Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct olefination of benzaldehydes into 1,3-diarylpropenes via a copper-catalyzed heterodomino Knoevenagel-decarboxylation-Csp3-H activation sequence†
Yaping
Zhao
,
Lu
Sun
,
Tieqiang
Zeng
,
Jiayi
Wang
,
Yanqing
Peng
and
Gonghua
Song
*
Shanghai Key Laboratory of Chemical Biology, Institute of Pesticides and Pharmaceuticals, East China University of Science and Technology, Shanghai 200237, P.R. China. E-mail: ghsong@ecust.edu.cn
First published on 26th March 2014
Abstract
Copper-catalyzed direct olefination of benzaldehydes into 1,3-diarylpropenes by a novel domino Knoevenagel-decarboxylation-Csp3-H activation sequence is reported. This method provides a concise and effective route toward the synthesis of unsymmetrical 1,3-diarylpropene derivatives.
Introduction
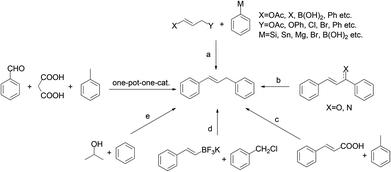
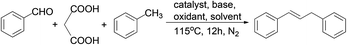
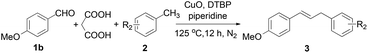
In the last two decades, there has been increasing interest in the development of more efficient and environmentally friendly methods for chemical syntheses.1 One of the related research areas is the development of sequential formation of multiple C–C bonds in one pot.2 In general, these processes eliminate intermediate recovery steps, thereby considerably decreasing the amount of waste generated.3 For the past few years, a number of notable domino sequence reactions involving decarboxylation have been developed using simpler substrates like benzaldehydes.4 However, to the best of our knowledge, examples of utilizing benzaldehydes for one pot methylenation coupling into 1,3-diarylpropenes have not been explored.Furthermore, 1,3-diarylpropenes are often known to be privileged structures or key intermediates in the synthesis of natural products and the development of biologically active compounds.5 Traditionally, strategies toward the syntheses of 1,3-diarylpropenes include allylic arylation/alkenylation (Scheme 1, route a),6 allylic selective defunctionalization (route b),7 decarboxylation of cinnamic acids (route c),8 cross-coupling reactions of potassium alkenyltrifluoroborates with benzyl halides (route d)9 and alkylation of benzene (route e).10 However, most of the above methods have to bear disadvantages such as limited substrates, multistep procedures and necessary prefunctionalization. Encouraged by the ecological and economic advantages of domino reactions, we wish to report herein the synthesis of unsymmetrical 1,3-diarylpropenes through a domino Knoevenagel-decarboxylation-Csp3-H activation sequence (Scheme 1).
Results and discussion
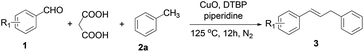
We started our research by using benzaldehyde (1a) as the standard substrate. The combination of malonic acid, CuO, di-t-butyl peroxide (DTBP) and piperidine in toluene at 115 °C (oil bath temperature, unless otherwise noted) gave the desired product 1,3-diarylpropenes (2a) in 68% GC yield within 12 h (Table 1, entry 1). Other copper catalysts (entries 2 and 3), oxidants (entry 6), bases (entry 7), or solvents (entry 8) decreased the yield.11 Employment of Fe3O4 or ferrocene resulted in a dramatic decrease in yield (entries 4 and 5). Modifying the quantity of oxidant, base, catalyst and time did not afford better results (entries 9–11 and 15).11 Higher yields were obtained when the reaction was carried out at an elevated temperature. Particularly, trace amounts of the target product were detected below the boiling point of toluene (entry 12) and a good result in 77% GC yield was achieved at 125 °C (entry 13). However, when the reaction was conducted under an air atmosphere, the yield decreased to 49% (entry 14). A control experiment showed that the domino reaction was poorly efficient when the reaction was carried out in the absence of a copper catalyst (entry 16).