Effect of configuration of 2-vinyldiazocarbonyl compounds on their reactivity: experimental and computational study†
Abstract
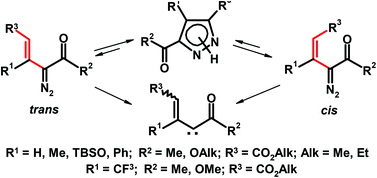
Non-fluorinated vinyldiazo compounds with trans-configuration irrespective of the nature of 3-R1-substituent (R1 = H, Me, TBSO) even under ambient conditions easily cyclize to produce pyrazoles, while cis-stereoisomers undergo similar ring closure only at elevated temperatures or decompose to produce vinyloxocarbene reaction products. The 3-CF3-substituted analogues with cis- or trans-configuration do not produce pyrazoles at all, but on heating furnish only vinylcarbene derived products. DFT calculations of theoretical energy barriers adequately explain the different experimental reactivity found for stereoisomeric vinyldiazocarbonyl compounds, and a new model for their interconversion through the corresponding pyrazoles has been suggested.

 Please wait while we load your content...
Please wait while we load your content...