7.23–7.14 (m, 3H, ArH), 7.12–7.05 (m, 2H, ArH), 6.96 (d, 1H, J = 8.8 Hz, ArH), 3.25 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.81 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.36 (s, 3H, Ar–CH3), 2.21–2.02 (m, 2H, CH2CH3), 0.90 (t, 3H, J = 7.3 Hz, CH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.4 (s, O–C
6 as the eluent) furnished the lactone 9q (107.5 mg, 85%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2970, 2924, 1764, 1598, 1491, 1259, 1197, 1124, 1060, 922, 823, 734, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.29 (dd, 2H, J = 7.8 and 7.3 Hz, ArH), 7.23–7.14 (m, 3H, ArH), 7.12–7.05 (m, 2H, ArH), 6.96 (d, 1H, J = 8.8 Hz, ArH), 3.25 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.81 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.36 (s, 3H, Ar–CH3), 2.21–2.02 (m, 2H, CH2CH3), 0.90 (t, 3H, J = 7.3 Hz, CH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.4 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.5 (s, ArC), 143.0 (s, ArC), 133.9 (s, ArC), 129.0 (d, ArCH), 128.8 (s, ArC), 128.6 (d, 2C, 2 × ArCH), 127.3 (d, ArCH), 126.9 (d, ArCH), 126.7 (d, 2C, 2 × ArCH), 117.2 (d, ArCH), 44.7 [s, ArC(CH2CO)Et], 39.7 (t, CH2CO), 32.1 (t, CH2CH3), 21.0 (q, Ar–CH3), 8.8 (q, CH2CH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1198.
O), 149.5 (s, ArC), 143.0 (s, ArC), 133.9 (s, ArC), 129.0 (d, ArCH), 128.8 (s, ArC), 128.6 (d, 2C, 2 × ArCH), 127.3 (d, ArCH), 126.9 (d, ArCH), 126.7 (d, 2C, 2 × ArCH), 117.2 (d, ArCH), 44.7 [s, ArC(CH2CO)Et], 39.7 (t, CH2CO), 32.1 (t, CH2CH3), 21.0 (q, Ar–CH3), 8.8 (q, CH2CH3) ppm. HR-MS (ESI+) m/z calculated for [C18H18NaO2]+ = [M + Na]+: 289.1199; found 289.1198.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 11a (54.9 mg, 35%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 3342, 2922, 2852, 1770, 1680, 1640, 1454, 1426, 1256, 1198, 1120, 1068, 1023, 762, 699, 653 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.52 (dd, 2H, J = 8.3 and 1.5 Hz, ArH), 7.42 (dd, 2H, J = 7.8 and 7.3 Hz, ArH), 7.37 (dd, 2H, J = 6.4 and 2.4 Hz, ArH), 7.32 (dd, 2H, J = 7.8 and 1.5 Hz, ArH), 7.24 (ddd, 5H, J = 7.8, 6.4 and 1.5 Hz, ArH), 3.32 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.86 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.78 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.2 (s, O–C
6 as the eluent) furnished the lactone 11a (54.9 mg, 35%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 3342, 2922, 2852, 1770, 1680, 1640, 1454, 1426, 1256, 1198, 1120, 1068, 1023, 762, 699, 653 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.52 (dd, 2H, J = 8.3 and 1.5 Hz, ArH), 7.42 (dd, 2H, J = 7.8 and 7.3 Hz, ArH), 7.37 (dd, 2H, J = 6.4 and 2.4 Hz, ArH), 7.32 (dd, 2H, J = 7.8 and 1.5 Hz, ArH), 7.24 (ddd, 5H, J = 7.8, 6.4 and 1.5 Hz, ArH), 3.32 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.86 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.78 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.2 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 148.0 (s, ArC), 144.0 (s, ArC), 136.6 (s, ArC), 131.5 (s, ArC), 130.8 (s, ArC), 130.4 (d, ArCH), 129.6 (d, 2C, 2 × ArCH), 128.8 (d, 2C, 2 × ArCH), 128.3 (d, 2C, 2 × ArCH), 127.6 (d, ArCH), 127.2 (d, ArCH), 126.3 (d, 2C, 2 × ArCH), 125.9 (s, ArCH), 124.6 (d, ArCH), 43.5 (t, CH2CO), 41.4 [s, ArC(CH2CO)CH3], 27.8 [q, ArC(CH2CO)CH3] ppm. HR-MS (APCI+) m/z calculated for [C22H19O2]+ = [M + H]+: 315.1380; found: 315.1372.
