One-pot formation of fluorescent γ-lactams having an α-phosphorus ylide moiety through three-component α(δ′)-Michael reactions of phosphines with an enyne and N-tosyl aldimines†
Yu-Wei
Lin
,
Jie-Cheng
Deng
,
You-Zung
Hsieh
and
Shih-Ching
Chuang
*
Department of Applied Chemistry, National Chiao Tung University, 1001 Ta Hsueh Road, Hsinchu, Taiwan 30010, Republic of China. E-mail: jscchuang@faculty.nctu.edu.tw
First published on 16th October 2013
Abstract
We demonstrate a straightforward synthesis of γ-lactams possessing an α-phosphorus ylide moiety from assembly of phosphines, N-tosyl aldimines and an enyne through an initial α(δ′)-attack of phosphines to an enyne in up to 79% yield. The investigated multicomponent reaction tolerates a variety of triarylphosphines and electron-poor aldimines to give γ-lactams in one pot. One of the lactams, with the tri(p-tol)phosphine and 4-cyanophenyl moiety, exhibits fluorescence emission at 447 nm with a quantum yield of 0.11.
Introduction
Multicomponent reactions (MCRs) are synthetic reactions showing expediency, molecular diversity and step-economy which are used to construct complex molecular structures from simple reactants in one pot.1 In this context, the initial reactive intermediates could be generated from nucleophilic attack of amine,2 phosphine3 or isocyanide4 species to electron-deficient acetylenes/allenes followed by subsequent addition to electrophiles. In recent years, versatile reactions using phosphine-catalysis have also been demonstrated for the syntheses of various heterocyclic natural products and bioactive compounds.5 For example, the highly functionalized coumarins,6 tetrahydropyridines,7 2-pyrones8 and bicarbocyclic skeletons9 can be prepared efficiently through the methodology of phosphine-catalysis. Recently, we have developed a three-component reaction (3CR) of phosphines, enynes 1 and aldehydes through an initial region-selective α(δ′)-attack of phosphines to enynes that form γ-lactones possessing an α-phosphorus ylide moiety (Scheme 1).10 The same methodology can also be used to react with [60]fullerene to give cyclopentenofullerene derivatives in one pot,11 and is also transferable to substrates such as dimethyl acetylenedicarboxylate (DMAD) in a particular molar ratio of the three reactants. The assembled products, γ-lactones possessing α-phosphorus ylides, are reactive toward electron-poor aldehydes as Wittig reagents to give substituted α-benzylidine lactones.12 In addition, primary and secondary amines also undergo α(δ′)-nucleophilic attack to enyne 1.13Due to our continuing interest in expanding this methodology for practical applications, we subsequently chose to develop the synthesis of the γ-lactam core structure by MCRs since we have noted that the natural products, isatin and its derivatives possessing a γ-lactam moiety, can be used as useful building blocks for the syntheses of other structurally relevant bioactive molecules.14 We are able to construct isatin derivatives through this developed α(δ′)-Michael addition.15 Further, the approaches to build up a γ-lactam moiety with multiple functional substituents in one step remain to be developed16 in addition to other previous examples.17 Herein, we wish to report the one-pot synthesis and characterization of fluorescent γ-lactams possessing α-phosphorus ylides through an initial α(δ′)-Michael addition of phosphines to an enyne by MCRs (Scheme 1).
Results and discussion
First of all, we briefly delineate the condition optimization of the synthesis of γ-lactams by three-component assembly of enyne 1, triphenylphosphine (2a), and aldimine 3a. We find that the reaction with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1
3a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gives a relatively better yield (32%) in the aprotic etherate solvent tetrahydrofuran (THF) at 60 °C for 2 h (Table 1, entries 1–5). When we increase the molar ratio of both 1 and 2a (1.5 equiv.) for generating relatively greater amounts of reactive 1,3-dipolar species, we observe an increase of reaction yield to 44% (entry 6). Further increment of the relative molar ratio of 1
1 gives a relatively better yield (32%) in the aprotic etherate solvent tetrahydrofuran (THF) at 60 °C for 2 h (Table 1, entries 1–5). When we increase the molar ratio of both 1 and 2a (1.5 equiv.) for generating relatively greater amounts of reactive 1,3-dipolar species, we observe an increase of reaction yield to 44% (entry 6). Further increment of the relative molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a to 2
3a to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gives the highest yield of 57% (entry 7). Other adjustments of the conditions such as time-shortening to 1 h (entry 8) or carrying out the reaction under milder conditions at r.t. (entries 9 and 10) do not improve the yields of the reaction notably.
