Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biomimetic aerobic oxidative hydroxylation of arylboronic acids to phenols catalysed by a flavin derivative†
Hana
Kotoučová
ab,
Iveta
Strnadová
b,
Martina
Kovandová
a,
Josef
Chudoba
c,
Hana
Dvořáková
c and
Radek
Cibulka
*a
aDepartment of Organic Chemistry, Institute of Chemical Technology, Prague, Technická 5, 16628 Prague 6, Czech Republic. E-mail: cibulkar@vscht.cz; Fax: +420 220 444 288; Tel: +420 220 443 688
bCharles University of Prague, Faculty of Education, M. D. Rettigové 4, 116 39 Prague 1, Czech Republic
cCentral Laboratories, Institute of Chemical Technology, Prague, Technická 5, 16628 Prague 6, Czech Republic
First published on 24th January 2014
Abstract
Flavin-catalysed oxidative hydroxylation of substituted arylboronic acids by molecular oxygen with the assistance of hydrazine or ascorbic acid resulted in phenols in high yields. This mild organocatalytic protocol is compatible with a variety of functional groups and it is alternatively usable for transformation of alkylboronic acids to alcohols. Reaction takes place also in water and fulfils criteria for a green procedure.
Introduction
Design of biomimetic systems inspired by enzymatic catalytic processes usually yields new efficient green methodologies in organic synthesis.1 Accordingly, oxygenation reactions occurring in flavin-dependent monooxygenases provided inspiration for artificial systems based on flavinium salts which are now used as powerful catalysts for chemoselective and stereoselective oxidations with oxygen or hydrogen peroxide.2–4 In monooxygenases, flavin cofactor Fl (FMN or FAD) reduced by NADPH reacts with oxygen to give flavin-4a-hydroperoxide FlOOH (Scheme 1), which oxidizes a substrate. After oxygen transfer, the cofactor is regenerated by water elimination.2,5 Artificial aerial oxidations catalysed by flavinium salts (e.g. by compound 1 in Scheme 1) in the presence of a sacrificial reducing agent proceed via the same mechanism.2,4 The use of 5-alkylflavinium salts instead of neutral flavin is necessary because the 5-alkyl group stabilizes hydroperoxide 1-OOH so that it can be utilized for oxidations.2b,6 Despite their considerable potential, flavinium catalysts have still been tested only in O2 oxidations of sulfides to sulfoxides,4a,f amines to N-oxides,4f and in Baeyer–Villiger (B.V.)4e and Dakin oxidations.4c Here, we report flavinium-catalysed oxidative hydroxylation of arylboronic acids, thus extending the portfolio of flavin-mediated oxidations and bringing an organocatalytic procedure for the transformation of arylboronic acids to phenols. Analogous biocatalytic oxidation of C–B bonds with Baeyer–Villiger oxygenases employing the flavin cofactor has been reported,7 but with a substrate scope focused on alkylboronates.7b | ||
| Scheme 1 Mechanism of aerobic oxidations of substrate S catalysed by neutral flavin Fl (in enzymes) or flavinium salt 1 (in artificial systems) with the assistance of reductant AH2. | ||
Currently, the hydroxylation of arylboronic acid represents a useful alternative to classical phenol synthesis methods, i.e. nucleophilic aromatic substitution of aryl halides or hydrolysis of arene diazonium salts, which often suffer from low functional group compatibility and poor accessibility of the starting compounds. Arylboronic acids can be hydroxylated by strong oxidizing agents such as hydrogen peroxide, oxone, or MCPBA which are usually used in stoichiometric amounts.8 Because there is a need for mild and environmentally friendly oxidation methods tolerating other functionalities, catalytic oxidative hydroxylations of boronic acids became a subject of intensive research in the last decade.9–12 As a result, oxidations with molecular oxygen catalysed by copper(II) or palladium(II) salts,9 copper-promoted electrochemical hydroxylation,10a reaction with electrochemically generated superoxide anions,10b and photocatalytic aerobic oxidative hydroxylation mediated by a ruthenium or methylene blue sensitizer and visible light have been developed.11 Recently, the metal-free mild oxidation with N-oxides has been reported, but it requires a stoichiometric amount of organic oxidant.12 Unexpected phenol production from arylboronic acid in the presence of oxygen and naphthoquinone is still the only example of an organocatalytic aerobic process mentioned in the literature.13