O), 148.0 (s, ArC), 144.0 (s, ArC), 136.6 (s, ArC), 131.5 (s, ArC), 130.8 (s, ArC), 130.4 (d, ArCH), 129.6 (d, 2C, 2 × ArCH), 128.8 (d, 2C, 2 × ArCH), 128.3 (d, 2C, 2 × ArCH), 127.6 (d, ArCH), 127.2 (d, ArCH), 126.3 (d, 2C, 2 × ArCH), 125.9 (s, ArCH), 124.6 (d, ArCH), 43.5 (t, CH2CO), 41.4 [s, ArC(CH2CO)CH3], 27.8 [q, ArC(CH2CO)CH3] ppm. HR-MS (APCI+) m/z calculated for [C22H19O2]+ = [M + H]+: 315.1380; found: 315.1372.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
6, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the lactone 11b (64.5 mg, 37%) as a white solid, and the solid was recrystallized with dichloromethane/hexane, m. p. 172–174 °C. IR (MIR-ATR, 4000–600 cm−1): νmax = 3342, 2922, 2852, 1770, 1680, 1640, 1454, 1426, 1256, 1198, 1120, 1068, 1023, 762, 699, 653 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.50 (ddd, 2H, J = 9.8, 8.8 and 1.5 Hz, ArH), 7.41 (dd, 2H, J = 7.3 and 1.5 Hz, ArH), 7.36 (dd, 2H, J = 6.4 and 2.9 Hz, ArH), 7.31 (dd, 2H, J = 7.8 and 1.5 Hz, ArH), 7.22 (dd, 5H, J = 8.3 and 2.0 Hz, ArH), 3.31 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.85 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.77 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.2 (s, O–C
6 as the eluent) furnished the lactone 11b (64.5 mg, 37%) as a white solid, and the solid was recrystallized with dichloromethane/hexane, m. p. 172–174 °C. IR (MIR-ATR, 4000–600 cm−1): νmax = 3342, 2922, 2852, 1770, 1680, 1640, 1454, 1426, 1256, 1198, 1120, 1068, 1023, 762, 699, 653 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.50 (ddd, 2H, J = 9.8, 8.8 and 1.5 Hz, ArH), 7.41 (dd, 2H, J = 7.3 and 1.5 Hz, ArH), 7.36 (dd, 2H, J = 6.4 and 2.9 Hz, ArH), 7.31 (dd, 2H, J = 7.8 and 1.5 Hz, ArH), 7.22 (dd, 5H, J = 8.3 and 2.0 Hz, ArH), 3.31 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.85 (d, 1H, J = 15.6 Hz, CHaHbCO), 1.77 [s, 3H, ArC(CH2CO)CH3] ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.2 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 147.9 (s, ArC), 143.9 (s, ArC), 136.5 (s, ArC), 131.5 (s, ArC), 130.5 (s, ArC), 130.3 (d, ArCH), 129.6 (d, 2C, 2 × ArCH), 128.7 (d, 2C, 2 × ArCH), 128.2 (d, 2C, 2 × ArCH), 127.5 (d, ArCH), 127.2 (d, ArCH), 126.2 (d, 2C, 2 × ArCH), 125.8 (d, ArCH), 124.5 (d, ArCH), 120.7 (s, ArC), 115.8 (s, ArC), 43.4 (t, CH2CO), 41.4 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3] ppm.
O), 147.9 (s, ArC), 143.9 (s, ArC), 136.5 (s, ArC), 131.5 (s, ArC), 130.5 (s, ArC), 130.3 (d, ArCH), 129.6 (d, 2C, 2 × ArCH), 128.7 (d, 2C, 2 × ArCH), 128.2 (d, 2C, 2 × ArCH), 127.5 (d, ArCH), 127.2 (d, ArCH), 126.2 (d, 2C, 2 × ArCH), 125.8 (d, ArCH), 124.5 (d, ArCH), 120.7 (s, ArC), 115.8 (s, ArC), 43.4 (t, CH2CO), 41.4 [s, ArC(CH2CO)CH3], 27.5 [q, ArC(CH2CO)CH3] ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
8, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the Michael addition ester 12a (94.0 mg, 52%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 3412, 2978, 2926, 1712, 1507, 1490, 1406, 1369, 1322, 1273, 1222, 1157, 1095, 1012, 829, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.50–7.40 (m, 3H, ArH), 7.36 (t, 1H, J = 7.3 Hz, ArH), 7.30–7.13 (m, 6H, ArH), 7.08 (d, 1H, J = 2.4 Hz, ArH), 7.04 (dd, 1H, J = 8.3 and 2.4 Hz, ArH), 6.86 (d, 1H, J = 8.3 Hz, ArH), 5.28 (s, 1H, ArOH), 3.88 (q, 2H, J = 7.3 Hz, OCH2CH3), 3.11 (s, 2H, CH2COOEt), 1.86 [s, 3H, ArC(CH2CO)CH3], 0.97 (t, 3H, J = 7.3 Hz, OCH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 171.4 (s, O–C
6 as the eluent) furnished the Michael addition ester 12a (94.0 mg, 52%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 3412, 2978, 2926, 1712, 1507, 1490, 1406, 1369, 1322, 1273, 1222, 1157, 1095, 1012, 829, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.50–7.40 (m, 3H, ArH), 7.36 (t, 1H, J = 7.3 Hz, ArH), 7.30–7.13 (m, 6H, ArH), 7.08 (d, 1H, J = 2.4 Hz, ArH), 7.04 (dd, 1H, J = 8.3 and 2.4 Hz, ArH), 6.86 (d, 1H, J = 8.3 Hz, ArH), 5.28 (s, 1H, ArOH), 3.88 (q, 2H, J = 7.3 Hz, OCH2CH3), 3.11 (s, 2H, CH2COOEt), 1.86 [s, 3H, ArC(CH2CO)CH3], 0.97 (t, 3H, J = 7.3 Hz, OCH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 171.4 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.6 (s, ArC), 148.5 (s, ArC), 140.5 (s, ArC), 137.4 (s, ArC), 129.1 (d, 2C, 2 × ArCH), 129.0 (d, 2C, 2 × ArCH), 128.9 (d, ArCH), 128.0 (d, ArCH), 127.9 (d, 2C, 2 × ArCH), 127.7 (d, ArCH), 127.4 (s, ArC), 127.0 (d, 2C, 2 × ArCH), 126.0 (d, ArCH), 115.3 (d, ArCH), 60.0 (t, OCH2CH3), 46.8 (t, CH2COOEt), 45.0 (s, ArCCH2COOEt), 28.5 [q, ArC(CH2COOEt)CH3], 13.9 (q, OCH2CH3) ppm. HR-MS (ESI+) m/z calculated for [C24H24NaO3]+ = [M + Na]+: 383.1618; found 383.1620.
