 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cooperative hydrolysis of aryl esters on functionalized membrane surfaces and in micellar solutions†
M.
Poznik
and
B.
König
Institut für Organische Chemie, Universität Regensburg, Universitätsstraße 31, D-93053 Regensburg, Germany. E-mail: burkhard.koenig@chemie.uni-regensburg.de; Fax: +49 941 943 1717; Tel: +49 941 943 4575
First published on 14th March 2014
Abstract
Catalytic hydrolysis of peptides, proteins, phosphates or carboxylate esters in nature is catalysed by enzymes, which are efficient, fast and selective. Most of the hydrolytic chemical catalysts published so far mimic the active site of enzymes and contain metal complexes and amino acid residues. Their synthesis can be laborious, while the hydrolytic activity is still limited compared to enzymes. We present an approach that uses fluid membranes of vesicles and micelles as a support for amphiphilic additives, which cooperatively cleave aryl ester bonds. The membrane anchored bis-Zn(II)-complex 1 is hydrolytically active and hydrolyses fluorescein diacetate (FDA) with a second order rate constant (k2) of 0.9 M−1 s−1. The hydrolytic activity is modulated by co-embedded membrane additives, bearing common amino acid side chain functional groups. With this approach, the hydrolytic activity of the system is enhanced up to 16 fold in comparison with cyclen 1 (k2 = 14.7 M−1 s−1). DOPC and DSPC lipids form at room temperature fluid or gel phase membranes, respectively. Omitting the lipid, micellar solutions were obtained with hydrolytic activity reaching k2 = 13.4 M−1 s−1. It is shown that cooperative hydrolysis is favoured in fluid membranes and micelles, allowing the active moieties to arrange freely. The embedding and dynamic self-assembly of membrane active components in fluid membranes and micelles provide facile access to hydrolytically active soft interfaces.
Introduction
Carboxyesters are ubiquitously found in nature. They are involved in many biological processes and are present in everyday life. Esters of drugs are better transported through membranes with respect to free drugs and cleaved inside cells by esterases. This is often used as a pro-drug concept in pharmaceutical sciences.1 Peptides, proteins, phosphates and carboxy esters, respectively, are catalytically hydrolysed by natural enzymes and many attempts to design artificial hydrolysing enzymes have been reported over time, but their efficiency is still limited when compared to the natural models.2,3 Most hydrolases contain metal ions such as zinc(II) (carboxypeptidase A, astacin) in their active centres.4 The stabilisation of the transition state during hydrolysis occurs by hydrogen bonding of the ester group to amino acid residues of the enzyme protein backbone. Histidine, tyrosine and glutamine often participate in the activation of the substrate. The crucial properties of the enzyme active centre are the appropriate distance and geometry of all participating groups, their dynamics during the reaction, the microenvironment created by the folded enzyme and the ability to bind the substrate. Combining all such features in one organic molecule is challenging or even impossible and covalent enzyme models may be incapable of adjusting the conformation for an efficient hydrolysis.2,5,6To investigate an alternative approach, we used functionalized membrane surfaces of vesicles and micelles for the cooperative hydrolysis of esters (Fig. 1) with zinc complex 1 (Scheme 1) as a hydrolytically active metal center. This complex is known to coordinate water molecules and due to its Lewis acidity it produces hydroxide anions used for hydrolysis reactions at neutral pH.7,8 The reaction rate was found to increase dramatically once complex 1 was embedded in a lipid membrane or in micelles.9 Embedded in membranes, complex 1 may also serve as a ligand for the molecular recognition of phosphate anions.10
The effect of micellar catalysis of surfactant solutions has been studied and described in detail in the literature.11,12 It was discovered that positively charged surfactants such as tetraammonium salts, amines, etc. embedded in micelles cleave p-nitrophenol esters in buffered solutions at pH 7 with a high efficiency.13–15 This effect is most likely caused by the high local concentration of the polar functional groups on the surface. Metallomicelles were also intensively studied for their increased hydrolytic activity with respect to a homogeneous solution.16,17
The proposed cooperative catalytic effect by combining embedded metal complexes and membrane additives on the surface of a vesicular membrane is depicted in Fig. 1. Soft interfaces are important for many biological processes and have found use also in artificial systems.18 A potential advantage of using a 2D functionalized membrane for hydrolysis is the non-covalent assembly of the components in a two dimensional fashion. In the fluid membrane of a vesicle, the hydrolytic active components may statistically arrange in distances optimal for hydrolysis. This concept offers easy and facile testing of different combinations of components and their possible cooperative action. Micellar solutions of cyclen 1 and membrane additives were also examined for their hydrolytic activity. However, an advantage of the vesicular systems is that their structure is better defined and the effects of different membrane additives can be compared more easily.
