Photoresponsive vesicle permeability based on intramolecular host–guest inclusion†
Ulrike
Kauscher‡
,
Avik
Samanta‡
and
Bart Jan
Ravoo
*
Organic Chemistry Institute and Graduate School of Chemistry, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany. E-mail: b.j.ravoo@uni-muenster.de
First published on 12th November 2013
Abstract
This article describes light-responsive vesicles that can release their contents in response to a light-sensitive molecular trigger. To this end, liposomes were equipped with amphiphilic β-cyclodextrin that was covalently labeled with azobenzene. Using dye encapsulation and confocal laser scanning microscopy, we show that the permeability of these vesicles strongly increases upon UV irradiation (λ = 350 nm) with concomitant isomerization of apolar trans-azobenzene to polar cis-azobenzene on the liposome surface.
Introduction
Compartmentalization of intracellular machineries and metabolic processes from the extracellular environment is a crucial role of the biological cell membrane. Over the last decade, multiple studies have aimed to mimic complex biological processes by constructing artificial nano-assemblies.1 In this context, vesicles are intriguing biomimetic, self-assembled structures which have been investigated as simple artificial cellular systems and nanocarriers for drug delivery.2 Such cell-mimetic nano-assemblies require transmembrane channels to achieve the connection between the internal compartment of the vesicle and the external environment. A liposomal membrane composed of conventional phospholipids is generally liquid crystalline in nature and the permeability for hydrophilic molecules such as amino acids, phosphates, etc. is very low.3 It is a challenge to modify the building blocks of the self-assembled vesicular system to improve the permeability and in particular to facilitate a controlled delivery of entrapped payload by these nanocontainers. Various studies have been performed to increase permeability of vesicles and liposomes by incorporating channel proteins,4,5 pore forming peptides6–8 or entirely synthetic channels9,10 in the membrane or fabricating organic–inorganic hybrid vesicles11 and polymersomes.12–14 Stimuli-responsive self-assembled nanocontainers are also interesting and widely used in the development of sophisticated controlled release systems.15,16 For example, the stimuli-regulated change in size, orientation or polarity of the building blocks can lead to open or close transmembrane channels. In this context, Gin and coworkers reported an elegant light-responsive cyclodextrin (CD) based ion channel embedded in liposomes.17In recent years, we have described a modular vesicular system composed of amphiphilic CDs.18 If the primary side of the CDs is functionalized with hydrophobic alkyl chains and additionally hydrophilic oligo(ethylene glycol) groups are installed at the secondary side, the resulting amphiphilic macrocycles form unilamellar bilayer vesicles in aqueous solution with an average diameter of around 100 nm. Such unilamellar bilayer vesicles resemble conventional liposomes, but the CD cavities at the outer surface of the vesicles make these nanocontainers more versatile. The CD vesicles can selectively form inclusion complexes with hydrophobic guest moieties (such as adamantanes, t-butylbenzenes, and azobenzenes) allowing them to be decorated with synthetically modified guest molecules such as peptides,19 proteins,20 DNA,21 hydrophobic ions22 and polymers23 simply by mixing the host vesicles with the desired mixture of guest-appended functional molecules. Recently, we have reported that mixed vesicles with essentially impermeable membranes can be obtained by the incorporation of amphiphilic β-CD into conventional liposomes consisting of dioleyl phosphatidyl ethanolamine (DOPE), dioleyl phosphatidyl choline (DOPC), and cholesterol.24 A unique feature of these mixed vesicles is their ability to form host–guest complexes with suitable guest molecules (similar to CD vesicles) even with a rather low concentration of CDs. It is also noteworthy that the permeability of the membrane increases with the percentage of amphiphilic CD. Thus, mixed vesicles with a small percentage of CD combine the attractive feature of selective host–guest chemistry at the vesicle surface with a low permeability of the vesicles.
In this contribution we report a new generation of stimulus-responsive vesicles of which the transmembrane permeability is triggered by light. To this end, amphiphilic β-CD was functionalized with an azobenzene moiety (Azo-CD) to obtain an intramolecular host–guest inclusion complex with the CD cavity. Azobenzenes are a well-known class of light-responsive guest molecules for CDs that can be reversibly isomerized from trans to cis by irradiation at 350 nm and from cis to trans by irradiation at 455 nm. The rod-like, apolar trans isomer forms a stable inclusion complex with β-CD, whereas the bent, polar cis isomer does not fit into CD cavities.25 The azobenzene labeled amphiphilic β-CD was incorporated in liposomes. It was our hypothesis that the isomerisation of the azobenzene moiety might change the polarity and water affinity of the lipid membrane so that transmembrane passages will open in response to light.