1 gives the highest yield of 57% (entry 7). Other adjustments of the conditions such as time-shortening to 1 h (entry 8) or carrying out the reaction under milder conditions at r.t. (entries 9 and 10) do not improve the yields of the reaction notably.
| Entry | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: a mixture of 1 (0.30 mmol), 2a (0.30 mmol) and 3a (0.30 mmol) under nitrogen in anhydrous solvents.
b Yield is determined by a 1H NMR spectroscopic method using mesitylene as an internal standard.
c Molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1.5 3a = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
d Molar ratio of 1 1.
d Molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 2 3a = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
e 1,2-Dichloroethane. 1.
e 1,2-Dichloroethane.
|
||||
| 1 | DCM | r.t. | 2 | 21 |
| 2 | THF | 60 | 2 | 32 |
| 3 | Toluene | 60 | 2 | 25 |
| 4 | MeCN | 60 | 2 | 27 |
| 5 | DCEe | 60 | 2 | 22 |
| 6c | THF | 60 | 2 | 44 |
| 7d | THF | 60 | 2 | 57 |
| 8d | THF | 60 | 1 | 48 |
| 9d | THF | r.t. | 24 | 48 |
| 10d | THF | r.t. | 48 | 51 |
We investigate the scope of currently developed three-component reactions with other triarylphosphines and electron-poor aldimines. As shown in Table 2, γ-lactams can be assembled with isolated yields ranging from 49 to 79%, with variously substituted triarylphosphines 2a–f and 4-nitrobenzaldimine (3a) (entries 1–6); among these phosphines, tris(4-chlorophenyl)phosphine (2c) performs the best to give 79% yield (entry 4) and the reaction with a non-aryl hexamethylphosphorus triamide (2f, HMPT) produces 4f in a comparable yield of 53% (entry 6) as those with phosphines 2a–f. We next evaluate the performance with other substituted aldimines 3b–d and find that the reactions proceed to give yields spanning from 22 to 71%. It is worthy to note that the present assembly reaction proceeds with phosphines such as the more nucleophilic P(cHex)3 (2h) and the less nucleophilic P(NMe2)3 (2f), but these two phosphines did not work well in the syntheses of the corresponding γ-lactones with aldehydes as substrates.9 The reaction with a more nucleophilic tricyclohexylphosphine (2h) gives a relatively poor yield (entry 16, 22%), likely due to the presence of a P(cHex)3 moiety that makes the corresponding product (4p) unstable. This notion is further evidenced from the fact that reaction products are not isolable when we use trialkylphosphines such as PMe3, PEt3, P(n-Pr)3 and P(n-Bu)3; with these trialkylphosphines, only a trace amount of products resulting from P(n-Bu)3 is observed.
| Entry | 2; PR3 | 3; R′ | 4 | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: a mixture of 1 (0.30 mmol), 2 (0.30 mmol) and 3 (0.15 mmol) under nitrogen in anhydrous THF.
b Yields (%) were determined by a 1H NMR spectroscopic method using mesitylene as an internal standard after isolation by flash SiO2 column chromatography.
c Room temperature.
d Molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 = 1 3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
||||
| 1 | 2a; PPh3 | 3a; 4-NO2 | 4a | 57 |
| 2 | 2b; P(pTol)3 | 3a; 4-NO2 | 4b | 49 |
| 3 | 2c; P(4-Cl-C6H4)3 | 3a; 4-NO2 | 4c | 57 |
| 4 | 2d; P(4-F-C6H4)3 | 3a; 4-NO2 | 4d | 79 |
| 5 | 2e; P(2-thienyl)3 | 3a; 4-NO2 | 4e | 56 |
| 6c,d | 2f; P(NMe2)3 | 3a; 4-NO2 | 4f | 53 |
| 7 | 2a; PPh3 | 3b; 3-NO2 | 4g | 59 |
| 8 | 2b; P(pTol)3 | 3b; 3-NO2 | 4h | 54 |
| 9 | 2g; PPh2(pTol) | 3b; 3-NO2 | 4i | 62 |
| 10 | 2d; P(4-FC6H4)3 | 3b; 3-NO2 | 4j | 61 |
| 11 | 2b; P(pTol)3 | 3c; 4-Cl-3-NO2 | 4k | 51 |
| 12 | 2c; P(4-Cl-C6H4)3 | 3c; 4-Cl-3-NO2 | 4l | 52 |
| 13 | 2d; P(4-F-C6H4)3 | 3c; 4-Cl-3-NO2 | 4m | 56 |
| 14 | 2b; P(pTol)3 | 3d; 4-CN | 4n | 71 |
| 15 | 2c; P(4-Cl-C6H4)3 | 3d; 4-CN | 4o | 54 |
| 16 | 2h; P(cHex)3 | 3d; 4-CN | 4p | 22 |
