N-Bromosuccinimide as an oxidant for the transition-metal-free synthesis of 2-aminobenzoxazoles from benzoxazoles and secondary amines†
Abstract
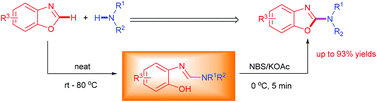
A facile and transition-metal-free method was developed through merging the ring opening of benzoxazoles with secondary amines and N-bromosuccinimide (NBS) mediated oxidative cyclization toward the synthesis of 2-aminobenzoxazoles. NBS was selected as a powerful oxidant in the oxidative cyclization of ring-opening amidines to provide the desirable 2-aminobenzoxazoles in excellent yields (up to 94%).

 Please wait while we load your content...
Please wait while we load your content...