To prove our assumption, we performed a set of experiments on large and giant unilamellar bilayer vesicles (LUVs and GUVs) composed of Azo-CD, DOPE, DOPC and cholesterol. The structure of these vesicles is shown in Fig. 1. The properties of the LUVs were investigated using dynamic light scattering (DLS) and dye encapsulation experiments. Photo-triggered permeability of giant unilamellar vesicles (GUVs) was observed via confocal laser scanning microscopic (CLSM) experiments. We also demonstrate that these vesicles can be aggregated in the presence of a divalent non-covalent linker (G1) via a light trigger.
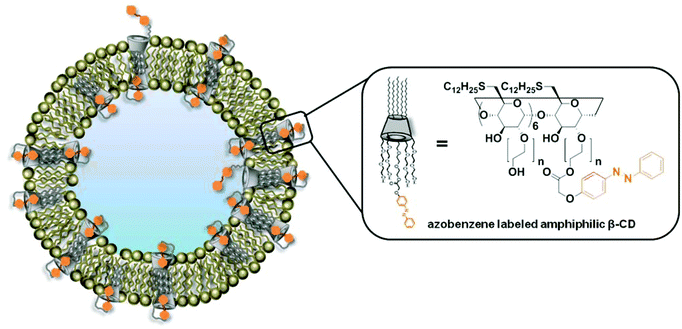 | ||
| Fig. 1 Schematic representation of vesicles prepared from a mixture of azobenzene labeled amphiphilic β-CD (Azo-CD), DOPE, DOPC and cholesterol. | ||
Methods
Materials
All chemicals used in this study were purchased from Acros Organics (Schwerte, Germany) or Sigma–Aldrich Chemie (Taufkirchen, Germany) and were used without further purification. β-CD was kindly donated by Wacker Chemie (Burghausen, Germany). All solvents were dried according to conventional methods if desired. The synthesis of G1 is described in the literature.26 The synthesis and characterization of Azo-CD are described in the ESI.†Preparation of vesicles
Unilamellar bilayer vesicles of Azo-CD and phospholipids with an average diameter of 80–100 nm were prepared by hydration of a lipid film in buffer solution (20 mM HEPES, 90 mM NaCl, pH 7.5) and extrusion with a Liposofast manual extruder through a polycarbonate membrane with a pore size of 100 nm. The lipid film was obtained by evaporating a chloroform solution containing Azo-CD mixed with the desired amount of lipid mixture (DOPE–DOPC–cholesterol 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Giant unilamellar vesicles (GUVs) were formed using the electroformation method.27 Azo-CD was combined with the required amount of a lipid mixture to make a 1 mM solution of amphiphiles in chloroform. 16 μL of the chloroform solution was spread on the electrode slides coated with ITO, and chloroform was evaporated in a vacuum oven at 50 °C. The film was hydrated with buffer solution (1 mM HEPES, 300 mM sucrose, pH 7.5). An alternating electric field was applied at 1 V and 10 Hz at 50 °C for 1 h. The GUVs were investigated with a Leica DMRE with a TCS SL scanning unit (Leica Microsystems Heidelberg GmbH, Mannheim, Germany). A buffer solution with 300 mM sucrose was used to improve the visualization of the GUVs by CLSM.
1). Giant unilamellar vesicles (GUVs) were formed using the electroformation method.27 Azo-CD was combined with the required amount of a lipid mixture to make a 1 mM solution of amphiphiles in chloroform. 16 μL of the chloroform solution was spread on the electrode slides coated with ITO, and chloroform was evaporated in a vacuum oven at 50 °C. The film was hydrated with buffer solution (1 mM HEPES, 300 mM sucrose, pH 7.5). An alternating electric field was applied at 1 V and 10 Hz at 50 °C for 1 h. The GUVs were investigated with a Leica DMRE with a TCS SL scanning unit (Leica Microsystems Heidelberg GmbH, Mannheim, Germany). A buffer solution with 300 mM sucrose was used to improve the visualization of the GUVs by CLSM.