We characterized these γ-lactams 4a–p by using infrared (IR) and 1H, 31P and 13C nuclear magnetic resonance (NMR) spectroscopy, electrospray ionization mass spectrometry (ESI-MS), and X-ray crystallography. All MS data corresponded to the expected formulae of the isolated γ-lactams. In their IR spectra, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, next to the ylidic carbanion, shows stretching bands at ca. 1629–1658 cm−1, lower than that of a normal C
O group, next to the ylidic carbanion, shows stretching bands at ca. 1629–1658 cm−1, lower than that of a normal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency due to electronic resonance. It is interesting to note that the C
O stretching frequency due to electronic resonance. It is interesting to note that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency of the lactam 4f with a HMPT moiety appears at 1658 cm−1 and that of lactam 4p with P(cHex)3 appears at 1629 cm−1. This indicated that P(cHex)3 behaves as a strong electron-donating group and HMPT as a strong electron-pulling group—such an effect renders strong and weak delocalizations of the ylide carbanion through resonance to the lactam carbonyl moiety, respectively. For the characterization of an example of compound 4a by NMR, we observe a signal at 12.7 ppm in its 31P NMR spectrum, corresponding to a typical α-ylidic γ-lactam. Its 1H NMR spectrum displays simple singlets at 2.46 and 3.38 ppm, corresponding to methyl and methoxy groups, respectively. Two signals at 165.7 and 167.0 (2JPC = 15.8 Hz) ppm correspond to the carbonyl resonances of ester and lactam in the 13C NMR spectrum. The ylidic carbon (C2, Fig. 1) appears at 61.3 ppm with one bond coupling to phosphorus P1 (1JPC = 129.2 Hz).
O stretching frequency of the lactam 4f with a HMPT moiety appears at 1658 cm−1 and that of lactam 4p with P(cHex)3 appears at 1629 cm−1. This indicated that P(cHex)3 behaves as a strong electron-donating group and HMPT as a strong electron-pulling group—such an effect renders strong and weak delocalizations of the ylide carbanion through resonance to the lactam carbonyl moiety, respectively. For the characterization of an example of compound 4a by NMR, we observe a signal at 12.7 ppm in its 31P NMR spectrum, corresponding to a typical α-ylidic γ-lactam. Its 1H NMR spectrum displays simple singlets at 2.46 and 3.38 ppm, corresponding to methyl and methoxy groups, respectively. Two signals at 165.7 and 167.0 (2JPC = 15.8 Hz) ppm correspond to the carbonyl resonances of ester and lactam in the 13C NMR spectrum. The ylidic carbon (C2, Fig. 1) appears at 61.3 ppm with one bond coupling to phosphorus P1 (1JPC = 129.2 Hz).
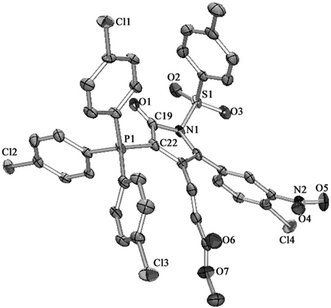
Further, we find that these isolated ylide compounds tend to crystallize by slow evaporation of their dichloromethane or chloroform solution. We obtain the crystal structure of compounds 4a18 (Fig. 1) and 4l19 (Fig. 2) by X-ray diffraction analysis. The phosphorus atom P1 is clearly covalently bonded to C2 and C22 with a bond length of 1.7319(18) and 1.7360(4) Å for 4a and 4l, respectively. Due to the delocalization of negative charge from ylidic carbon C2 and C22 to the lactam carbonyl π bond (C1–O1 and C19–O1), the C1–C2 and C19–C22 bond lengths 1.4170(2) and 1.4280(7) Å for 4a and 4l, respectively, are shorter than a normal carbon to carbon single bond. The bond lengths of C1–O1 and C19–O1, 1.2350(2) and 1.2120(6) Å for 4a and 4l, respectively, are longer than a normal carbon to oxygen double bond. The relatively shorter C19–O1 in 4l may be inferred from the slightly larger electron-pulling ability of the tris(4-chlorophenyl)phosphine than a triphenylphosphine moiety, making the α-carbanion show lesser extent of resonance toward the carbonyl group.
We account for the formation of lactam ylide 4 by an initial nucleophilic attack of phosphine PR3 (2) at α(δ′)-position of the enyne 1 (Scheme 2), generating a reactive zwitterionic species Ia bearing a carbenoid moiety at β(γ′)-carbon. Nucleophilic addition of Ia to the aldiminyl carbon of aldimines 3 generates Ib. Intramolecular cyclization of Ib gives Ic followed by release of a methoxide molecule. Finally, deprotonation on Id by the methoxide takes place to form the product 4.