| Entry | Catalyst | Oxidant | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Catalytic conditions: benzaldehyde (0.3 mmol), malonic acid (0.5 mmol), toluene (0.5 mmol), solvent (2 mL), base (0.2 mmol), catalyst (20 mol%), oxidant (4 equiv.), 115 °C, 12 h, N2 atmosphere. b GC yields were given using dodecane as the internal standard. c The reaction was conducted within 24 h. d 0.1 mmol of piperidine was used. e 2 equiv. of DTBP was used. f The reaction was conducted at 105 °C under a N2 atmosphere. g The reaction was conducted at 125 °C under a N2 atmosphere. h The reaction was conducted at 125 °C under an air atmosphere. i 10 mol% of CuO was used. | |||||
| 1 | CuO | DTBP | Piperidine | Toluene | 68 |
| 2 | CuBr2 | DTBP | Piperidine | Toluene | 53 |
| 3 | CuI | DTBP | Piperidine | Toluene | 50 |
| 4 | Fe3O4 | DTBP | Piperidine | Toluene | 30 |
| 5 | Ferrocene | DTBP | Piperidine | Toluene | Trace |
| 6 | CuO | TBHP | Piperidine | Toluene | 42 |
| 7 | CuO | DTBP | DBU | Toluene | 24 |
| 8 | CuO | DTBP | Piperidine | DMSO | 11 |
| 9c | CuO | DTBP | Piperidine | Toluene | 69 |
| 10d | CuO | DTBP | Piperidine | Toluene | 8 |
| 11e | CuO | DTBP | Piperidine | Toluene | 33 |
| 12f | CuO | DTBP | Piperidine | Toluene | NR |
| 13g | CuO | DTBP | Piperidine | Toluene | 77 |
| 14h | CuO | DTBP | Piperidine | Toluene | 49 |
| 15i | CuO | DTBP | Piperidine | Toluene | 55 |
| 16 | — | DTBP | Piperidine | Toluene | 11 |
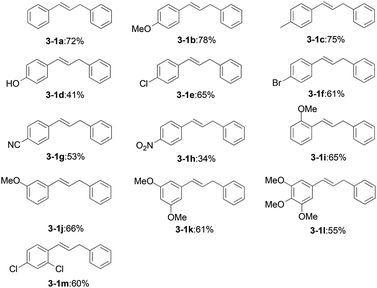
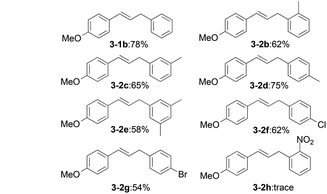
Under the optimized reaction conditions, the allylation of a variety of benzaldehyde derivatives was examined. As shown in Table 2, benzaldehydes bearing a variety of substituents were found to afford exclusively 1,3-diarylpropenes in moderate to good yields (3-1a–3-1h). Obviously, an electron-donating group at the para-position, such as the methoxy substituent in 3-1b, afforded a higher yield compared with an electron-withdrawing group, such as a cyano group substituent in 3-1g. para-Substituted benzaldehydes (3-1b) gave a superior product yield compared to that of ortho- or meta-substituted benzaldehydes (3-1i and 3-1j). The domino reaction with 4-nitrobenzaldehyde (3-1h) was also successful. OH-, Cl- and Br-substituted compounds (3-1d, 3-1e and 3-1f) were also well tolerated. It turned out that multiple substituent groups (3-1k, 3-1l and 3-1m) would decrease the reaction efficiency.
Subsequently, we surveyed the substrate scope of benzylic hydrocarbons (Table 3). All kinds of xylenes and mesitylene (3-2b–3-2e) offered the mono-coupling products in moderate yields. The toluenes substituted by electron-withdrawing groups (3-2f, 3-2g and 3-2h) were less reactive than xylenes (3-2b and 3-2d).
Considering the effects of electronic parameters on the reaction, it was found that the yield roughly decreased with the increase of Hammett constant (σ) values of the substituents on benzaldehyde. For example, the yield of 3-1b (p-CH3O, σp = −0.27) is 78%, and the yield of 3-1g (p-CN, σp = 0.66) is 53% as shown in Table 4. The exceptional substrates are p-hydroxyl benzaldehyde and m,m,p-tri-methoxyl benzaldehyde, which might be dominated by other factors such as hydrogen bonds and steric hindrance. However, a similar approach for benzylic hydrocarbons is not applicable.