Results and discussion
When searching for suitable conditions for the flavin-based oxidative hydroxylation of arylboronic acids, we evaluated a range of reducing agents and solvents from efficient protocols designed for aerobic sulfoxidations and B.V. oxidations.4a,d–f We used simple flavinium catalyst 2 (Scheme 2) which is readily available by a two-step procedure from commercial material.4d We initially employed the oxidation of phenylboronic acid (3a) with oxygen (1 atm., balloon) with 5 mol% of the catalyst as a model reaction (Table 1).| Entry | Catalyst | Reducing agent | Solvent + additives | Conv.b 10 min. [%] |
|---|---|---|---|---|
a Conditions: phenylboronic acid (0.079 mmol), 2 (5 mol% unless otherwise indicated), reducing agent (0.106 mmol), oxygen (1 atm., balloon), solvent 0.6 mL, R.T. (for details see procedure A in the Experimental section).
b Conversion after 10 minutes (conversion after 3 hours in brackets) determined by 1H NMR.
c Air (1 atm., balloon) used instead of oxygen.
d 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
e 2 1.
e 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
f 7 1.
f 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. 2.
|
||||
| 1 | 2 | N2H4·H2O | CH3OH | 5 |
| 2 | 2 | N2H4·H2O | CH3CN | 35 |
| 3 | 2 | N2H4·H2O | CH3CN–EtOAc–H2Od | 12 |
| 4 | 2 | N2H4·H2O | CF3CH2OH–CH3OHe | 95 |
| 5 | 2 (1 mol%) | N2H4·H2O | CF3CH2OH–CH3OHe | 10 |
| 6c | 2 | N2H4·H2O | CF3CH2OH–CH3OHe | 15 |
| 7 | — | N2H4·H2O | CF3CH2OH–CH3OHe | 0(0) |
| 8 | 2 | N2H4·H2O | H2O (pH = 7.8) | 7(95) |
| 9 | 2 | Zn | CH3OH | 39 |
| 10 | 2 | Zn | CH3CN–EtOAc–H2Od | 12 |
| 11 | 2 | Zn | CH3CN–EtOAc–H2Od + CH3COONa (1 equiv.) | 13 |
| 12 | 2 | Ascorbic acid | CF3CH2OH–CH3OH–H2Of | 6 |
| 13 | 2 | Ascorbic acid | CF3CH2OH–CH3OH–H2Of + CH3COONa (1 equiv.) | 8 |
| 14 | 2 | Ascorbic acid | H2O (pH = 7.8) | 27 |
When hydrazine is used as a sacrificial reducing agent, the choice of solvent is essential (entries 1–4). In methanol, which dissolves phenylboronic acid well, only 5% conversion to phenol was observed after 10 min. Reaction in acetonitrile and in an acetonitrile–ethyl acetate–water mixture led to 35% and 12% conversions, respectively. Therefore we turned our attention to trifluoroethanol which is considered to be a suitable solvent for aerobic oxidations due to high solubility of oxygen.4f Addition of the methanol co-solvent was necessary to homogenize the reaction mixture. In the resulting trifluoroethanol–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system we observed the best result by far with a conversion of 95% after 10 min of oxidation (entry 4). As expected, lower catalyst loading as well as the use of air instead of oxygen led to deceleration of the process (entries 5 and 6). It is important to note that oxidation in the absence of the flavin catalyst does not take place (entry 7). Hydroxylation also proceeds with zinc or ascorbic acid as sacrificial reductants; however, the rates were lower than that with hydrazine under optimized conditions (entries 9–14). Reactions with zinc or ascorbic acid failed to accelerate either by changing the solvent or by adding sodium acetate to generate ascorbate with a higher reduction power4a and/or by generating the flavin hydroperoxide anion which is more powerful for nucleophilic oxidations4e,14 (entries 11 and 13). Interestingly, flavin-based hydroxylations take place also in aqueous solutions (entries 8 and 14). The reaction is slower compared to that in trifluoroethanol. Most probably, the reaction rate in water is negatively influenced by low solubility of oxygen which is approx. 10 times lower as compared with organic and fluorinated solvents.15 Even so, quantitative conversion was almost observed in aqueous medium with hydrazine as the reductant after extended time (3 h).