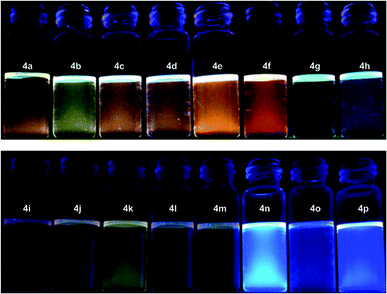
These isolated lactam compounds 4a–4p exhibit remarkable visible colors from light yellow to orange. As a result, we measure the UV-vis absorption of compounds 4a–p, and these absorption data are shown in the ESI (Fig. S33 to S36†). In Fig. S33,† we find that benzaldimines equipped with the 4-NO2 group display absorptions spanning from 330 to 600 nm. Among these compounds (4a–f), their maximum absorptions in the visible region are blue-shifted for phosphines with more electron-releasing groups—compound 4b with P(pTol)3 shows absorption maxima at 458 nm and those of compounds 4a (with PPh3), 4f (with P(NMe2)3), 4e (with P(2-thienyl)3), 4d (with P(4-F-C6H4)3) and 4c (with P(4-Cl-C6H4)3) appear at 452, 452, 444, 441 and 436 nm, respectively (Fig. S33†). However, the switch of 4-nitro to 3-nitro substitution (compounds 4g–j) causes an apparent blue shift of the absorption maxima spanning from 376 to 400 nm, with compound 4j showing the most blue-shift (Fig. S33–S34†). Further, lactams with 3-chloro-4-nitro and 4-cyano substitutions (4k–4p) exhibit a pale-yellow solution in CHCl3 and do not show intense absorptions (Fig. S34†).
Interestingly, we note that lactams 4a–p were fluorescent and measure their fluorescent emission spectra with a solution concentration of 5.0 × 10−5 M. As shown in Fig. 3, while compounds 4a–m and 4o show extremely poor fluorescent emission with quantum yields less than 0.01, compounds 4n and 4p exhibit relatively observable blue fluorescence. Their fluorescence quantum yields, determined by using anthracene as a reference standard (Φ = 0.27 in EtOH), are 0.112 and 0.038 with maximum emission wavelengths of 447 and 445 nm when excited at 363 nm, respectively (Fig. 4). Further, we note that the fluorescence of compound 4n is concentration-dependent in CHCl3—the fluorescence emission is bathochromic-shifted while the concentration of solutions increases (Fig. 5). The emission wavelength maxima for solutions A to E of 4n are 447, 486, 491, 490 and 494 nm, respectively. However, this concentration-dependent notion is minute when 4n is dissolved in THF or dichloromethane. This typical change is attributed to more ordered packing of 4n in CHCl3 than in THF or dichloromethane—consistent with the notion that 4n shows higher propensity for crystallization in CHCl3.
 | ||
| Fig. 5 Concentration-dependent emission phenomenon of compound 4n at concentrations of 5.0 × 10−5 (A), 7.5 × 10−5 (B), 1.0 × 10−4 (C), 2.0 × 10−4 (D), and 4.0 × 10−4 (E), respectively. | ||
It is noteworthy that the assembled γ-lactam 4n with multiple functional groups exhibits fluorescent properties. We perform semiempirical calculations to retrieve the HOMO−1, HOMO, LUMO and LUMO+1 molecular orbitals of 4n. As shown in Fig. 6, the HOMO−1 and HOMO orbitals are primarily located at the lactam moiety while the LUMO and LUMO+1 orbitals are distributed over the 4-cyanophenyl and ester moiety. The electronic excitation may be contributed by the electron excited from the lactam core to the outer moiety to facilitate the subsequent fluorescent emission.