UV/vis spectroscopy
Optical density measurements were carried out in 1.5 mL disposable PMMA cuvettes with dimensions 12.5 × 12.5 × 45 mm and 10 mm path length using a Uvikon 923 double-beam spectrophotometer. The time dependent optical density was measured at λ = 600 nm (OD600), which is far from the absorption of the azobenzene chromophore. Measurements were performed for 40 to 60 min; with data points collected every 12 s. For the aggregation study, 1 mL solution of vesicles (10, 20 or 30 mol% of Azo-CD in lipid mixture) in aqueous buffer (20 mM HEPES, 90 mM NaCl, pH 7.5) were taken in a cuvette. After 2 min a DMSO solution of G1 (10 to 20 μM) was added with slight mixing within one interval of 12 s.Irradiation experiments
A Rayonet photochemical reactor (The Southern New England Ultraviolet Company) equipped with 16 RPR-3500 lamps used to generate UV light (350 nm) was utilized for irradiation experiments for the isomerization of Azo-CD. Samples were irradiated for 15 min.Dye encapsulation
A 20 mM solution of sulforhodamine B was prepared in buffer solution (20 mM HEPES, 90 mM NaCl, pH 7.5). Vesicles were obtained by the hydration of a film of Azo-CD and phospholipids with sulforhodamine B containing buffer solution. The total concentration of amphiphiles was 1 mM. The dye encapsulated vesicle solution (500 μL) was loaded onto a Sephadex G-50 size-exclusion column with buffer solution (20 mM HEPES, 90 mM NaCl, pH 7.5) as an eluent to separate the vesicles and the free dye. A fraction of around 2 mL was collected and filled up to 5 mL to yield an amphiphile concentration of around 0.1 mM. The fluorescence spectrum of the fraction was measured using a JASCO FP6500 spectrofluorometer at an excitation wavelength of 490 nm. A reference sample was prepared by the addition of 0.1% Triton X-100.Results and discussion
The synthesis of Azo-CD was carried out using an esterification of the oligo(ethylenglycol) chains of an amphiphilic β-CD substituted with n-dodecyl groups on the primary side. Details of the synthesis are reported in the ESI.† The analytical data are consistent with the molecular structure shown in Fig. 1 and the NMR and mass spectra indicate an average degree of substitution of a single azobenzene unit on each Azo-CD.Our initial aim was the preparation of unilamellar vesicles composed exclusively of Azo-CD by hydration of a dried film and extrusion. However, these experiments resulted in unstable and turbid suspensions indicating the formation of multilamellar vesicles (MLV) or aggregated LUVs. It should be emphasized that amphiphilic CDs without the azobenzene substituent can be readily dispersed as small or large unilamellar vesicles by sonication or extrusion, respectively.22 The instability of the Azo-CD vesicles can be explained by the formation of inclusion complexes between azobenzene units on one vesicle and Azo-CDs on another vesicle (intervesicular binding) instead of the Azo-CD to which the azobenzene linker is also covalently bound (intramolecular binding or self-inclusion). We thus pursued a different approach for the formation of stable unilamellar vesicles. It was our hypothesis that a decrease in the number of CDs per vesicle would favour intramolecular inclusion of azobenzene at the expense of intervesicular inclusion. To this end, the vesicles which were used in the following experiments were obtained via the formation of a lipid film containing a minority mole fraction (max. 30 mol%) of Azo-CD and a majority fraction of phospholipids. The lipid mixture consisted of 50 mol% DOPC, 25 mol% DOPE and 25 mol% cholesterol and remained constant in all experiments. The percentage of Azo-CD in the vesicles indicated in the following refers to the molar ratio of Azo-CD and total lipid (DOPC plus DOPE plus cholesterol), so that for example “20 mol% Azo-CD” implies a molar ratio of Azo-CD/DOPC/DOPE/cholesterol of 20/40/20/20.
In a first set of experiments we verified the formation of mixed vesicles of Azo-CD and phospholipids by DLS. Vesicles were prepared with 10, 20 or 30 mol% Azo-CD. The resulting lipid film was hydrated with buffer (20 mM HEPES, 90 mM NaCl, pH 7.5) and the obtained dispersion of MLVs was extruded through a 0.1 μm polycarbonate membrane to provide LUVs. The total amphiphile concentration was 0.2 mM. DLS measurements indicate the formation of vesicles with an average size of 100 nm irrespective of the Azo-CD content. The vesicles are solubilized upon addition of Triton X-100 (0.1 wt%) and the formation of micelles with a size of around 5 nm is indicated by DLS (Fig. 2).