O), 150.6 (s, ArC), 148.5 (s, ArC), 140.5 (s, ArC), 137.4 (s, ArC), 129.1 (d, 2C, 2 × ArCH), 129.0 (d, 2C, 2 × ArCH), 128.9 (d, ArCH), 128.0 (d, ArCH), 127.9 (d, 2C, 2 × ArCH), 127.7 (d, ArCH), 127.4 (s, ArC), 127.0 (d, 2C, 2 × ArCH), 126.0 (d, ArCH), 115.3 (d, ArCH), 60.0 (t, OCH2CH3), 46.8 (t, CH2COOEt), 45.0 (s, ArCCH2COOEt), 28.5 [q, ArC(CH2COOEt)CH3], 13.9 (q, OCH2CH3) ppm. HR-MS (ESI+) m/z calculated for [C24H24NaO3]+ = [M + Na]+: 383.1618; found 383.1620.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96
8, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 94
4 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 as the eluent) furnished the Michael addition ester 12b (80.0 mg, 54%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 3429, 2978, 2922, 1712, 1507, 1490, 1464, 1406, 1369, 1273, 1222, 1157, 1095, 1012, 829, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.52–7.30 (m, 5H, ArH), 7.22 (d, 2H, J = 8.8 Hz, ArH), 7.15 (d, 2H, J = 8.8 Hz, ArH), 7.03 (d, 1H, J = 2.4 Hz, ArH), 7.00 (dd, 1H, J = 8.8 and 2.4 Hz, ArH), 6.84 (d, 1H, J = 8.8 Hz, ArH), 5.38 (br. s, 1H, ArOH), 3.88 (q, 2H, J = 7.3 Hz, OCH2CH3), 3.07 (s, 2H, CH2COOEt), 1.83 [s, 3H, ArC(CH2COOEt)CH3], 0.98 (t, 3H, J = 7.3 Hz, OCH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 171.2 (s, O–C
6 as the eluent) furnished the Michael addition ester 12b (80.0 mg, 54%) as a colorless viscous liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 3429, 2978, 2922, 1712, 1507, 1490, 1464, 1406, 1369, 1273, 1222, 1157, 1095, 1012, 829, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.52–7.30 (m, 5H, ArH), 7.22 (d, 2H, J = 8.8 Hz, ArH), 7.15 (d, 2H, J = 8.8 Hz, ArH), 7.03 (d, 1H, J = 2.4 Hz, ArH), 7.00 (dd, 1H, J = 8.8 and 2.4 Hz, ArH), 6.84 (d, 1H, J = 8.8 Hz, ArH), 5.38 (br. s, 1H, ArOH), 3.88 (q, 2H, J = 7.3 Hz, OCH2CH3), 3.07 (s, 2H, CH2COOEt), 1.83 [s, 3H, ArC(CH2COOEt)CH3], 0.98 (t, 3H, J = 7.3 Hz, OCH2CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 171.2 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.8 (s, ArC), 146.9 (s, ArC), 140.1 (s, ArC), 137.2 (s, ArC), 131.8 (s, ArC), 129.1 (d, 2C, 2 × ArCH), 129.0 (d, 2C, 2 × ArCH), 128.8 (d, ArCH), 128.5 (d, 2C, 2 × ArCH), 128.1 (d, 2C, 2 × ArCH), 127.8 (d, 2C, ArCH), 127.6 (s, ArC), 115.5 (d, ArCH), 60.2 (t, OCH2CH3), 46.6 (t, CH2COOEt), 44.7 [s, ArC(CH2COOEt)CH3], 28.5 [q, ArC(CH2COOEt)CH3], 13.9 (q, OCH2CH3) ppm. HR-MS (ESI+) m/z calculated for [C24H23ClNaO3]+ = [M + Na]+: 417.1228; found 417.1229.
