Brønsted acid catalyzed intramolecular benzylic cyclizations of alkylpyridines†
Abstract
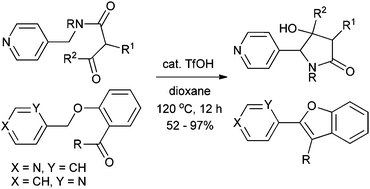
Aldehyde and ketone electrophiles incorporated into the side chains of 2- and 4-alkylpyridines participate in intramolecular aldol-like condensations with pyridine benzylic carbons in the presence of Brønsted acid catalysts. Pyridines featuring β-ketoamide side chains undergo cyclization in the presence of 10 mol% TfOH to afford pyridyl-substituted hydroxy lactams in good yield. These products were found to be resistant to further dehydration under a variety of conditions, however treatment with thionyl chloride elicited an unusual dehydration/oxidation reaction sequence. In contrast, acid-catalyzed cyclization of pyridines tethered to aliphatic aldehydes with amine linkers gives pyridyl-substituted dehydro-piperidine products. Similarly, intramolecular condensation of salicylaldehyde- and salicylketone-substituted pyridines affords pyridyl-substituted benzofurans.

 Please wait while we load your content...
Please wait while we load your content...