 | ||
| Fig. 6 HOMO−1 (−0.35227 eV), HOMO (−0.29164 eV), LUMO (−0.21441 eV) and LUMO+1 (−0.20384 eV) energy levels of 4n calculated by a semiempirical AM1 method. | ||
Conclusions
We have demonstrated a one-pot multicomponent reaction for the synthesis of γ-lactams possessing an α-phosphorus ylide moiety from assembly of phosphines, N-tosyl aldimines and an enyne through an initial α(δ′)-Michael addition of phosphines to an enyne in up to 79% yield. The investigated MCRs tolerate a variety of phosphines such as triarylphosphines, tricyclohexylphosphine and hexamethylphosphorus triamide with electron-deficient aldimines, providing γ-lactams having α-phosphorus ylides in a one-pot procedure. One of these compounds, with the tri(p-tol)phosphine and 4-cyanophenyl moiety, exhibits blue fluorescence with a quantum yield of 0.112.Experimental section
General methods
All reactions were performed under argon. Anhydrous benzene and THF were distilled from sodium/benzophenone under argon. The chemical shifts of 31P NMR were taken with reference to 85% H3PO4 in D2O and that of 1H and 13C with reference to TMS or CHCl3.Typical procedure for the synthesis of 4a–p
A benzene solution containing 1 (0.30 mmol) and aldimines 3 (0.15 mmol) was distilled three times to remove water using a Dean–Stark apparatus. Then, 8 mL of THF was added to the resulting mixture followed by phosphines 2 (0.30 mmol). The mixture was heated at 60 °C and monitored by thin layer chromatography (TLC). Upon completion of the reaction, THF was removed under reduced pressure and subjected to flash chromatography. Elution first with DCM–EA (3/1) gave products 4.Measurement of fluorescence spectroscopy
The quantum yield was calculated according to the following equation: ΦS/ΦR = (AS/AR) × (AbsR/AbsS) × (ŋS2/ŋR2), where ΦS and ΦR are the fluorescence quantum yields of the sample and the reference, respectively; AS and AR are the emission areas of the sample and the reference; AbsS and AbsR are the corresponding absorbances of the sample and the reference solution at the wavelength of excitation; ŋS and ŋR are the refractive indices of the sample and the reference.20(E)-Methyl 3-(2-(4-nitrophenyl)-5-oxo-1-tosyl-4-(tri phenylphosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4a)
Red solid. M.p. 174–175 °C. Rf = 0.27 (DCM–EA, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 57% (0.0600 g). 1H NMR (600 MHz, CDCl3, 25 °C): δ = 2.46 (3H, s, Me), 3.38 (3H, s, CO2Me), 5.01 (1H, d, J = 16.1 Hz, CH), 6.35 (1H, d, J = 16.0 Hz, CH), 7.25 (2H, d, J = 8.8 Hz, Ph), 7.38–7.42 (6H, m, Ph), 7.45 (6H, t, J = 7.8 Hz, Ph), 7.50 (2H, d, J = 8.5 Hz, Ph), 7.56, (3H, t, J = 7.1 Hz, Ph), 7.67 (2H, d, J = 8.1 Hz, Ph), 8.13 (2H, d, J = 8.6 Hz, Ph) ppm. 13C NMR (150 MHz, CDCl3, 25 °C): δ = 21.6, 51.0, 61.3 (d, 1JPC = 129.2 Hz), 121.9, 122.6 (d, 1JPC = 92.3 Hz), 122.7, 122.8, 124.0 (d, 3JPC = 11.9 Hz), 128.0, 129.0, 129.1 (d, 3JPC = 12.8 Hz), 130.8, 133.1 (d, 4JPC = 2.7 Hz), 133.8 (d, 2JPC = 10.5 Hz), 135.7, 136.3, 140.0, 143.8, 146.1, 165.7, 167.0 (d, 2JPC = 15.8 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 12.7 ppm. FTIR (KBr):
1). Isolated yield 57% (0.0600 g). 1H NMR (600 MHz, CDCl3, 25 °C): δ = 2.46 (3H, s, Me), 3.38 (3H, s, CO2Me), 5.01 (1H, d, J = 16.1 Hz, CH), 6.35 (1H, d, J = 16.0 Hz, CH), 7.25 (2H, d, J = 8.8 Hz, Ph), 7.38–7.42 (6H, m, Ph), 7.45 (6H, t, J = 7.8 Hz, Ph), 7.50 (2H, d, J = 8.5 Hz, Ph), 7.56, (3H, t, J = 7.1 Hz, Ph), 7.67 (2H, d, J = 8.1 Hz, Ph), 8.13 (2H, d, J = 8.6 Hz, Ph) ppm. 13C NMR (150 MHz, CDCl3, 25 °C): δ = 21.6, 51.0, 61.3 (d, 1JPC = 129.2 Hz), 121.9, 122.6 (d, 1JPC = 92.3 Hz), 122.7, 122.8, 124.0 (d, 3JPC = 11.9 Hz), 128.0, 129.0, 129.1 (d, 3JPC = 12.8 Hz), 130.8, 133.1 (d, 4JPC = 2.7 Hz), 133.8 (d, 2JPC = 10.5 Hz), 135.7, 136.3, 140.0, 143.8, 146.1, 165.7, 167.0 (d, 2JPC = 15.8 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 12.7 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1640, 1716 cm−1. λmax(CHCl3): 452 nm. HRMS (ESI+): calcd for C39H31N2O7PS [M+] 702.1590; found 702.1585.