| Substituents on benzaldehyde | σ | Yield |
|---|---|---|
| p-OH | −0.37 | 41% |
| p-OCH3 | −0.27 | 78% |
| p-CH3 | −0.17 | 75% |
| m,m,p-tri-OCH3 | −0.03 | 55% |
| p-H | 0 | 72% |
| o-OCH3 | 0.04 | 65% |
| m-OCH3 | 0.12 | 66% |
| p-Cl | 0.23 | 65% |
| p-Br | 0.23 | 61% |
| m,m-di-OCH3 | 0.24 | 61% |
| o,p-di-Cl | 0.63 | 60% |
| p-CN | 0.66 | 53% |
| p-NO2 | 0.78 | 34% |
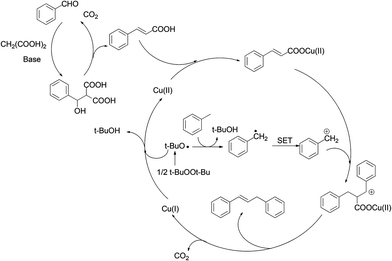
Based on previous observations and literature reports,4h,8 we proposed a plausible catalytic cycle (Scheme 2). The reaction involves a domino anionic-metal catalyzed pathway,2a,4h wherein an incipient cinnamic acid (formed in situ from the K-D reaction) continuously undergoes copper catalyzed cross coupling and decarboxylation, leading to 1,3-diarylpropenes in one pot.8
Conclusions
In summary, we have developed the first copper-catalyzed one step direct olefination of benzaldehydes into 1,3-diarylpropenes via a novel domino Knoevenagel-decarboxylation-Csp3-H activation sequence. The unsymmetrical 1,3-diarylpropenes were obtained in moderate to good yields. All of the substrates were economical, simple and readily available.Experimental
General information
All reactions were carried out under an N2 atmosphere. CuO was purchased from Aladdin-reagent with high purity (99.5%). All reagents were used as supplied without further purification and drying. Flash column chromatography was performed over silica gel (48–75 μm) and reactions were monitored by thin layer chromatography (TLC) using UV light (254 nm). 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Avance 400 MHz NMR spectrometer using d6-DMSO as a solvent and tetramethylsilane as an internal standard (s = singlet, d = doublet, t = triplet, m = multiplet). MS analyses were performed on an Agilent 5975 GC-MS instrument (EI). HRMS analyses were performed on a Waters Micromass GCT instrument (EI).General procedures for copper-catalyzed allylation of benzaldehydes
Malonic acid (52 mg, 0.5 mmol) and CuO (4.8 mg, 0.06 mol) were added into a 10 mL Schlenk flask. Then toluene (2 mL), benzaldehyde (31 μl, 0.3 mmol), DTBP (120 μl, 1.2 mmol), and piperidine (20 μl, 0.2 mmol) were added at room temperature. The reaction vessel was purged with N2 three times. The mixture was stirred at 125 °C for 12 h. After cooling to room temperature, the mixture was diluted with CH2Cl2 and water. The organic phase was washed with brine, dried with MgSO4, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (petroleum ether–ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the corresponding product.
1) to afford the corresponding product.
Acknowledgements
The financial support for this study from the National Basic Research Program of China (973 Program) (grant 2010CB126101) is gratefully acknowledged.Notes and references
- P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- For a seminal review on domino reactions, see: (a) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; for representative examples; (b) S. W. Li and R. A. Batey, Chem. Commun., 2007, 36, 3759 RSC; (c) K. Alex, A. Tillack, N. Schwarz and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 2304 CrossRef CAS PubMed; (d) A. Trejos, A. Fardost, S. Yahiaoui and M. Larhed, Chem. Commun., 2009, 48, 7587 RSC; (e) D. M. Souza, F. Rominger and T. J. J. Muller, Chem. Commun., 2006, 39, 4096 RSC.
- A. Bruggink, R. Schoevaart and T. Kieboom, Org. Process Res. Dev., 2003, 7, 622 CrossRef CAS.