1) solvent system we observed the best result by far with a conversion of 95% after 10 min of oxidation (entry 4). As expected, lower catalyst loading as well as the use of air instead of oxygen led to deceleration of the process (entries 5 and 6). It is important to note that oxidation in the absence of the flavin catalyst does not take place (entry 7). Hydroxylation also proceeds with zinc or ascorbic acid as sacrificial reductants; however, the rates were lower than that with hydrazine under optimized conditions (entries 9–14). Reactions with zinc or ascorbic acid failed to accelerate either by changing the solvent or by adding sodium acetate to generate ascorbate with a higher reduction power4a and/or by generating the flavin hydroperoxide anion which is more powerful for nucleophilic oxidations4e,14 (entries 11 and 13). Interestingly, flavin-based hydroxylations take place also in aqueous solutions (entries 8 and 14). The reaction is slower compared to that in trifluoroethanol. Most probably, the reaction rate in water is negatively influenced by low solubility of oxygen which is approx. 10 times lower as compared with organic and fluorinated solvents.15 Even so, quantitative conversion was almost observed in aqueous medium with hydrazine as the reductant after extended time (3 h).
Series of boronic acids with an electron-withdrawing or electron-donating group were screened in preparative experiments to investigate the substrate scope of the reaction (Table 2; see the Experimental section and ESI† for details). Most substituted phenols 4 were obtained in quantitative conversion and with good to excellent yields from arylboronic acids 3 using our optimized protocol, i.e. the 2/O2 (1 atm.)/hydrazine system in trifluoroethanol–methanol. It should be mentioned that the procedure is suitable also for ortho-substituted and even for ortho,ortho′-disubstituted derivatives with only longer reaction time being required. The reaction is slower also for ortho- and para nitro derivatives 3g and 3i. For oxidation of o-nitrophenylboronic acid (3i), 51% conversion was only observed even after extended reaction time. This is probably caused (in addition to a steric effect in the case of 3i) by relatively high acidity of the resulting nitrophenols16 which are able to protonate hydrazine. Hydrazine acts as a flavin reducing agent and a base generating flavinhydroperoxide anion. Both these processes could be decelerated by hydrazinium/hydrazine equilibrium. Addition of a weak base,18e.g. sodium bicarbonate or sodium acetate, speeds up the reaction significantly: quantitative and 85% conversion was detected for 3g and 3i, respectively, in the presence of 5 equivalents of sodium acetate after 2 h.
a Conditions: boronic acid (0.79 mmol), catalyst 2 (5 mol%), hydrazine (1.06 mmol), oxygen (1 atm., balloon), CF3CH2OH–CH3OH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6 mL), R.T. (see procedure B in the Experimental section).
b Conversion >95%.
c Conversion 51%.
d 5 equiv. of sodium acetate added (see procedure D in the Experimental section).
e Conversion 85%.
f Ascorbic acid + sodium acetate instead of hydrazine (see procedure C in the Experimental section).
g Conversion 76%. 1, 6 mL), R.T. (see procedure B in the Experimental section).
b Conversion >95%.
c Conversion 51%.
d 5 equiv. of sodium acetate added (see procedure D in the Experimental section).
e Conversion 85%.