O), 150.8 (s, ArC), 146.9 (s, ArC), 140.1 (s, ArC), 137.2 (s, ArC), 131.8 (s, ArC), 129.1 (d, 2C, 2 × ArCH), 129.0 (d, 2C, 2 × ArCH), 128.8 (d, ArCH), 128.5 (d, 2C, 2 × ArCH), 128.1 (d, 2C, 2 × ArCH), 127.8 (d, 2C, ArCH), 127.6 (s, ArC), 115.5 (d, ArCH), 60.2 (t, OCH2CH3), 46.6 (t, CH2COOEt), 44.7 [s, ArC(CH2COOEt)CH3], 28.5 [q, ArC(CH2COOEt)CH3], 13.9 (q, OCH2CH3) ppm. HR-MS (ESI+) m/z calculated for [C24H23ClNaO3]+ = [M + Na]+: 417.1228; found 417.1229.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97
05, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 95
3 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 as the eluent) furnished the spiro-lactone 13a (52.8 mg, 40%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1774, 1484, 1449, 1252, 1197, 1068, 920 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.30–7.12 (m, 4H, ArH), 7.10 (dd, 1H, J = 8.3 and 1.0 Hz, ArH), 7.05 (d, 1H, J = 7.8 Hz, ArH), 6.98 (ddd, 1H, J = 7.8, 7.3 and 1.5 Hz, ArH), 6.64 (dd, 1H, J = 7.8 and 1.5 Hz, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.95 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.88 (dd, 2H, J = 7.8 and 5.4 Hz, ArCH2CH2CH2), 1.95 (dd, 2H, J = 5.9 and 5.4 Hz, ArCH2CH2CH2), 1.87–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C
5 as the eluent) furnished the spiro-lactone 13a (52.8 mg, 40%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1774, 1484, 1449, 1252, 1197, 1068, 920 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.30–7.12 (m, 4H, ArH), 7.10 (dd, 1H, J = 8.3 and 1.0 Hz, ArH), 7.05 (d, 1H, J = 7.8 Hz, ArH), 6.98 (ddd, 1H, J = 7.8, 7.3 and 1.5 Hz, ArH), 6.64 (dd, 1H, J = 7.8 and 1.5 Hz, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.95 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.88 (dd, 2H, J = 7.8 and 5.4 Hz, ArCH2CH2CH2), 1.95 (dd, 2H, J = 5.9 and 5.4 Hz, ArCH2CH2CH2), 1.87–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.8 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.8 (s, ArC), 138.4 (s, ArC), 137.9 (s, ArC), 132.4 (s, ArC), 129.6 (d, ArCH), 128.6 (d, ArCH), 128.3 (d, ArCH), 128.1 (d, ArCH), 127.1 (d, ArCH), 126.5 (d, ArCH), 124.3 (d, ArCH), 117.0 (d, ArCH), 43.4 (t, CH2CO), 41.2 (s, ArCCH2CO), 36.0 (t, CH2), 30.0 (t, CH2), 18.6 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C18H17O2]+ = [M + H]+: 265.1223; found 265.1216.
O), 150.8 (s, ArC), 138.4 (s, ArC), 137.9 (s, ArC), 132.4 (s, ArC), 129.6 (d, ArCH), 128.6 (d, ArCH), 128.3 (d, ArCH), 128.1 (d, ArCH), 127.1 (d, ArCH), 126.5 (d, ArCH), 124.3 (d, ArCH), 117.0 (d, ArCH), 43.4 (t, CH2CO), 41.2 (s, ArCCH2CO), 36.0 (t, CH2), 30.0 (t, CH2), 18.6 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C18H17O2]+ = [M + H]+: 265.1223; found 265.1216.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97
5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 95
3 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 as the eluent) furnished the lactone 13b (86.2 mg, 62%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2920, 2851, 1769, 1578, 1490, 1448, 1163, 1065, 759 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.25–7.09 (m, 3H, ArH), 7.05 (d, 1H, J = 7.8 Hz, ArH), 6.91 (d, 1H, J = 1.0 Hz, ArH), 6.80 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.52 (d, 1H, J = 7.8 Hz, ArH), 3.17 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.93 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.87 (dd, 2H, J = 7.8 and 5.4 Hz, ArCH2CH2CH2), 2.32 (s, 3H, ArCH3), 1.92 (dd, 2H, J = 6.4 and 5.4 Hz, ArCH2CH2CH2), 1.85–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.0 (s, O–C
5 as the eluent) furnished the lactone 13b (86.2 mg, 62%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2920, 2851, 1769, 1578, 1490, 1448, 1163, 1065, 759 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.25–7.09 (m, 3H, ArH), 7.05 (d, 1H, J = 7.8 Hz, ArH), 6.91 (d, 1H, J = 1.0 Hz, ArH), 6.80 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.52 (d, 1H, J = 7.8 Hz, ArH), 3.17 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.93 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.87 (dd, 2H, J = 7.8 and 5.4 Hz, ArCH2CH2CH2), 2.32 (s, 3H, ArCH3), 1.92 (dd, 2H, J = 6.4 and 5.4 Hz, ArCH2CH2CH2), 1.85–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.0 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.7 (s, ArC), 138.5 (s, ArC), 138.4 (s, ArC), 138.1 (s, ArC), 129.5 (d, ArCH), 129.3 (s, ArC), 128.3 (d, ArCH), 128.1 (d, ArCH), 127.0 (d, ArCH), 126.4 (d, ArCH), 125.0 (d, ArCH), 117.4 (d, ArCH), 43.5 (t, CH2CO), 40.9 (s, ArCCH2CO), 36.1 (t, CH2), 30.0 (t, CH2), 20.9 (q, ArCH3), 18.6 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C19H19O2]+ = [M + H]+: 279.1380; found 279.1371.