= 1640, 1716 cm−1. λmax(CHCl3): 452 nm. HRMS (ESI+): calcd for C39H31N2O7PS [M+] 702.1590; found 702.1585.
(E)-Methyl 3-(2-(4-nitrophenyl)-5-oxo-1-tosyl-4-(tri-p-tolylphosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4b)
Red solid. M.p. 72–76 °C. Rf = 0.33 (DCM–EA, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 49% (0.0547 g). 1H NMR (500 MHz, CDCl3, 25 °C): δ = 2.40 (9H, s, Me), 2.51 (3H, s, Me), 3.45 (3H, s, CO2Me), 5.00 (1H, d, J = 16.0 Hz, CH), 6.39 (1H, d, J = 15.5 Hz, CH), 7.21 (6H, dd, J = 3.0, 8.0 Hz, Ph), 7.28 (2H, d, J = 5.0 Hz, Ph), 7.33 (6H, dd, J = 8.5, 12.5 Hz, Ph), 7.51 (2H, d, J = 8.5 Hz, Ph), 7.73 (2H, d, J = 8.5 Hz, Ph), 8.17 (2H, d, J = 8.5 Hz, Ph) ppm. 13C NMR (125 MHz, CDCl3, 25 °C): δ = 21.56, 21.62, 51.1, 62.2 (d, 1JPC = 129.3 Hz), 119.6 (d, 1JPC = 95.4 Hz), 121.7, 122.7, 122.8, 124.5 (d, 3JPC = 12.2 Hz), 128.1, 129.0, 129.9 (d, 3JPC = 13.3 Hz), 130.9, 133.8 (d, 2JPC = 11.1 Hz), 135.9, 136.7, 140.2, 143.7, 144.0, 146.1, 166.0, 167.0 (d, 2JPC = 15.5 Hz), ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 11.8 ppm. λmax(CHCl3): 458 nm. FTIR (KBr):
1). Isolated yield 49% (0.0547 g). 1H NMR (500 MHz, CDCl3, 25 °C): δ = 2.40 (9H, s, Me), 2.51 (3H, s, Me), 3.45 (3H, s, CO2Me), 5.00 (1H, d, J = 16.0 Hz, CH), 6.39 (1H, d, J = 15.5 Hz, CH), 7.21 (6H, dd, J = 3.0, 8.0 Hz, Ph), 7.28 (2H, d, J = 5.0 Hz, Ph), 7.33 (6H, dd, J = 8.5, 12.5 Hz, Ph), 7.51 (2H, d, J = 8.5 Hz, Ph), 7.73 (2H, d, J = 8.5 Hz, Ph), 8.17 (2H, d, J = 8.5 Hz, Ph) ppm. 13C NMR (125 MHz, CDCl3, 25 °C): δ = 21.56, 21.62, 51.1, 62.2 (d, 1JPC = 129.3 Hz), 119.6 (d, 1JPC = 95.4 Hz), 121.7, 122.7, 122.8, 124.5 (d, 3JPC = 12.2 Hz), 128.1, 129.0, 129.9 (d, 3JPC = 13.3 Hz), 130.9, 133.8 (d, 2JPC = 11.1 Hz), 135.9, 136.7, 140.2, 143.7, 144.0, 146.1, 166.0, 167.0 (d, 2JPC = 15.5 Hz), ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 11.8 ppm. λmax(CHCl3): 458 nm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1646, 1717 cm−1. HRMS (ESI+): calcd for C42H37N2O7PS [M+] 744.2059; found 744.2058.
= 1646, 1717 cm−1. HRMS (ESI+): calcd for C42H37N2O7PS [M+] 744.2059; found 744.2058.