f Ascorbic acid + sodium acetate instead of hydrazine (see procedure C in the Experimental section).
g Conversion 76%.
|
|---|

|
Special attention has been paid to arylboronic acids possessing moieties sensitive to oxidation: aldehyde group, pyridine nitrogen, double bond and (hydroxymethyl)phenyl group. As expected, hydrazine is not compatible with aldehyde function and hydrazone 5 is formed by the original protocol from p-formylphenylboronic acid (3p). On the other hand, the procedure with ascorbic acid gave p-hydroxybenzaldehyde (4p) in an almost quantitative yield. This indicates that the 2/O2/ascorbic acid system is also useful on the preparative scale and, moreover, the procedure is chemoselective leaving the aldehyde function non-oxidized.19 The protocol with ascorbic acid was efficiently applied also to hydroxylation of o-nitrophenylboronic acid (3i) and 4-vinylphenylboronic acid (3m). The hydrazine based procedure is excluded for 3m to avoid double bond reduction by diimide which can be formed from hydrazine by the action of flavinium salts.20 On the other hand, the procedure with hydrazine succeeded in producing corresponding hydroxy derivatives from pyridin-3-ylboronic acid (3n) and 4-(hydroxymethyl)phenylboronic acid (3o) in quantitative conversion and with good yields. No side-oxidation of the double bond, pyridine nitrogen, as well as (hydroxymethyl)phenyl group was ever observed. Finally, cyclohexyl- (6a) and dodecylboronic acid (6b) were oxidized to the corresponding alcohols 7a and 7b by the unchanged protocol with hydrazine showing its applicability to alkylboronic acids.
In a preliminary mechanistic study on the course of oxidative hydroxylation, we tried to vary the amount of hydrazine relative to the substrate. We observed the quantitative production of phenol with only 0.5 equivalent of hydrazine showing that, similar to sulfoxidations,4d,f one equivalent of hydrazine generates two equivalents of dihydroflavine (the precursor of flavin hydroperoxide) in the catalytic cycle. During the first reduction step, hydrazine is oxidized to diimide which is still strong enough to reduce the second molecule of flavinium salt while it itself is oxidized to molecular nitrogen.4d1H and 11B NMR monitoring of the p-methylphenylboronic acid (3d) hydroxylation mediated by the 2/ascorbic acid/CH3COONa system21 in CF3CH2OH–CD3CN–D2O revealed a simple reaction course with no by-products (Fig. 1; see also ESI†). It also shows that the likely intermediates, e.g. adduct of 2-OOH on arylboronic acid and the corresponding arylborate, are readily converted to the reaction products (phenol and boric acid). Similarly, a simple course of hydroxylation was observed in aqueous solution (see ESI† for details).
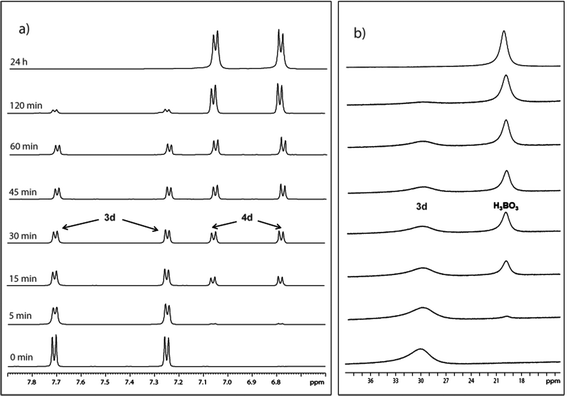 | ||
Fig. 1 Course of aerial oxidation of 3d to 4d mediated by the 2/ascorbic acid/CH3COONa system in CF3CH2OH–CD3CN–D2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) monitored by 1H (a) and 11B (b) NMR; for full spectra, see ESI.† 3) monitored by 1H (a) and 11B (b) NMR; for full spectra, see ESI.† | ||
Conclusions