The membrane active amphiphilic molecules are, most likely, not randomly distributed in the membrane, but form clusters and patches. Complete phase separation or limited mobility in the membrane might inhibit the cooperative action (Fig. 2).19
To support this concept a well-described FRET pair of two amphiphilic fluorescent dyes was co-embedded in membranes (Fig. 3).20 The amphiphilic dye TAMRA exhibits in close proximity to amphiphilic carboxyfluorescein CF an emission at 580 nm after FRET, if irradiated at 495 nm. Both amphiphilic dyes were embedded in DOPC and DSPC membranes, respectively (Scheme 1). The unsaturated lipid DOPC forms a fluid membrane at room temperature due to its transition temperature of −20 °C. DSPC yields a rigid gel phase membrane at room temperature (transition temp. 55 °C). The FRET is observed only in the fluid membrane of DOPC lipids, which indicates partially mixed patch formation under these conditions (Fig. 3).
 | ||
| Fig. 3 Emission spectra of a CF (0.025 mM) and TAMRA (0.025 mM) mixture in various lipids (λexc = 495 nm) and the mechanism of FRET. | ||
Membrane additives (Scheme 2) were designed to support the hydrolysis reaction resembling functional groups that are present in the active centers of hydrolytic enzymes, e.g. imidazole, phenol, acids or amides. Micellar hydrolytic activity has been reported previously for amines, oximes and histidine.21,22 The series was extended to cover a larger range of acidic, neutral, basic and cationic functional groups. Amphiphiles are grouped in Scheme 2 according to increasing pKa values of the corresponding non-amphiphilic molecules (see ESI† for reported literature values).
 | ||
| Scheme 2 Membrane additives investigated for cooperative hydrolysis in their expected protonation state at pH 7.4, grouped by pKa values of the functional group. | ||
Results and discussion
Vesicular hydrolysis
The hydrolytic activity was monitored using fluorescein diacetate (FDA, Scheme 1), a substrate for membrane esterases, often used for evaluating the viability of cell samples.23,24 FDA is a non-fluorescent diaryl ester whose fluorescence is triggered by the cleavage of its ester groups. The often used p-nitrophenol esters are more labile towards hydrolysis and their photometric determination is less sensitive than the measurement of fluorescence.All measurements were performed under pseudo first-order-reaction conditions and the rate constant of hydrolysis (kobs) was derived from the slope of the increased fluorescein concentration over 20 min (s−1) and from the initial FDA concentration. The concentration of the released fluorescein was determined using a recorded calibration curve in vesicular solutions not exceeding its linear region (see ESI†). All reported membrane additives were tested in vesicular membranes prepared by sonication above their transition temperature from both DOPC and DSPC in the presence of bis-Zn-cyclen 1. To properly examine the hydrolysis, the best composition of the membrane was found to be 5 mol% of 1, 10 mol% of the respective membrane additive and 85 mol% of phospholipid. Hydrolytic activity was determined also for vesicles containing only the zinc cyclen complex 1 (5 mol%) or exclusively non-metal ion containing membrane additives (10 mol%). The measurements indicate which species is mainly responsible for the hydrolysis. All data are summarized in Table 1 (values sorted according to Scheme 2). Bis-Zn-cyclen 1 embedded in the membrane showed a higher activity towards hydrolysis than a non-amphiphilic solution of 1 (data not shown, see ESI†), which confirms previous observations for bis(4-nitrophenyl) phosphate.9 Non-amphiphilic models of membrane additives were also tested in hydrolysis, but were found inactive (see ESI†). We found that 1 is much more active in rigid DSPC- than in fluid DOPC-membranes. Compounds A9, A10, A11, A13, A14 and A15, respectively, exhibit high rates of hydrolysis in the absence of 1 in the membrane, often exceeding the rate of sole cyclen 1. These compounds have functional groups that have been previously reported to be hydrolytically active in micellar solutions.