(E)-Methyl 3-(2-(4-nitrophenyl)-5-oxo-1-tosyl-4-(tris(4-chlorophenyl)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4c)
Red solid. M.p. 174–175 °C. Rf = 0.30 (hexanes–EA, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 79% (0.0953 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.52 (3H, s, Me), 3.51 (3H, s, CO2Me), 5.02 (1H, d, J = 16.2 Hz, CH), 6.36 (1H, d, J = 15.9 Hz, CH), 7.30 (2H, d, J = 8.1 Hz, Ph), 7.35–7.45 (12H, m, Ph), 7.51 (2H, d, J = 9.0 Hz, Ph), 7.73 (2H, d, J = 8.1 Hz, Ph), 8.18 (2H, d, J = 8.7 Hz, Ph) ppm. 13C NMR (100 MHz, CDCl3, 25 °C): δ = 21.7, 51.5, 60.3 (d, 1JPC = 130.9 Hz), 120.5 (d, 1JPC = 95.2 Hz), 122.1, 122.6 (d, 2JPC = 12.1 Hz), 122.8, 123.6 (d, 3JPC = 12.6 Hz), 128.2, 129.1, 129.8 (d, 3JPC = 13.7 Hz), 131.0, 134.9 (d, 2JPC = 11.8 Hz), 135.6, 136.0, 139.6, 140.7 (d, 4JPC = 3.8 Hz), 144.2, 146.4, 165.6, 166.9 (d, 2JPC = 15.9 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 12.1 ppm. FTIR (KBr):
1). Isolated yield 79% (0.0953 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.52 (3H, s, Me), 3.51 (3H, s, CO2Me), 5.02 (1H, d, J = 16.2 Hz, CH), 6.36 (1H, d, J = 15.9 Hz, CH), 7.30 (2H, d, J = 8.1 Hz, Ph), 7.35–7.45 (12H, m, Ph), 7.51 (2H, d, J = 9.0 Hz, Ph), 7.73 (2H, d, J = 8.1 Hz, Ph), 8.18 (2H, d, J = 8.7 Hz, Ph) ppm. 13C NMR (100 MHz, CDCl3, 25 °C): δ = 21.7, 51.5, 60.3 (d, 1JPC = 130.9 Hz), 120.5 (d, 1JPC = 95.2 Hz), 122.1, 122.6 (d, 2JPC = 12.1 Hz), 122.8, 123.6 (d, 3JPC = 12.6 Hz), 128.2, 129.1, 129.8 (d, 3JPC = 13.7 Hz), 131.0, 134.9 (d, 2JPC = 11.8 Hz), 135.6, 136.0, 139.6, 140.7 (d, 4JPC = 3.8 Hz), 144.2, 146.4, 165.6, 166.9 (d, 2JPC = 15.9 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 12.1 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1640, 1717 cm−1. λmax(CHCl3): 436 nm. HRMS (ESI+): calcd for C39H28Cl3N2O7PS [M+] 804.0420; found 804.0428.
= 1640, 1717 cm−1. λmax(CHCl3): 436 nm. HRMS (ESI+): calcd for C39H28Cl3N2O7PS [M+] 804.0420; found 804.0428.
(E)-Methyl 3-(2-(4-nitrophenyl)-5-oxo-1-tosyl-4-(tris(4-fluorophenyl)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4d)
Red solid. M.p. 122–124 °C. Rf = 0.30 (hexanes–EA, 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 57% (0.0646 g). 1H NMR (500 MHz, CDCl3, 25 °C): δ = 2.47 (3H, s, Me), 3.44 (3H, s, CO2Me), 5.00 (1H, d, J = 16.0 Hz, CH), 6.28 (1H, d, J = 16.0 Hz, CH), 7.12 (6H, td, J = 2.5, 9.0 Hz, Ph), 7.26 (2H, d, J = 7.5 Hz, Ph), 7.44–7.48 (8H, m, Ph), 7.69 (2H, d, J = 8.5 Hz, Ph), 8.13 (2H, d, J = 8.5 Hz, Ph) ppm. 13C NMR (125 MHz, CDCl3, 25 °C): δ = 21.6, 51.3, 61.3 (d, 1JPC = 132.1 Hz), 117.0 (dd, 3JPC = 14.3, 2JFC = 21.0 Hz), 118.2 (d, 1JPC = 97.7 Hz), 122.0, 122.8, 123.3 (d, 3JPC = 12.2 Hz), 126.2, 128.1, 129.1, 131.0, 135.7, 136.0, 136.3, (dd, 2JPC = 12.2, 3JFC = 21.0 Hz), 139.7, 144.1, 146.4, 165.7, 165.8 (dd, 4JPC = 3.3, 1JFC = 258.6 Hz), 166.8 (d, 2JPC = 15.6 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 11.4 ppm. FTIR (KBr):
1). Isolated yield 57% (0.0646 g). 1H NMR (500 MHz, CDCl3, 25 °C): δ = 2.47 (3H, s, Me), 3.44 (3H, s, CO2Me), 5.00 (1H, d, J = 16.0 Hz, CH), 6.28 (1H, d, J = 16.0 Hz, CH), 7.12 (6H, td, J = 2.5, 9.0 Hz, Ph), 7.26 (2H, d, J = 7.5 Hz, Ph), 7.44–7.48 (8H, m, Ph), 7.69 (2H, d, J = 8.5 Hz, Ph), 8.13 (2H, d, J = 8.5 Hz, Ph) ppm. 13C NMR (125 MHz, CDCl3, 25 °C): δ = 21.6, 51.3, 61.3 (d, 1JPC = 132.1 Hz), 117.0 (dd, 3JPC = 14.3, 2JFC = 21.0 Hz), 118.2 (d, 1JPC = 97.7 Hz), 122.0, 122.8, 123.3 (d, 3JPC = 12.2 Hz), 126.2, 128.1, 129.1, 131.0, 135.7, 136.0, 136.3, (dd, 2JPC = 12.2, 3JFC = 21.0 Hz), 139.7, 144.1, 146.4, 165.7, 165.8 (dd, 4JPC = 3.3, 1JFC = 258.6 Hz), 166.8 (d, 2JPC = 15.6 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 11.4 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1640, 1721 cm−1. λmax(CHCl3): 441 nm. HRMS (ESI+): calcd for C39H28F3N2O7PS [M+] 756.1307; found 756.1298.