In conclusion, flavinium salt 2, in the presence of a sacrificial reducing agent, catalysed the oxidative hydroxylation of arylboronic acids to phenols with molecular oxygen which is a new synthetic application for the flavin-based biomimetic systems. The flavinium-based method is organocatalytic, thus distinguishing it from other catalytic procedures for the hydroxylation of arylboronic acids. The method is characterized by a broad substrate scope, including ortho-substituted arylboronic acids and derivatives with groups sensitive to oxidation. It is also applicable for the transformation of alkylboronic acids to alcohols. The reaction is performed under mild conditions with potential use of water as a solvent. The use of oxygen as a stoichiometric oxidant and hydrazine as a sacrificial reducing agent producing water and nitrogen as the only by-products ranks this method among the green procedures. The efficiency of the described catalytic system should be further increased by optimizing the structure of the flavin catalyst, and this is now underway in our laboratory.Experimental
Materials and methods
NMR spectra were recorded on a Varian Mercury Plus 300 (299.97 MHz for 1H, and 75.44 MHz for 13C) and a Bruker Avance DRX 500 (500.13 MHz for 1H, 125.77 MHz for 13C and 160.4 MHz for 11B) at 298 K unless otherwise indicated. Chemical shifts δ are given in ppm using residual solvent or tetramethylsilane as an internal standard for 1H and 13C NMR and BF3·Et2O as an external standard for 11B. Elemental analyses (C, H, N) were performed on a Perkin-Elmer 240 analyser. High-resolution mass spectra were obtained on an LTQ Orbitrap Velos (Thermo Fisher Scientific), equipped with an orbitrap mass analyzer. The mass spectrometer was operated in ESI mode (ESI source temperature 250 °C, potential 3000 V) with a mass range from 200 to 2000 a.m.u. TLC analyses were carried out on a DC Alufolien Kieselgel 60 F254 (Merck). Preparative column chromatography separations were performed on a silica gel Kieselgel 60 (0.040–0.063 mm) (Merck). Melting points were measured on a Boetius melting point apparatus and are uncorrected. Starting materials, reagents and substrates were obtained from commercial suppliers and used without further purification. The solvents were purified and dried using standard procedures. Catalyst 2 was prepared according to the modified protocol from the literature4d (see ESI† for details and characterization).General procedures for oxidative hydroxylations
Characterization of products of oxidative hydroxylations
Phenols 4a–4p and 5 and alcohols 7a and 7b resulting from oxidative hydroxylations of boronic acids were characterized by 1H and 13C NMR and the HR-MS technique. Spectral data correspond to those published in the literature (see ESI† for details).Acknowledgements
This work was financially supported by the Czech Science Foundation (grant no. P207/12/0447).Notes and references
- L. Marchetti and M. Levine, ACS Catal., 2011, 1, 1090–1118 CrossRef CAS.
- Applications of flavinium salts in catalysis are reviewed in: (a) G. de Gonzalo and M. W. Fraaije, ChemCatChem, 2013, 5, 403–415 CrossRef CAS; (b) F. G. Gelalcha, Chem. Rev., 2007, 107, 3338–3361 CrossRef CAS PubMed; (c) Y. Imada and T. Naota, Chem. Rec., 2007, 7, 354–361 CrossRef CAS PubMed.