| DOPC | DSPC | |||
|---|---|---|---|---|
| k obs (10−5 s−1) | k 0obs (10−5 s−1) | k obs (10−5 s−1) | k 0obs (10−5 s−1) | |
| a k obs: 10 mol% of amphiphiles (0.05 mM) and 5 mol% of cyclen 1 (0.025 mM) relative to the lipids in DOPC and DSPC membranes; k0ob: hydrolytic rates for systems without bis-Zn-cyclen 1. | ||||
| Zn-cyclen 1 [5 mol%] | 0.26 | — | 2.14 | — |
| With a membrane additive [10 mol%] | ||||
| A1 | 0.07 | 0.01 | 0.6 | 0.01 |
| A2 | 0.9 | 0.03 | 2.1 | 0.02 |
| A3 | 0.3 | 0.02 | 2.5 | 0.02 |
| A4 | 2.4 | 0.03 | 2.2 | 0.01 |
| A5 | 0.4 | 0.03 | 1.9 | 0.02 |
| A6 | 0.4 | 0.02 | 2.6 | 0.02 |
| A7 | 0.5 | 0.04 | 1.4 | 0.02 |
| A8 | 0.4 | 0.05 | 1.9 | 0.02 |
| A9 | 4.0 | 0.5 | 21.6 | 0.8 |
| A10 | 4.4 | 0.9 | 6.0 | 0.3 |
| A11 | 11.9 | 3.3 | 9.3 | 1.2 |
| A12 | 0.9 | 0.05 | 4.2 | 0.03 |
| A13 | 10.9 | 4.0 | 5.0 | 0.1 |
| A14 | 17.2 | 9.8 | 5.3 | 0.08 |
| A15 | 7.6 | 2.5 | 4.2 | 0.05 |
| A16 | 1.6 | 0.05 | 3.3 | 0.04 |
| A17 | 0.9 | 0.03 | 3.9 | 0.03 |
Only palmitic acid A1 caused a notable drop in hydrolytic activity. The coordination of its carboxylate group to bis-Zn-cyclen 1 may provide a rational explanation for the inhibition. Amphiphiles which are expected to be neutral at the investigated pH = 7.4, A7, A8, A3, A5 and A6, respectively (for DSPC also A2, A4), induced only small changes in the hydrolytic activity. High-polar and basic membrane additives exhibited the highest hydrolytic activity in combination with cyclen 1. A comparison of hydrolytic rates of membranes containing only cyclen 1 or its combination with a membrane additive (Fig. 4) clearly indicates cooperative effects.
Fig. 4 shows that selected membrane additives (A4, A9, A10, A11, A13, A14, A15, A16, and A17) are hydrolytically more active in fluid membranes. With the exception of A9 a larger increase of the hydrolytic rate is observed in DOPC membranes than in DSPC. The effect is most pronounced for membrane additives that are only hydrolytically active in combination with complex 1, such as oxime A4 (ninefold higher activity), phenol A2 (threefold) and guanidinium A17 (threefold). A rationale behind this may be the higher dynamics of embedded amphiphiles in the membrane. All membrane additives that increase the hydrolytic activity of complex 1 have some common structural features. All compounds bear a N–O or H–O bond and are at pH 7 either neutral with a pKa value close to 7 (imidazole: 6.95, phenol: 8.05) or are protonated (amines, guanidinium). Protonated membrane additives A with more hydrogen atoms tend to accelerate the hydrolysis more: the addition of the primary octadecylamine A14 induced a more than 10 times faster hydrolysis compared to the tertiary amine A12 in DOPC.
The hydrolytic effect of micellar solutions with polar head groups is often credited to the high local concentration of positive charge on the surface, therefore creating a local high concentration of hydroxide ions, but the example of trimethylammonium salt A17 shows that this effect is only small in comparison with cations generated by protonation (amines, guanidinium). The observations indicate that the rate enhancing effect of some membrane additives may have its origin in creation of hydrogen bonds, which stabilize the transition state of the substrate hydrolysis reaction.