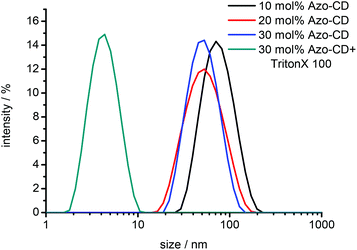 | ||
| Fig. 2 Dynamic light scattering (DLS) of mixed vesicles and vesicles dissolved by Triton X-100. Total amphiphile concentration: 0.2 mM. | ||
The photo-isomerization of Azo-CD was measured using UV-vis spectroscopy. The absorbance spectra for vesicles containing 30 mol% Azo-CD are shown in Fig. 3. These vesicles show an absorption peak at 350 nm consistent with the predominant trans-state of the azobenzene. Upon irradiation with UV light (λ = 350 nm) for 15 min the azobenzenes are converted into the cis-isomer and the absorption band at 350 nm disappears. Instead, a (weaker) absorbance maximum at 450 nm is observed, consistent with the formation of the cis-isomer. Irradiation of the vesicles with visible light (λ = 455 nm) for 15 min switches the azobenzene back into the trans-state.
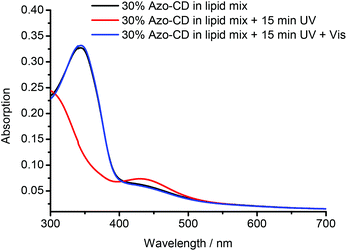 | ||
| Fig. 3 UV-vis absorbance measurements of mixed vesicles containing 30 mol% Azo-CD (as prepared, irradiated at 350 nm, and irradiated at 455 nm). | ||
In order to obtain more information about the structure of the vesicles, GUVs were formed via the electroformation method. GUVs with 20 mol% of Azo-CD were obtained successfully as can be seen in Fig. 4a. The GUVs are polydisperse with diameters ranging from 5 to 250 μm. After we were able to verify the formation of GUV with Azo-CD, our next concern was the investigation of the vesicle stability during photo-isomerization of the azobenzene moiety between its trans- and cis-state. To this end, the GUVs were irradiated for 10 min with light of 350 nm wavelength. Fig. 4b shows that the irradiation has no influence on the average size, shape or stability of the GUVs.
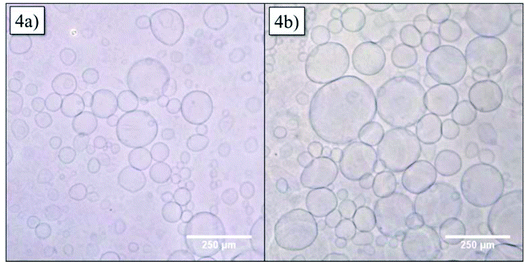 | ||
| Fig. 4 Optical microscopy of giant unilamellar vesicles (GUVs) containing 20 mol% Azo-CD (a) before and (b) after irradiation at 350 nm for 10 min. | ||
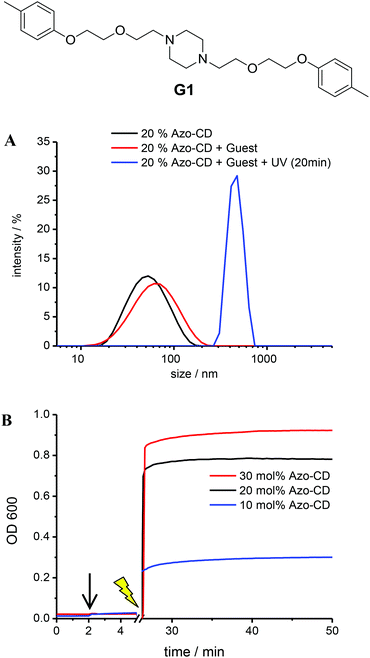
Further experiments were carried out to investigate the availability of the CD cavities as a function of the Azo-CD photoisomerization. It is reasonable to assume that most CD cavities are occupied due to intramolecular complexation in case the azobenzene is in the (binding) trans-state, whereas the CD cavities should be unoccupied and available for other guests if the azobenzene is in the (nonbinding) cis-state. Inspired by experiments described in a previous publication,26 the divalent guest molecule G1 (Fig. 5) was selected to verify the light-induced binding of a competitive guest. Guest G1 can cause aggregation of the vesicles, since it can connect CD cavities on two different vesicles, so that the vesicles are cross-linked.26 To this end, LUVs with 10, 20 or 30 mol% of Azo-CD were prepared. The concentration of G1 was invariably twice the concentration of Azo-CD so that G1 would be able to saturate all available cavities exposed in the outer surface of the vesicles. As can be seen from Fig. 5, the addition of the divalent guest G1 to vesicles containing Azo-CD did not cause any aggregation: the average vesicle size remains constant and the optical density (OD600) of the solution is unaffected. This is consistent with the scenario that all CD cavities are blocked by intramolecular complexation with the azobenzene moieties. However, upon irradiation with UV light (λ = 350 nm) for 15 min, the average vesicles size (as measured by DLS, Fig. 5a) and the optical density (Fig. 5b) steeply increase, indicating the photo-triggered aggregation of the vesicles. These observations strongly suggest that when the azobenzenes are photo-isomerized into the cis-form, the intermolecular inclusion complex dissociates and the CD cavities are vacated, so that G1 can bind in an intervesicular fashion and induce aggregation of the vesicles due to the formation of multiple noncovalent cross-links. This behaviour was observed for every mole fraction of Azo-CD (10–30 mol%) investigated and is fully consistent with our previous investigations on competitive host–guest interactions of CD vesicles.26
