K. Mahender Reddy, J. Shashidhar and Subhash Ghosh
Org. Biomol. Chem., 2014,12, 4002-4012
DOI:
10.1039/C4OB00250D,
Paper
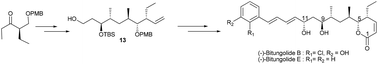
The first total synthesis of (−)-bitungolide B and a second-generation total synthesis of (−)-bitungolide E are described. The cornerstone of the approach comprises a convergent and flexible route involving Brown crotylation, highly diastereoselective substrate controlled Paterson anti-aldol reaction, hydroxyl-directed 1,3-syn/anti reduction, Barton–McCombie deoxygenation and RCM reactions. Via this route, a common intermediate 13 is readily accessible for the synthesis of the family of bitungolides A–E and franklinolides A–C.