Micellar hydrolysis
Kinetic measurements of micellar solutions were done under the same conditions as those described for the vesicular solutions. The concentration of every component was kept constant and only the lipids were omitted in the preparation step. The concentrations of amphiphiles were exceeding their cmc values (cmc for cyclen 1 is less than 0.01 mM, see ESI†). Fluorescence is strongly quenched in the co-micellar solutions and the hydrolysis was therefore monitored by absorption spectroscopy. Pseudo first-order-reaction rate constants of hydrolysis (kobs) were calculated from the slope of the increased fluorescein absorption band (λmax = 505 nm) over 3 min (M s−1) divided by the initial FDA concentration (see ESI†).For micellar hydrolysis only the membrane additives A9, A11, and A14 were tested, because they gave the best results in vesicular systems. An enhancement of the hydrolytic activity was observed for micelles of cyclen 1 with respect to the vesicular solution. In contrast, the membrane additives without the lipid were less active (A11, A14) or insoluble (A9). Co-micellar solutions gave high catalytic activity towards hydrolysis in all three tested systems exceeding the activity of vesicles. An average 3-fold increase of activity in comparison with micellar cyclen 1 solution without a membrane additive implies the existence of co-micelles and cooperative effects in the hydrolysis.
Mechanism and kinetics
For mechanistic studies, various concentrations of vesicular and micellar solutions were used and the observed pseudo-first-order kinetic rate constants were plotted against the overall cyclen 1 concentration in the solutions (Fig. 5). The reaction mechanism with embedded cyclen 1 for DSPC and micelles tends to be different from that for DOPC membranes. Two kinetic models were used: second-order kinetics were observed in the case of DSPC vesicles and micelles with kobs linearly dependent on the concentration of cyclen 1 in solution. In contrast, DOPC vesicles show a Michaelis–Menten type behaviour of saturation kinetics. Apparently, a complex of a substrate and a catalyst is formed, which then undergoes the hydrolysis reaction. The kinetic data were non-linearly fitted using eqn (1) according to previously published methods.25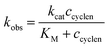 | (1) |
 | ||
| Fig. 5 Second order kinetics for DSPC vesicles and micelles (left) and saturation kinetics for DOPC (right) containing cyclen 1. | ||
In a fluid DOPC membrane, Zn-cyclen 1 shows saturation kinetics even without the presence of a membrane additive, but in a rigid DSPC membrane and in micelles, the hydrolysis rate increases linearly with the Zn-cyclen 1 concentration.
The kinetics of functionalized membranes with the highest hydrolytic activity consisting of cyclen 1 and membrane additives A4, A14, A9 or A11 were recorded and fitted to linear and non-linear (1) models. The derived constants are summarized in Table 2 (for data evaluation, see ESI†). For a better comparison of second order rate constants and reactions following a Michaelis–Menten kinetics, the initial slope was calculated by dividing kcat by KM (k′2). For most of the functionalized DOPC membranes, non-linear fitting gave better results as they followed not a single kinetic model, whereas rigid DSPC systems showed mostly linear correlations. The membrane additive A9 behaves differently from the other functionalized lipids and shows a linear second-order kinetics also in the DOPC. Embedded amine A14 leads to a saturation kinetics also in DSPC vesicles. Micelles of cyclen 1 and membrane additives showed the best hydrolytic activity (Table 3).
| k obs (10−5 s−1) | k 0obs (10−5 s−1) | |
|---|---|---|
| a k obs: rate of the system with cyclen 1 (0.025 mM) and membrane additives (0.05 mM), k0obs: micellar solutions of membrane additives. The same concentration as that for vesicular measurements is used. | ||
| Zn-cyclen 1 | 8.6 | — |
| With an additive | ||
| A9 | 34.6 | n.d. |
| A11 | 30.7 | 0.5 |
| A14 | 22.4 | 1.7 |
| k cat (10−5 s−1) | K M (10−5 M) | k ′2 (kcat/KM) (M−1 s−1) | k 2 (M−1 s−1) | ||
|---|---|---|---|---|---|
| a Only best performing systems were selected. | |||||
| DOPC | Only cyclen 1 (5%) | 0.3 | 0.7 | 0.5 | — |
| 5% 1 + 10% A4 | 10.4 | 8.4 | 1.2 | — | |
| 5% 1 + 10% A9 | — | — | — | 1.6 | |
| 5% 1 + 10% A11 | 40.9 | 9.3 | 4.4 | — | |
| 5% 1 + 10% A14 | 44.2 | 3.0 | 14.7 | — | |
| DSPC | Only cyclen 1 (5%) | — | — | — | 0.9 |
| 5% 1 + 10% A4 | — | — | — | 0.9 | |
| 5% 1 + 10% A9 | — | — | — | 8.4 | |
| 5% 1 + 10% A11 | — | — | — | 3.7 | |
| 5% 1 + 10% A14 | 11.1 | 2.7 | 4.1 | — | |
| Micelles | Only cyclen 1 | — | — | — | 3.2 |
| 1 + A9 | — | — | — | 13.4 | |
| 1 + A11 | — | — | — | 12.9 | |
| 1 + A14 | — | — | — | 7.8 | |
To study the effect of lipophilicity and steric hindrance of the ester group on the hydrolysis rate, a series of fluorescein esters was prepared (Table 4). All these compounds were tested under the same conditions as those previously described and pseudo-first order hydrolytic rates were recorded for the three best performing membrane compositions with DSPC and DOPC lipids (Tables 1 and 2).