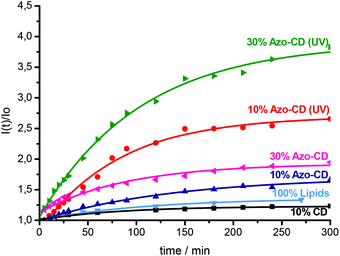
Finally we investigated the permeability of LUVs and GUVs containing Azo-CD. LUVs were prepared in a solution of sulforhodamine B at a self-quenching concentration (20 mM) and separated from the free dye by gel filtration on a column of Sephadex G50. After gel filtration, the vesicles were diluted to approximately 0.1 mM total amphiphile concentration and the sulforhodamine B fluorescence intensity was measured over time and finally after the addition of Triton X-100. Triton X-100 solubilizes the vesicles and leads to a maximum fluorescence intensity due to complete relief of self-quenching (see ESI† for the fluorescence spectra). Using 10 mol% of Azo-CD, the encapsulation of sulforhodamine B was quite efficient and the permeability of the vesicles is rather low, even though such vesicles are somewhat more permeable than vesicles with 10 mol% of amphiphilic CD lacking the azobenzene or liposomes without any CD (Fig. 6). However, the leakage of sulforhodamine B from the vesicles can be significantly accelerated by UV irradiation (λ = 350 nm). Vesicles with 30 mol% of Azo-CD are slightly more permeable than vesicles with 10 mol% of Azo-CD. Nevertheless, more importantly, UV irradiation results in a faster and more extensive release of entrapped sulforhodamine B (Fig. 6). These experiments clearly show that the permeability of the vesicles can be regulated via a light trigger and that the entrapped dye is released faster if the vesicles have a higher Azo-CD content.
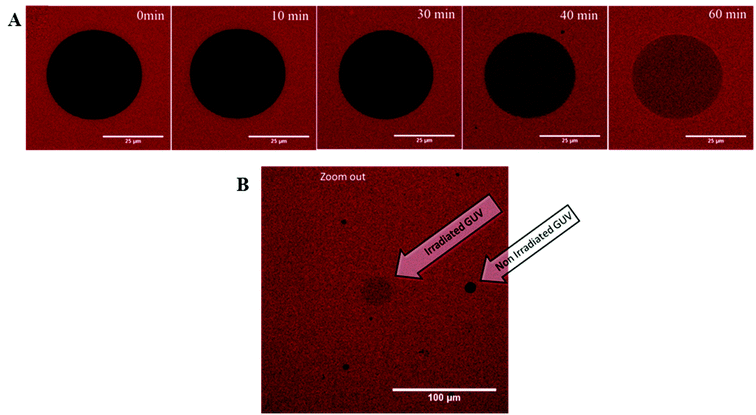
An additional experiment was done to visualize the light triggered permeability of the vesicles by confocal microscopy. In this case, GUVs were prepared in buffer solution and sulforhodamine B was added to the GUVs. Hence, the dye stained the solution surrounding the GUVs while the GUV interior does not show significant fluorescence. Upon irradiation at 350 nm (in this case, the Hg-lamp of the microscope was used as the light source), the permeability of the GUVs increases and the dye diffuses into the GUVs (Fig. 7A). Permeation proceeds until the fluorescence intensity (i.e. the dye concentration) inside the vesicle is of roughly the same value as the intensity outside the vesicles. A low magnification snap shot of the vicinity of the irradiated vesicle shows that the fluorescence intensity of neighbouring vesicles which had not been irradiated remains negligible (Fig. 7B). This experiment was repeated for numerous GUVs and the irradiation time required to obtain the same fluorescence intensity inside and outside the vesicles differs between 20 min and 150 min (see Fig. S3 in the ESI†). These results reflect the fact that the experiments with GUVs are in principle single vesicle experiments, in which statistical variations due to differences in size and composition of each GUV as well as local defects induced by the adhesion of the GUVs to the microscopy slides lead to a rather broad range of influx times observed by confocal microscopy. On the other hand, it is evident from a comparison of the data for LUVs presented in Fig. 6 and the observations for GUVs shown in Fig. 7 as well as Fig. S3† that the efflux of dye from LUVs and the influx of dye into GUVs occur on a similar time scale and that equilibrium is typically reached after about 2 h in either case.