O), 150.7 (s, ArC), 138.5 (s, ArC), 138.4 (s, ArC), 138.1 (s, ArC), 129.5 (d, ArCH), 129.3 (s, ArC), 128.3 (d, ArCH), 128.1 (d, ArCH), 127.0 (d, ArCH), 126.4 (d, ArCH), 125.0 (d, ArCH), 117.4 (d, ArCH), 43.5 (t, CH2CO), 40.9 (s, ArCCH2CO), 36.1 (t, CH2), 30.0 (t, CH2), 20.9 (q, ArCH3), 18.6 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C19H19O2]+ = [M + H]+: 279.1380; found 279.1371.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97
5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 95
3 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 as the eluent) furnished the lactone 13c (61.2 mg, 44%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2851, 1766, 1578, 1446, 1202, 1162, 962, 729 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.25–7.10 (m, 3H, ArH), 7.05 (d, 1H, J = 7.3 Hz, ArH), 7.03 (dd, 1H, J = 8.3 and 1.5 Hz, ArH), 6.98 (d, 1H, J = 8.3 Hz, ArH), 6.43 (d, 1H, J = 1.5 Hz, ArH), 3.15 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.92 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.91–2.80 (m, 2H, ArCH2CH2CH2), 2.18 (s, 3H, ArCH3), 1.93 (dd, 2H, J = 5.9 and 5.4 Hz, ArCH2CH2CH2), 1.87–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.9 (s, O–C
5 as the eluent) furnished the lactone 13c (61.2 mg, 44%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2851, 1766, 1578, 1446, 1202, 1162, 962, 729 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.25–7.10 (m, 3H, ArH), 7.05 (d, 1H, J = 7.3 Hz, ArH), 7.03 (dd, 1H, J = 8.3 and 1.5 Hz, ArH), 6.98 (d, 1H, J = 8.3 Hz, ArH), 6.43 (d, 1H, J = 1.5 Hz, ArH), 3.15 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.92 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.91–2.80 (m, 2H, ArCH2CH2CH2), 2.18 (s, 3H, ArCH3), 1.93 (dd, 2H, J = 5.9 and 5.4 Hz, ArCH2CH2CH2), 1.87–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.9 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 148.9 (s, ArC), 138.4 (s, ArC), 138.1 (s, ArC), 133.9 (s, ArC), 132.1 (s, ArC), 129.6 (d, ArCH), 128.8 (d, ArCH), 128.7 (d, ArCH), 128.2 (d, ArCH), 127.0 (d, ArCH), 126.5 (d, ArCH), 116.8 (d, ArCH), 43.6 (t, CH2CO), 41.3 (s, ArCCH2CO), 36.1 (t, CH2), 30.0 (t, CH2), 20.8 (q, ArCH3), 18.7 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C19H19O2]+ = [M + H]+: 279.1380; found 279.1369.
O), 148.9 (s, ArC), 138.4 (s, ArC), 138.1 (s, ArC), 133.9 (s, ArC), 132.1 (s, ArC), 129.6 (d, ArCH), 128.8 (d, ArCH), 128.7 (d, ArCH), 128.2 (d, ArCH), 127.0 (d, ArCH), 126.5 (d, ArCH), 116.8 (d, ArCH), 43.6 (t, CH2CO), 41.3 (s, ArCCH2CO), 36.1 (t, CH2), 30.0 (t, CH2), 20.8 (q, ArCH3), 18.7 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C19H19O2]+ = [M + H]+: 279.1380; found 279.1369.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97
5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 95
3 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 as the eluent) furnished the lactone 13d (54.2 mg, 39%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2851, 1773, 1462, 1243, 1192, 1085, 920 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.25–7.12 (m, 3H, ArH), 7.08 (d, 1H, J = 7.3 Hz, ArH), 7.05 (d, 1H, J = 7.8 Hz, ArH), 6.87 (dd, 1H, J = 7.8 and 7.3 Hz, ArH), 6.45 (d, 1H, J = 7.8 Hz, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.92 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.87 (dd, 2H, J = 7.3 and 5.9 Hz, ArCH2CH2CH2), 2.35 (s, 3H, ArCH3), 2.05–1.90 (m, 2H, ArCH2CH2CH2), 1.85–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.9 (s, O–C
5 as the eluent) furnished the lactone 13d (54.2 mg, 39%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2851, 1773, 1462, 1243, 1192, 1085, 920 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.25–7.12 (m, 3H, ArH), 7.08 (d, 1H, J = 7.3 Hz, ArH), 7.05 (d, 1H, J = 7.8 Hz, ArH), 6.87 (dd, 1H, J = 7.8 and 7.3 Hz, ArH), 6.45 (d, 1H, J = 7.8 Hz, ArH), 3.20 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.92 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.87 (dd, 2H, J = 7.3 and 5.9 Hz, ArCH2CH2CH2), 2.35 (s, 3H, ArCH3), 2.05–1.90 (m, 2H, ArCH2CH2CH2), 1.85–1.65 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 167.9 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.1 (s, ArC), 138.4 (s, ArC), 138.2 (s, ArC), 132.2 (s, ArC), 129.8 (d, ArCH), 129.6 (d, ArCH), 128.1 (d, ArCH), 127.0 (d, ArCH), 126.4 (d, ArCH), 126.2 (1s and 1d, 2C, ArC and ArCH), 123.6 (d, ArCH), 43.3 (t, CH2CO), 41.2 (s, ArCCH2CO), 35.8 (t, CH2), 30.0 (t, CH2), 18.6 (t, CH2), 15.9 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C19H19O2]+ = [M + H]+: 279.1380; found 279.1362.