Fig. 6 shows that the trends in hydrolysis for different esters remain the same in different vesicular systems (for data, see ESI†); the molecular structure of the ester does not significantly affect the mechanism. The reactivity of the different esters is not affected by the membrane fluidity (DOPC vs. DSPC). The highest hydrolytic activity was reached for FDA, the least lipophilic ester (Fig. 6). With increasing lipophilicity of the esters, the hydrolytic activity decreases. The lowest rates of hydrolysis were recorded for phenyl or tBu esters (F-Ph, F-tBu), which are more stable due to steric hindrance.
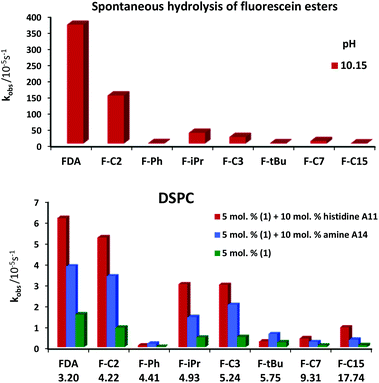 | ||
| Fig. 6 Hydrolysis of fluorescein esters (0.02 mM) spontaneously at pH 10.15 and by functionalized DSPC membranes at pH 7.4. | ||
The trend in vesicular catalytic hydrolysis of the esters follows the reactivity for spontaneous hydrolysis. The stabilizing effect of increasing lipophilicity is less pronounced for reactions in vesicular solutions.
Comparison with hydrolytic enzymes
A direct comparison of kinetic data from the literature can be challenging due to different substrates and variations in the reaction conditions. Therefore commercially available hydrolytic enzymes were used under identical conditions to benchmark the hydrolytic rate of the functionalized vesicles. Porcine liver esterase and the lipase Candida rugosa, typical enzymes used in organic synthesis, were selected. Kinetic data were derived using the same non-linear fit as that for vesicles to obtain kcat and KM. Since the enzymes are produced by extraction and no exact purity is given by the supplier, kinetic constants were calculated in weight concentration (mg ml−1) for enzymes and vesicles. For vesicles the weight of cyclen complex 1, membrane additive A and the lipid was considered (see ESI† for data).DOPC membranes functionalized with cyclen 1 and amine A14 reach the activity of the two purchased enzymes (Table 5). However, the catalytic activity of the enzyme active site still remains much higher when compared to cyclen complex 1 and membrane additives, as the vesicles contain many hydrolytic active sites on their surface, while each enzyme has only one active centre.
| k cat (10−5 s−1) | K M (10−1 mg ml−1) | k ′2 (kcat/KM) (10−4 (mg ml−1)−1 s−1) | |
|---|---|---|---|
| Porcine liver esterase | 68.7 | 0.08 | 858 |
| Lipase Candida rugosa | 73.2 | 0.4 | 166 |
| DOPC 5% 1 + 10% A14 | 44.2 | 0.2 | 391 |
Conclusions
Soft surfaces with ester hydrolysis activity were obtained by co-embedding of bis-Zn-cyclen complex 1 and amphiphilic membrane additives into a phospholipid membrane of a vesicle or into a micellar solution. For many combinations of membrane additives and bis-Zn-cyclen complex 1 an increase of the hydrolytic activity up to 25 fold in comparison with complex 1 was observed. DOPC membranes that are fluid at the reaction temperature lead to more pronounced cooperative action of the membrane embedded amphiphiles, and Michaelis–Menten saturation kinetics was observed for such membranes. Micellar solutions showed also higher activity when metal complexes and additives are present. The functionalized vesicles with the highest hydrolytic activity were compared with two commercially available enzymes under identical reaction conditions. Considering the overall weight of the catalytic systems their hydrolysis activity towards fluorescein diesters is comparable.In conclusion, we observed a significantly increased hydrolytic activity of functionalized vesicles and micelles at neutral pH towards carboxylesters, if Lewis acidic bis-Zn-cyclen complex 1 and functionalized amphiphiles are used concertedly. Their assembly has to be dynamic, as in DOPC membranes or micelles, to gain a cooperative hydrolytic effect.