- (a) M. Koichi and O. Hirofumi, Chem. Commun., 2002, 22, 2626 Search PubMed; (b) P. Frederic, C. Sebastien, S. Morgane and D. Adam, J. Org. Chem., 2008, 73, 1975 CrossRef PubMed; (c) S. Amit, W. Roy, K. Naama and S. Doron, J. Am. Chem. Soc., 2008, 130, 5434 CrossRef PubMed; (d) X.-C. Huang, F. Wang, Y. Liang and J.-H. Li, Org. Lett., 2009, 11, 1139 CrossRef CAS PubMed; (e) M. Liang, S. Cecilia, P. Chiara and W. Peter, Tetrahedron Lett., 2009, 50, 6810 CrossRef CAS PubMed; (f) B. Alicia, H. Dacil and H. Rosendo, Eur. J. Org. Chem., 2010, 20, 3847 Search PubMed; (g) P. B. S. Dawadi and L. Johan, Synth. Commun., 2010, 40, 2539 CrossRef CAS; (h) S. Abhishek, S. Naina, K. Rakesh, S. Amit and A. K. Sinha, Chem. Commun., 2010, 46, 3283 RSC; (i) D. Zhao, C. Gao, X. Su, Y. He, J. You and Y. Xue, Chem. Commun., 2010, 46, 9049 RSC; (j) S. H. Kim, Y. M. Kim, H. S. Lee and J. N. Kim, Tetrahedron Lett., 2010, 51, 1592 CrossRef CAS PubMed; (k) L. J. Cotterill, R. W. Harrington, C. William and M. J. Hall, J. Org. Chem., 2010, 75, 4604 CrossRef CAS PubMed; (l) W. Xu and H. Fu, J. Org. Chem., 2011, 76, 3846 CrossRef CAS PubMed; (m) A. Carlos, J.-O. Gonzalo, A. Alberto, J. H. Busto, J. M. Peregrina and M. M. Zurbano, J. Org. Chem., 2011, 76, 6990 CrossRef PubMed; (n) K.-P. Claire, D. M. Alba, O. Julie, P. Guillaume, M. Pedro and P. Giovanni, J. Organomet. Chem., 2012, 714, 53 CrossRef PubMed; (o) G. Steven, L. Frederic, P. Guillaume, W. Benoit, S. Mathieu, C. Yves, M. Andre and P. Giovanni, Chem. Commun., 2012, 48, 5889 RSC; (p) P. Thanasekaran and M. Shanmugam, Tetrahedron Lett., 2012, 53, 4248 CrossRef PubMed; (q) T. David, M.-A. Gabriela, C. Leandro and G.-T. Fernando, Chem. – Eur. J., 2012, 18, 3468 CrossRef PubMed; (r) W. Reina, K. Takashi, K. Robert, O. Hiromichi, U. Daisuke, Y. Shosuke and M. Kenji, Tetrahedron Lett., 2013, 54, 1921 CrossRef PubMed; (s) W. Xu, U. Kloeckner and B. J. Nachtsheim, J. Org. Chem., 2013, 78, 6065 CrossRef CAS PubMed; (t) P. Svetlana, T. Tony, L. Vincent and J.-F. Briere, Adv. Synth. Catal., 2013, 355, 2513 CrossRef.
- (a) B. Kramer and S. R. Waldvogel, Angew. Chem., Int. Ed., 2004, 43, 2446 CrossRef CAS PubMed; (b) B. Kramer and S. R. Waldvogel, Angew. Chem., Int. Ed., 2004, 116, 2501 CrossRef; (c) J. C. Anderson, C. Headly, P. D. Stapleton and P. W. Taylor, Tetrahedron, 2005, 61, 7703 CrossRef CAS PubMed; (d) S. B. Wan, Bioorg. Med. Chem., 2005, 13, 2177 CrossRef CAS PubMed; (e) C. R. Su, Y. C. Shen, P. C. Kuo, Y. L. Leu, A. G. Damu, Y. H. Wang and T. S. Wu, Bioorg. Med. Chem. Lett., 2006, 16, 6155 CrossRef CAS PubMed; (f) M. Belley, C. C. Chan, Y. Gareau, M. Gallant, H. Juteau, K. Houde, N. Lachance, M. Labelle, N. Sawyer, N. Tremblay, S. Limontage, M. C. Carrière, D. Denis, G. M. Greig, D. Slipez, R. Gordon, N. Chauret, C. Lo, R. J. Zamboni and K. M. Metters, Bioorg. Med. Chem. Lett., 2006, 16, 5639 CrossRef CAS PubMed; (g) C. Ito, M. Itoigawa, T. Kanematsu, Y. Imamura, H. Tokuda, H. Nishino and H. Furukawa, Eur. J. Med. Chem., 2007, 42, 902 CrossRef CAS PubMed; (h) K. N. Mewett, S. P. Fernandez, A. K. Pasricha, A. Pong, S. O. Devenish, D. E. Hibbs, M. Chebib, G. A. R. Johnston and J. R. Hanrahan, Bioorg. Med. Chem., 2009, 17, 7156 CrossRef CAS PubMed; (i) K. Umehara, K. Nemoto, A. Matsushita, E. Terada, O. Monthakantirat, W. De-Eknamkul, T. Miyase, T. Warashina, M. Degawa and H. J. Noguchi, Nat. Prod., 2009, 72, 2163 CrossRef CAS PubMed; (j) S. Cheenpracha, C. Karalai, C. Ponglimanont and A. J. Kanjana-Opas, Nat. Prod., 2009, 72, 1395 CrossRef CAS PubMed; (k) F. M. Abdel Bar, M. A. Khanfar, A. Y. Elnagar, F. A. Badria, A. M. Zaghloul, K. F. Ahmad, P. W. Sylvester and K. A. El Sayed, Bioorg. Med. Chem., 2010, 18, 496 CrossRef CAS PubMed; (l) M. Namara and M. Yvonne, Bioorg. Med. Chem., 2011, 19, 1328–1348 CrossRef PubMed; (m) J. C. Anderson, R. McCarthy, S. Paulin and P. W. Taylor, Bioorg. Med. Chem. Lett., 2011, 21, 6996 CrossRef CAS PubMed; (n) M. Moriyasu, N. Nakatani, M. Ichimaru, Y. Nishiyama, A. Kato, S. G. Mathenge, F. D. Juma and P. B. C. Mutiso, J. Nat. Med., 2011, 65, 313 CrossRef CAS PubMed.