O), 149.1 (s, ArC), 138.4 (s, ArC), 138.2 (s, ArC), 132.2 (s, ArC), 129.8 (d, ArCH), 129.6 (d, ArCH), 128.1 (d, ArCH), 127.0 (d, ArCH), 126.4 (d, ArCH), 126.2 (1s and 1d, 2C, ArC and ArCH), 123.6 (d, ArCH), 43.3 (t, CH2CO), 41.2 (s, ArCCH2CO), 35.8 (t, CH2), 30.0 (t, CH2), 18.6 (t, CH2), 15.9 (q, ArCH3) ppm. HR-MS (ESI+) m/z calculated for [C19H19O2]+ = [M + H]+: 279.1380; found 279.1362.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97
5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 95
3 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 as the eluent) furnished the lactone 13e (80.7 mg, 51%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2917, 2849, 1773, 1600, 1461, 1210, 1174, 1025, 812 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.79 (d, 1H, J = 9.3 Hz, ArH), 7.78 (d, 1H, J = 8.3 Hz, ArH), 7.28 (ddd, 1H, J = 8.3, 7.8 and 1.5 Hz, ArH), 7.27 (d, 1H, J = 9.3 Hz, ArH), 7.22 (d, 1H, J = 7.8 Hz, ArH), 7.15 (ddd, 1H, J = 7.8, 7.8 and 1.5 Hz, ArH), 7.08 (ddd, 1H, J = 8.8, 8.3 and 1.5 Hz, ArH), 7.05 (d, 1H, J = 8.8 Hz, ArH), 6.95 (dd, 1H, J = 8.3 and 7.8 Hz, ArH), 6.77 (d, 1H, J = 7.8 Hz, ArH), 3.31 (d, 1H, J = 15.6 Hz, CHaHbCO), 3.13–2.92 (m, 2H, ArCH2CH2CH2), 2.98 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.35–2.20 (m, 1H, CHaHb), 2.16–1.90 (m, 3H, CHaHb and CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 166.7 (s, O–C
5 as the eluent) furnished the lactone 13e (80.7 mg, 51%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2917, 2849, 1773, 1600, 1461, 1210, 1174, 1025, 812 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.79 (d, 1H, J = 9.3 Hz, ArH), 7.78 (d, 1H, J = 8.3 Hz, ArH), 7.28 (ddd, 1H, J = 8.3, 7.8 and 1.5 Hz, ArH), 7.27 (d, 1H, J = 9.3 Hz, ArH), 7.22 (d, 1H, J = 7.8 Hz, ArH), 7.15 (ddd, 1H, J = 7.8, 7.8 and 1.5 Hz, ArH), 7.08 (ddd, 1H, J = 8.8, 8.3 and 1.5 Hz, ArH), 7.05 (d, 1H, J = 8.8 Hz, ArH), 6.95 (dd, 1H, J = 8.3 and 7.8 Hz, ArH), 6.77 (d, 1H, J = 7.8 Hz, ArH), 3.31 (d, 1H, J = 15.6 Hz, CHaHbCO), 3.13–2.92 (m, 2H, ArCH2CH2CH2), 2.98 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.35–2.20 (m, 1H, CHaHb), 2.16–1.90 (m, 3H, CHaHb and CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 166.7 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.1 (s, ArC), 141.8 (s, ArC), 136.0 (s, ArC), 132.1 (s, ArC), 130.1 (d, ArCH), 130.0 (s, ArC), 129.5 (d, ArCH), 128.9 (d, ArCH), 128.3 (d, ArCH), 126.9 (d, ArCH), 126.7 (d, ArCH), 125.8 (d, ArCH), 125.6 (d, ArCH), 124.3 (d, ArCH), 123.5 (s, ArC), 117.7 (d, ArCH), 44.5 (t, CH2CO), 42.4 (s, ArCCH2CO), 33.4 (t, CH2), 29.8 (t, CH2), 18.8 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C22H19O2]+ = [M + H]+: 315.1380; found 315.1369.
O), 150.1 (s, ArC), 141.8 (s, ArC), 136.0 (s, ArC), 132.1 (s, ArC), 130.1 (d, ArCH), 130.0 (s, ArC), 129.5 (d, ArCH), 128.9 (d, ArCH), 128.3 (d, ArCH), 126.9 (d, ArCH), 126.7 (d, ArCH), 125.8 (d, ArCH), 125.6 (d, ArCH), 124.3 (d, ArCH), 123.5 (s, ArC), 117.7 (d, ArCH), 44.5 (t, CH2CO), 42.4 (s, ArCCH2CO), 33.4 (t, CH2), 29.8 (t, CH2), 18.8 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C22H19O2]+ = [M + H]+: 315.1380; found 315.1369.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97
5, UV detection)]. Purification of the residue on a silica gel column (petroleum ether–ethyl acetate 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 95
3 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 as the eluent) furnished the lactone 13f (62.8 mg, 43%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2853, 1773, 1621, 1503, 1452, 1416, 1212, 1167, 963, 814 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.07 (d, 1H, J = 7.8 Hz, ArH), 7.01 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.91 (d, 1H, J = 1.0 Hz, ArH), 6.85 (s, 1H, ArH), 6.80 (d, 1H, J = 7.8 Hz, ArH), 6.52 (d, 1H, J = 7.8 Hz, ArH), 3.18 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.91 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.82 (dd, 2H, J = 5.9 and 5.4 Hz, ArCH2CH2CH2), 2.32 (s, 3H, ArCH3), 2.23 (s, 3H, ArCH3), 1.89 (dd, 2H, J = 6.3 and 5.4 Hz, ArCH2CH2CH2), 1.84–1.62 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.1 (s, O–C
5 as the eluent) furnished the lactone 13f (62.8 mg, 43%) as a liquid. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2853, 1773, 1621, 1503, 1452, 1416, 1212, 1167, 963, 814 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.07 (d, 1H, J = 7.8 Hz, ArH), 7.01 (dd, 1H, J = 7.8 and 1.0 Hz, ArH), 6.91 (d, 1H, J = 1.0 Hz, ArH), 6.85 (s, 1H, ArH), 6.80 (d, 1H, J = 7.8 Hz, ArH), 6.52 (d, 1H, J = 7.8 Hz, ArH), 3.18 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.91 (d, 1H, J = 15.6 Hz, CHaHbCO), 2.82 (dd, 2H, J = 5.9 and 5.4 Hz, ArCH2CH2CH2), 2.32 (s, 3H, ArCH3), 2.23 (s, 3H, ArCH3), 1.89 (dd, 2H, J = 6.3 and 5.4 Hz, ArCH2CH2CH2), 1.84–1.62 (m, 2H, ArCH2CH2CH2) ppm. 13C NMR (CDCl3, 100 MHz): δ = 168.1 (s, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.7 (s, ArC), 138.5 (s, ArC), 137.8 (s, ArC), 135.9 (s, ArC), 135.3 (s, ArC), 129.5 (s, ArC), 129.4 (d, ArCH), 128.4 (d, ArCH), 128.3 (d, ArCH), 128.0 (d, ArCH), 125.0 (d, ArCH), 117.3 (d, ArCH), 43.5 (t, CH2CO), 40.9 (s, ArCCH2CO), 36.2 (t, CH2), 29.6 (t, CH2), 21.1 (q, ArCH3), 20.9 (q, ArCH3), 18.7 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C20H21O2]+ = [M + H]+: 293.1536; found 293.1525.