It should be emphasized that it is not realistic to assume that the permeability of vesicles with Azo-CD is photo-triggered because the CDs operate as a light-gated channel. Although it is well known that CDs can function as (photoactive) synthetic ion channels,17 it can be ruled out that a large molecule such as sulforhodamine B can pass through the cavity of a β-CD macrocycle. Indeed, it can be seen in Fig. 6 that vesicles with amphiphilic CD lacking the azobenzene unit are less permeable for this dye than vesicles with Azo-CD. Thus, the photo-triggered permeability of vesicles with Azo-CD must have a different origin. We propose that the azobenzene units in Azo-CD have a small yet significant destabilizing effect on the CD vesicles, leading to more permeable vesicles (compared to vesicles containing CDs without azobenzene) even if the trans-azobenzene is mostly bound in an intramolecular complex. We speculate that even the formation of such intramolecular complexes will lead to small and local defects in the vesicle membranes, resulting in enhanced permeability compared to vesicles containing CDs without azobenzene. Moreover, upon photo-isomerization of the azobenzene to the cis-state, the polar cis-azobenzene units confined to the membrane lead to severe disruption of the bilayer packing and high permeability even for large polar molecules such as sulforhodamine B. Hence, the photo-triggered permeability should be explained in terms of photo-induced membrane defects, not by a light-gated channel. We emphasize that such defects are not a consequence of phase separation in the bilayer membrane, since we have previously shown that homogeneous GUVs of amphiphilic CDs and lipids are obtained independent of the CD content.24
Conclusion
In this paper we have described vesicles with photo-triggered membrane permeability. We have shown that phospholipid vesicles can be equipped with a photoactive CD (Azo-CD) which can be isomerized from a trans-state to a cis-state. We have also shown that the size, shape and stability of these vesicles are not affected by the photoisomerization of Azo-CD. However, the photoisomerization of Azo-CD from trans to cis regulates the availability of the CD cavities on the vesicles surface for complexation with other guest molecules. In addition, the permeability of the vesicles is much higher when the Azo-CD is isomerized to the cis-state, even for a rather large dye molecule such as sulforhodamine B. The efflux of the dye from small vesicles and the influx from giant vesicles occur on a similar time scale. We contend that the stimulus-responsive supramolecular system described here is of relevance to the development of sophisticated light-triggered drug delivery systems.Acknowledgements
We are grateful to the Graduate School of Chemistry for a fellowship for A. S. This work was supported by COST CM1005 “Supramolecular Chemistry in Water”.References
- J. W. Szostak, D. P. Bartel and P. L. Luisi, Nature, 2001, 409, 387 CrossRef CAS PubMed.
- D. M. Vriezema, M. Comellas Aragonés, J. A. Elemans, J. J. Cornelissen, A. E. Rowan and R. J. Nolte, Chem. Rev., 2005, 105, 1445 CrossRef CAS PubMed.
- A. C. Chakrabarti and D. W. Deamer, Biochim. Biophys. Acta, 1992, 1111, 171 CrossRef CAS.
- A. Graff, M. Winterhalter and W. Meier, Langmuir, 2001, 17, 919 CrossRef CAS.