- (a) U. Yasuhiro, D. Hiroshi and H. Tamio, J. Org. Chem., 1999, 64, 3384 CrossRef; (b) C. Jordi, M. M. Marcial and P. Roser, Eur. J. Org. Chem., 2000, 2, 239 Search PubMed; (c) R. Martin and A. Fürstner, Angew. Chem., Int. Ed., 2004, 43, 3955 CrossRef CAS PubMed; (d) A. Fürstner, R. Martin, H. Krause, G. Seidel, R. Goddard and C. W. Lehmann, J. Am. Chem. Soc., 2008, 130, 8773 CrossRef PubMed; (e) M. Takashi, K. Kenji, S. Yoshiaki, S. Masami and F. Tsutomu, Synlett, 2008, 17, 2711 Search PubMed; (f) M. A. Y. Yoichi, W. Toshihiro, T. Kaoruand and U. Yasuhiro, Chem. Commun., 2009, 37, 5594 Search PubMed; (g) M. Takashi, K. Taketo, A. Taichi, K. Tomoko, F. Tsutomu and S. Masami, J. Org. Chem., 2009, 74, 2321 CrossRef PubMed; (h) R. Ghosh, N. N. Adarsh and A. Sarkar, J. Org. Chem., 2010, 75, 5320 CrossRef CAS PubMed; (i) A. M. Lauer, F. Mahmud and J. Wu, J. Am. Chem. Soc., 2011, 133, 9119 CrossRef CAS PubMed; (j) B. Yao, Y. Liu, M. K. Wang, J. H. Li, R. Y. Tang, X. G. Zhang and C. L. Deng, Adv. Synth. Catal., 2012, 354, 1069 CrossRef CAS; (k) C. Li, J. Xing, J. Zhao, P. Huynh, W. Zhang, P. Jiang and Y. J. Zhang, Org. Lett., 2012, 14, 390 CrossRef CAS PubMed; (l) Y. M. A. Yamada, S. M. Sarkar and Y. Uozumi, J. Am. Chem. Soc., 2012, 134, 3190 CrossRef CAS PubMed; (m) M. Takashi, K. Taketo, A. Taichi, K. Tomoko, F. Tsutomu and S. Masami, Eur. J. Org. Chem., 2013, 8, 1501 Search PubMed.
- (a) J. Wang, W. Huang, Z. Zhang, X. Xiang, R. Liu and X. Zhou, J. Org. Chem., 2009, 74, 3299 CrossRef CAS PubMed; (b) B. L. Yang and S. K. Tian, Chem. Commun., 2010, 46, 6180 RSC; (c) G. G. K. S. N. Kumar and K. K. Laali, Org. Biomol. Chem., 2012, 10, 7347 RSC.
- (a) H. Yang, P. Sun, Y. Zhu, H. Yan, L. Lu, X. Qu, T. Li and J. Mao, Chem. Commun., 2012, 48, 7847 RSC; (b) H. Yang, H. Yan, P. Sun, Y. Zhu, L. Lu, D. Liu, G. Rong and J. Mao, Green Chem., 2013, 15, 976 RSC.
- E. Alacid and C. Nájera, J. Org. Chem., 2009, 74, 2321 CrossRef CAS PubMed.
- P. Makowski, R. Rothe, A. Thomas, M. Niederberger and F. Goettmann, Green Chem., 2009, 11, 34 RSC.
- For a more detailed condition screening table, see the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization data, copies of 1H-NMR and 13C-NMR spectra. See DOI: 10.1039/c4ob00155a |
| This journal is © The Royal Society of Chemistry 2014 |