O), 150.7 (s, ArC), 138.5 (s, ArC), 137.8 (s, ArC), 135.9 (s, ArC), 135.3 (s, ArC), 129.5 (s, ArC), 129.4 (d, ArCH), 128.4 (d, ArCH), 128.3 (d, ArCH), 128.0 (d, ArCH), 125.0 (d, ArCH), 117.3 (d, ArCH), 43.5 (t, CH2CO), 40.9 (s, ArCCH2CO), 36.2 (t, CH2), 29.6 (t, CH2), 21.1 (q, ArCH3), 20.9 (q, ArCH3), 18.7 (t, CH2) ppm. HR-MS (ESI+) m/z calculated for [C20H21O2]+ = [M + H]+: 293.1536; found 293.1525.
Acknowledgements
Financial support by the Council of Scientific and Industrial Research [(CSIR), 02(0018)/11/EMR-II], New Delhi is gratefully acknowledged. P.N thanks CSIR [(CSIR), 02(0018)/11/EMR-II] New Delhi for the financial support. B.V.R. thanks CSIR for the award of a fellowship.Notes and references
- (a) J. Staunton, in Comprehensive Organic Chemistry, ed. P. G. Sammes, Pergamon, Oxford, 1979, vol. 4, p. 651 Search PubMed; (b) U. Matern, P. Lüer and D. Kreusch, in Comprehensive Natural Products Chemistry, ed. U. Sankawa, Pergamon, Oxford, 1999, vol. 1, p. 623 Search PubMed.
- X. Zhang, H. Wang, Y. Song, L. Nie, L. Wang, B. Liu, P. Shen and Y. Liua, Bioorg. Med. Chem. Lett., 2006, 16, 949 CrossRef CAS PubMed.
- M. Takechi, Y. Tanaka, M. Takehara, G.-I. Nonaka and I. Nishioka, Phytochemistry, 1985, 24, 2245 CrossRef CAS.
- M. Iinuma, T. Tanaka, M. Mizuno, T. Katsuzaki and H. Ogawa, Chem. Pharm. Bull., 1989, 37, 1813 CrossRef CAS.
- F. L. Hsu, G.-I. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1985, 33, 3142 CrossRef CAS.
- (a) T. Yoshida, G. Ohbayashi, K. Ishihara, W. Ohwashi, K. Haba, Y. Okano, T. Shingu and T. Okuda, Chem. Pharm. Bull., 1991, 39, 2233 CrossRef CAS; (b) T. Okuda, T. Hatano and K. Yakazi, Chem. Pharm. Bull., 1983, 31, 333 CrossRef CAS; (c) R. Saijio, G.-I. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1989, 37, 2063 CrossRef; (d) M. Iinuma, T. Tanaka and F. Asai, Phytochemistry, 1994, 36, 941 CrossRef CAS; (e) M. Iinuma, T. Tanaka, M. Mizuno, T. Katsuzaki and H. Ogawa, Chem. Pharm. Bull., 1989, 37, 1813 CrossRef CAS; (f) F.-L. Hsu, G.-I. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1985, 33, 3142 CrossRef CAS; (g) G.-I. Nonaka, O. Kawahara and I. Nishioka, Chem. Pharm. Bull., 1982, 30, 4277 CrossRef CAS.
- M. A. McGuire, S. C. Shilcrat and E. Sorenson, Tetrahedron Lett., 1999, 40, 3293 CrossRef CAS.
- G. Chen, N. Tokunaga and T. Hayashi, Org. Lett., 2005, 7, 2285 CrossRef CAS PubMed.
- K. A. De Castro, J. Ko, D. Park, S. Park and H. Rhee, Org. Process Res. Dev., 2007, 11, 918 CrossRef CAS.
- (a) G. Speranza, A. Di Meo, S. Zanzola, G. Fontana and P. Mannito, Synthesis, 1997, 931 CrossRef CAS PubMed; (b) M. Z. B. Bezerra, I. L. Machado, S. M. De Morais and R. Braz-Filho, J. Braz. Chem. Soc., 1997, 8, 229 CrossRef CAS PubMed.