Notes and references
- B. Testa and J. M. Mayer, in Hydrolysis in Drug and Prodrug Metabolism, Verlag Helvetica Chimica Acta, 2006, pp. 1–9 Search PubMed.
- J. Chin, Acc. Chem. Res., 1991, 24, 145–152 CrossRef CAS.
- Y. Murakami, J.-i. Kikuchi, Y. Hisaeda and O. Hayashida, Chem. Rev., 1996, 96, 721–758 CrossRef CAS PubMed.
- D. W. Christianson and J. D. Cox, Annu. Rev. Biochem., 1999, 68, 33–57 CrossRef CAS PubMed.
- E. Kimura, Curr. Opin. Chem. Biol., 2000, 4, 207–213 CrossRef CAS.
- F. Mancin, P. Scrimin and P. Tecilla, Chem. Commun., 2012, 48, 5545–5559 RSC.
- M. Subat, K. Woinaroschy, S. Anthofer, B. Malterer and B. König, Inorg. Chem., 2007, 46, 4336–4356 CrossRef CAS PubMed.
- M. Subat, K. Woinaroschy, C. Gerstl, B. Sarkar, W. Kaim and B. König, Inorg. Chem., 2008, 47, 4661–4668 CrossRef CAS PubMed.
- B. Gruber, E. Kataev, J. Aschenbrenner, S. Stadlbauer and B. König, J. Am. Chem. Soc., 2011, 133, 20704–20707 CrossRef CAS PubMed.
- B. Gruber and B. König, Chem. – Eur. J., 2013, 19, 438–448 CrossRef CAS PubMed.
- R. A. Moss and W. L. Sunshine, J. Org. Chem., 1974, 39, 1083–1089 CrossRef CAS.
- F. Mancin, P. Scrimin, P. Tecilla and U. Tonellato, Coord. Chem. Rev., 2009, 253, 2150–2165 CrossRef CAS PubMed.
- C. A. Bunton, E. J. Fendler, G. L. Sepulveda and K.-U. Yang, J. Am. Chem. Soc., 1968, 90, 5512–5518 CrossRef CAS.
- R. A. Moss and W. L. Sunshine, J. Org. Chem., 1974, 39, 1083–1089 CrossRef CAS.
- T. Kunitake, Y. Okahata and T. Sakamoto, J. Am. Chem. Soc., 1976, 98, 7799–7806 CrossRef CAS.
- P. Scrimin, P. Tecilla and U. Tonellato, J. Org. Chem., 1991, 56, 161–166 CrossRef CAS.
- J. Zhang, X.-G. Meng, X.-C. Zeng and X.-Q. Yu, Coord. Chem. Rev., 2009, 253, 2166–2177 CrossRef CAS PubMed.
- P. Scrimin and P. Tecilla, Curr. Opin. Chem. Biol., 1999, 3, 730–735 CrossRef CAS.
- A. Grochmal, E. Ferrero, L. Milanesi and S. Tomas, J. Am. Chem. Soc., 2013, 135, 10172–10177 CrossRef CAS PubMed.
- B. Gruber, S. Balk, S. Stadlbauer and B. König, Angew. Chem., Int. Ed., 2012, 51, 10060–10063 CrossRef CAS PubMed.
- J.-S. You, X.-Q. Yu, X.-Y. Su, T. Wang, Q.-X. Xiang, M. Yang and R.-G. Xie, J. Mol. Catal. A: Chem., 2003, 202, 17–22 CrossRef CAS.
- J. Epstein, J. Kaminski, N. Bodor, R. Enever, J. Sowa and T. Higuchi, J. Org. Chem., 1978, 43, 2816–2821 CrossRef CAS.
- R. Swisher and G. Carroll, Microb. Ecol., 1980, 6, 217–226 CrossRef CAS PubMed.
- N. Steward, R. Martin, J. M. Engasser and J. L. Goergen, Plant Cell Rep., 1999, 19, 171–176 CrossRef CAS.
- D. H. Kim and S. S. Lee, Biorg. Med. Chem., 2000, 8, 647–652 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and compounds characterisation data, kinetic measurements data. See DOI: 10.1039/c4ob00247d |
| This journal is © The Royal Society of Chemistry 2014 |