- Very recent papers on H2O2 oxidations catalyzed by flavinium salts: (a) Y. Imada, T. Kitagawa, S. Iwata, N. Komiya and T. Naota, Tetrahedron, 2014, 70, 495–501 CrossRef CAS; (b) P. Ménová, H. Dvořáková, V. Eigner, J. Ludvík and R. Cibulka, Adv. Synth. Catal., 2013, 355, 3451–3462 CrossRef; (c) R. Jurok, J. Hodačová, V. Eigner, H. Dvořáková, V. Setnička and R. Cibulka, Eur. J. Org. Chem., 2013, 7724–7738 CrossRef CAS; (d) M. J. Pouy, E. M. Milczek, T. M. Figg, B. M. Otten, B. M. Prince, T. B. Gunnoe, T. R. Cundari and J. T. Groves, J. Am. Chem. Soc., 2012, 134, 12920–12923 CrossRef CAS PubMed; (e) A. T. Murray, P. Matton, N. W. G. Fairhurst, M. P. John and D. R. Carbery, Org. Lett., 2012, 14, 3656–3659 CrossRef CAS PubMed; (f) P. Ménová and R. Cibulka, J. Mol. Catal. A: Chem., 2012, 363–364, 362–370 CrossRef; (g) T. Hartman, V. Herzig, M. Buděšínský, J. Jindřich, R. Cibulka and T. Kraus, Tetrahedron: Asymmetry, 2012, 23, 1571–1583 CrossRef CAS.
- Papers on aerial oxidations catalyzed by flavinium salts: (a) Y. Imada, T. Kitagawa, H.-K. Wang, N. Komiya and T. Naota, Tetrahedron Lett., 2013, 54, 621–624 CrossRef CAS; (b) S. Chen, M. S. Hossain and F. W. Foss, ACS Sust. Chem. Eng., 2013, 1, 1045–1051 CrossRef CAS; (c) S. Chen and F. W. Foss, Org. Lett., 2012, 14, 5150–5153 CrossRef CAS PubMed; (d) Y. Imada, H. Iida, S. Ono, Y. Masui and S.-I. Murahashi, Chem.–Asian. J., 2006, 1, 136–147 CrossRef CAS PubMed; (e) Y. Imada, H. Iida, S.-I. Murahashi and T. Naota, Angew. Chem., Int. Ed., 2005, 44, 1704–1706 CrossRef CAS PubMed; (f) Y. Imada, H. Iida, S. Ono and S.-I. Murahashi, J. Am. Chem. Soc., 2003, 125, 2868–2869 CrossRef CAS PubMed.
- C. T. Walsh and T. A. Wencewicz, Nat. Prod. Rep., 2012, 175–200 Search PubMed.
- G. Merenyi and J. Lind, J. Am. Chem. Soc., 1991, 113, 3146–3153 CrossRef CAS.
- (a) B. P. Branchaud and C. T. Walsh, J. Am. Chem. Soc., 1985, 107, 2153–2161 CrossRef CAS; (b) P. B. Brondani, H. Dudek, J. S. Reis, M. W. Fraaije and L. H. Andrade, Tetrahedron: Asymmetry, 2012, 23, 703–708 CrossRef CAS.
- (a) D.-S. Chen and J.-M. Huang, Synlett, 2013, 499–501 CAS; (b) N. Mulakayala, Ismail, K. M. Kumar, R. K. Rapolu, B. Kandagatla, P. Rao, S. Oruganti and M. Pal, Tetrahedron Lett., 2012, 53, 6004–6007 CrossRef CAS; (c) A. Gogoi and U. Bora, Synlett, 2012, 1079–1081 CAS; (d) G. K. S. Prakash, S. Chacko, C. Panja, T. E. Thomas, L. Gurung, G. Rasul, T. Mathew and G. A. Olah, Adv. Synth. Catal., 2009, 351, 1567–1574 CrossRef CAS; (e) B. R. Travis, B. P. Ciaramitaro and B. Borhan, Eur. J. Org. Chem., 2002, 3429–3434 CrossRef CAS; (f) J. Simon, S. Salzbrunn, G. K. Surya Prakash, N. A. Petasis and G. A. Olah, J. Org. Chem., 2000, 66, 633–634 CrossRef; (g) K. S. Webb and D. Levy, Tetrahedron Lett., 1995, 36, 5117–5118 CrossRef CAS; (h) M. Hawthorne, J. Org. Chem., 1957, 22, 1001–1001 CrossRef CAS.