- W. H. Santos and L. C. Silva-Filho, Synthesis, 2012, 3361 Search PubMed.
- X.-F. Zhang, L. Xie, Y. Liu, J.-F. Xiang, L. Li and Y.-L. Tang, J. Mol. Struct., 2008, 888, 145 CrossRef CAS PubMed.
- X.-M. Li, M. Lin, Y.-H. Wang and X. Liu, Planta Med., 2004, 70, 160 CrossRef CAS PubMed.
- (a) L. Dhooghe, S. Maregesi, I. Mincheva, D. Ferreira, J. P. J. Marais, F. Lemière, A. Matheeussen, P. Cos, L. Maes, A. Vlietinck, S. Apers and L. Pieters, Phytochemistry, 2010, 71, 785 CrossRef CAS PubMed; (b) J. Hokkanen, S. Mattila, L. Jaakola, A. M. Pirttila and A. Tolonen, J. Agric. Food Chem., 2009, 57, 9437 CrossRef CAS PubMed; (c) N. Tabanca, R. S. Pawar, D. Ferreira, J. P. J. Marais, S. I. Khan, V. Joshi, D. E. Wedge and I. A. Khan, Planta Med., 2007, 73, 1107 CrossRef CAS PubMed; (d) X.-F. Zhang, H.-M. Wang, Y.-L. Song, L.-H. Nie, L.-F. Wang, B. Liu, P. Shen and Y. Liu, Bioorg. Med. Chem. Lett., 2006, 16, 949 CrossRef CAS PubMed; (e) C.-S. Yao, M. Lin and L. Wang, Chem. Pharm. Bull., 2006, 54, 1053 CrossRef CAS.
- K. Vosmann, P. Wittkamp and N. Weber, J. Agric. Food Chem., 2006, 54, 2969 CrossRef CAS PubMed and references cited therein.
- (a) R. Noyori, Asymmetric Catalysis in Organic Synthesis, John Wiley and Sons, New York, 1994 Search PubMed; (b) M. A. McGuire, S. C. Shilcrat and E. Sorenson, Tetrahedron Lett., 1999, 40, 3293 CrossRef CAS; (c) J. O. Park and S. W. Youn, Org. Lett., 2010, 12, 2258 CrossRef CAS PubMed.
- (a) C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2001, 34, 633 CrossRef CAS PubMed; (b) V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731 CrossRef CAS PubMed; (c) K. Li, L. N. Foresee and J. A. Tunge, J. Org. Chem., 2005, 70, 2881 CrossRef CAS PubMed; (d) A. R. Jagdale and A. Sudalai, Tetrahedron Lett., 2007, 48, 4895 CrossRef CAS PubMed; (e) S. Aoki, C. Amamoto, J. Oyamada and T. Kitamura, Tetrahedron, 2005, 61, 9291 CrossRef CAS PubMed.
- (a) E. Fillion, A. M. Dumas, B. A. Kuropatwa, N. R. Malhotra and T. C. Sitler, J. Org. Chem., 2006, 71, 409 CrossRef CAS PubMed; (b) C. E. Rodrigues-Santos and A. Echevarria, Tetrahedron Lett., 2007, 48, 4505 CrossRef CAS PubMed; (c) S. Duan, R. Jana and J. A. Tunge, J. Org. Chem., 2009, 74, 4612 CrossRef CAS PubMed.
- (a) Y. Gu and K. Xue, Tetrahedron Lett., 2010, 51, 192 CrossRef CAS PubMed; (b) A. Kumar, P. Kumar, V. D. Tripathi and S. Srivastava, RSC Adv., 2012, 2, 11641 RSC; (c) M. C. Laufer, H. Hausmann and W. F. Holderich, J. Catal., 2003, 218, 315 CrossRef CAS; (d) E. Tang, W. Li, Z. Y. Gao and X. Gu, Chin. Chem. Lett., 2012, 23, 631 CrossRef CAS PubMed; (e) D. P. Kamat, S. G. Tilve and V. P. Kamat, Tetrahedron Lett., 2012, 53, 4469 CrossRef CAS PubMed; (f) C. R. Reddy, B. Srikanth, N. N. Rao and D.-S. Shin, Tetrahedron, 2008, 64, 11666 CrossRef CAS PubMed.
- E. Fillion, A. M. Dumas, B. A. Kuropatwa, N. R. Malhotra and T. C. Sitler, J. Org. Chem., 2006, 71, 409 CrossRef CAS PubMed.
- (a) H. Poras, E. Stephan, G. Pourcelot and P. Cresson, Chem. Ind., 1993, 206 CAS; (b) A. Patra and S. K. Misra, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1990, 29, 66 Search PubMed; (c) A. I. Scott, P. A. Dodsox, F. McCapra and M. B. Meyers, J. Am. Chem. Soc., 1963, 85, 3702 CrossRef CAS.
- Z. Zhang, Y. Ma and Y. Zhao, Synlett, 2008, 1091 CAS.
- B. V. Ramulu, A. G. K. Reddy and G. Satyanarayana, Synlett, 2013, 863 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR data of the known compounds and the copies of 1H and 13C-NMR spectra are provided. See DOI: 10.1039/c4ob00490f |
| This journal is © The Royal Society of Chemistry 2014 |