- G. Huysmans, A. Ranquin, L. Wyns, J. Steyaert and P. Van Gelder, J. Controlled Release, 2005, 102, 171 CrossRef CAS PubMed.
- B. Okumus, S. Arslan, S. M. Fengler, S. Myong and T. Ha, J. Am. Chem. Soc., 2009, 131, 14844 CrossRef CAS PubMed.
- V. Noireaux and A. Libchaber, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17669 CrossRef CAS PubMed.
- R. Ferdani, R. Q. Li, R. Pajewski, J. Pajewska, R. K. Winter and G. W. Gokel, Org. Biomol. Chem., 2007, 5, 2423 CAS.
- M. Yano, C. C. Tong, M. E. Light, F. P. Schmidtchen and P. A. Gale, Org. Biomol. Chem., 2010, 8, 4356 CAS.
- T. M. Fyles and H. Luong, Org. Biomol. Chem., 2009, 7, 733 CAS.
- K. Yasuhara, T. Kawataki, S. Okuda, S. Oshima and J. Kikuchi, Chem. Commun., 2013, 49, 665 RSC.
- K. T. Kim, J. J. Cornelissen, R. J. Nolte and J. C. van Hest, Adv. Mater., 2009, 21, 2787 CrossRef CAS.
- A. Koide, A. Kishimura, K. Osada, W. D. Jang, Y. Yamasaki and K. Kataoka, J. Am. Chem. Soc., 2006, 128, 5988 CrossRef CAS PubMed.
- J. Montenegro, J. Braun, O. Fischer-Onaca, W. Meier and S. Matile, Org. Biomol. Chem., 2011, 9, 6623 CAS.
- G. K. Such, E. Tjipto, A. Postma, A. P. R. Johnston and F. Caruso, Nano Lett., 2007, 7, 1706 CrossRef CAS PubMed.
- L. J. De Cock, S. De Koker, B. G. De Geest, J. Grooten, C. Vervaet, J. P. Remon, G. B. Sukhorukov and M. N. Antipina, Angew. Chem., Int. Ed., 2010, 49, 6954 CrossRef CAS PubMed.
- P. V. Jog and M. S. Gin, Org. Lett., 2008, 10, 3693 CrossRef CAS PubMed.
- B. J. Ravoo and R. Darcy, Angew. Chem., Int. Ed., 2000, 39, 4324 CrossRef CAS.
- F. Versluis, I. Tomatsu, S. Kehr, C. Fregonese, A. W. J. W. Tepper, M. C. A. Stuart, B. J. Ravoo, R. I. Koning and A. Kros, J. Am. Chem. Soc., 2009, 131, 13186 CrossRef CAS PubMed.
- A. Samanta, M. C. A. Stuart and B. J. Ravoo, J. Am. Chem. Soc., 2012, 134, 19909 CrossRef CAS PubMed.
- S. K. M. Nalluri, J. Voskuhl, J. L. Bultema, E. J. Boekema and B. J. Ravoo, Angew. Chem., Int. Ed., 2011, 50, 9747 CrossRef CAS PubMed.
- P. Falvey, C. W. Lim, R. Darcy, T. Revermann, U. Karst, M. Giesbers, A. T. M. Marcelis, A. Lazar, A. W. Coleman, D. N. Reinhoudt and B. J. Ravoo, Chem.–Eur. J., 2005, 11, 1171 CrossRef CAS PubMed.
- B. J. Ravoo, J. C. Jacquier and G. Wenz, Angew. Chem., Int. Ed., 2003, 42, 2066 CrossRef CAS PubMed.
- U. Kauscher, M. C. A. Stuart, P. Drücker, H. J. Galla and B. J. Ravoo, Langmuir, 2013, 29, 2692–2699 CrossRef PubMed.
- Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823 CrossRef CAS PubMed.
- S. K. M. Nalluri, J. B. Bultema, E. J. Boekema and B. J. Ravoo, Chem.–Eur. J., 2011, 17, 10297 CrossRef CAS PubMed.
- M. I. Angelova and D. S. Dimitrov, Faraday Discuss., 1986, 81, 303 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: the synthesis of Azo-CD, NMR spectra and fluorescence spectra are provided as ESI. See DOI: 10.1039/c3ob41893f |
| ‡ These authors contributed equally to this work. Alphabetical order. |
| This journal is © The Royal Society of Chemistry 2014 |