- (a) H. Yang, Y. Li, M. Jiang, J. Wang and H. Fu, Chem.–Eur. J., 2011, 17, 5652–5660 CrossRef CAS PubMed; (b) K. Inamoto, K. Nozawa, M. Yonemoto and Y. Kondo, Chem. Commun., 2011, 47, 11775–11777 RSC; (c) A. D. Chowdhury, S. M. Mobin, S. Mukherjee, S. Bhaduri and G. K. Lahiri, Eur. J. Inorg. Chem., 2011, 2011, 3232–3239 CrossRef CAS; (d) J. Xu, X. Wang, C. Shao, D. Su, G. Cheng and Y. Hu, Org. Lett., 2010, 12, 1964–1967 CrossRef CAS PubMed.
- (a) H.-L. Qi, D.-S. Chen, J.-S. Ye and J.-M. Huang, J. Org. Chem., 2013, 78, 7482–7487 CrossRef CAS PubMed; (b) K. Hosoi, Y. Kuriyama, S. Inagi and T. Fuchigami, Chem. Commun., 2010, 46, 1284–1286 RSC.
- (a) Y.-Q. Zou, J.-R. Chen, X.-P. Liu, L.-Q. Lu, R. L. Davis, K. A. Jørgensen and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 784–788 CrossRef CAS PubMed; (b) S. P. Pitre, C. D. McTiernan, H. Ismaili and J. C. Scaiano, J. Am. Chem. Soc., 2013, 135, 13286–13289 CrossRef CAS PubMed.
- C. Zhu, R. Wang and J. R. Falck, Org. Lett., 2012, 14, 3494–3497 CrossRef CAS PubMed.
- A. N. Cammidge, V. H. M. Goddard, C. P. J. Schubert, H. Gopee, D. L. Hughes and D. Gonzalez-Lucas, Org. Lett., 2011, 13, 6034–6037 CrossRef CAS PubMed.
- S.-I. Murahashi, S. Ono and Y. Imada, Angew. Chem., Int. Ed., 2002, 41, 2366–2368 CrossRef CAS.
- S. L. Murov, I. Carmichael and G. L. Hug, Handbook of Photochemistry, New York, 2nd edn, 1993 Search PubMed.
- pKa values for 3g, 3h, 3i and hydrazinium are 7.23, 8.40, 7.15 and 8.10, respectively; see ref. 17.
- (a) M. D. Liptak, K. C. Gross, P. G. Seybold, S. Feldgus and G. C. Shields, J. Am. Chem. Soc., 2002, 124, 6421–6427 CrossRef CAS PubMed; (b) S. P. Mezyk, M. Tateishi, R. MacFarlane and D. M. Bartels, J. Chem. Soc., Faraday Trans., 1996, 92, 2541–2545 RSC.
- Stronger bases, e.g. sodium carbonate, caused decomposition of flavinium catalyst.
- Oxidation of aromatic aldehydes to carboxylic acids or corresponding phenols (Dakin oxidation) mediated by flavins has been described; see ref. 3b and 4c.
- (a) Y. Imada, H. Iida and T. Naota, J. Am. Chem. Soc., 2005, 127, 14544–14555 CrossRef CAS PubMed; (b) C. Smit, M. W. Fraaije and A. J. Minnaard, J. Org. Chem., 2008, 73, 9482–9485 CrossRef CAS PubMed; (c) Y. Imada, T. Kitagawa, T. Ohno, H. Iida and T. Naota, Org. Lett., 2010, 12, 32–35 CrossRef CAS PubMed; (d) J. F. Teichert, T. den Hartog, M. Hanstein, C. Smit, B. ter Horst, V. Hernandez-Olmos, B. L. Feringa and A. J. Minnaard, ACS Catal., 2011, 1, 309–315 CrossRef CAS.
- Since hydrazine interaction with boronic acid results in signal broadening (see ESI†), we used ascorbic acid as a reducing agent.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of catalyst 2, characterization of products of oxidative hydroxylations and NMR studies. See DOI: 10.1039/c3ob42081g |
| This journal is © The Royal Society of Chemistry 2014 |