= 1640, 1721 cm−1. λmax(CHCl3): 441 nm. HRMS (ESI+): calcd for C39H28F3N2O7PS [M+] 756.1307; found 756.1298.
(E)-Methyl 3-(2-(4-nitrophenyl)-5-oxo-1-tosyl-4-(tri(thiophen-2-yl)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4e)
Red solid. M.p. 165–168 °C. Rf = 0.28 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Isolated yield 56% (0.0604 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.47 (3H, s, Me), 3.48 (3H, s, CO2Me), 5.14 (1H, d, J = 16.2 Hz, CH), 6.57 (1H, d, J = 16.2 Hz, CH), 7.19 (3H, ddd, J = 2.1, 3.6, 4.7 Hz, Ph), 7.28 (2H, d, J = 7.2 Hz, Ph), 7.51–7.56 (5H, m, Ph), 7.74 (2H, d, J = 8.4 Hz, Ph), 7.86 (3H, td, J = 0.9, 4.8 Hz, Ph), 8.20 (2H, d, J = 8.7 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.7, 51.3, 63.2 (d, 1JPC = 142.6 Hz), 121.3, 122.6 (d, 2JPC = 13.6 Hz), 122.9, 123.9 (d, 3JPC = 13.3 Hz), 124.6 (d, 1JPC = 117.9 Hz), 128.1, 129.1 (d, 3JPC = 15.9 Hz), 129.2, 131.0, 135.7, 135.8, 137.0 (d, 2JPC = 6.0 Hz), 139.8, 140.0 (d, 3JPC = 12.1 Hz), 143.9, 146.5, 166.6, 166.7 (d, 2JPC = 18.9 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = −10.6 ppm. FTIR (KBr):
3). Isolated yield 56% (0.0604 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.47 (3H, s, Me), 3.48 (3H, s, CO2Me), 5.14 (1H, d, J = 16.2 Hz, CH), 6.57 (1H, d, J = 16.2 Hz, CH), 7.19 (3H, ddd, J = 2.1, 3.6, 4.7 Hz, Ph), 7.28 (2H, d, J = 7.2 Hz, Ph), 7.51–7.56 (5H, m, Ph), 7.74 (2H, d, J = 8.4 Hz, Ph), 7.86 (3H, td, J = 0.9, 4.8 Hz, Ph), 8.20 (2H, d, J = 8.7 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.7, 51.3, 63.2 (d, 1JPC = 142.6 Hz), 121.3, 122.6 (d, 2JPC = 13.6 Hz), 122.9, 123.9 (d, 3JPC = 13.3 Hz), 124.6 (d, 1JPC = 117.9 Hz), 128.1, 129.1 (d, 3JPC = 15.9 Hz), 129.2, 131.0, 135.7, 135.8, 137.0 (d, 2JPC = 6.0 Hz), 139.8, 140.0 (d, 3JPC = 12.1 Hz), 143.9, 146.5, 166.6, 166.7 (d, 2JPC = 18.9 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = −10.6 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1645, 1716 cm−1. λmax(CHCl3): 444 nm. HRMS (ESI+): calcd for C33H25N2O7PS4 [M+] 720.0282; found 720.0278.
= 1645, 1716 cm−1. λmax(CHCl3): 444 nm. HRMS (ESI+): calcd for C33H25N2O7PS4 [M+] 720.0282; found 720.0278.
(E)-Methyl 3-(2-(4-nitrophenyl)-5-oxo-1-tosyl-4-(tris(dimethylamino)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4f)
Red solid. M.p. 100–102 °C. Rf = 0.21 (EA). Isolated yield 53% (0.0480 g). Recrystallization from hexanes–EA gave pure products. 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.37 (3H, s, Me), 2.58 (18H, d, J = 9.7 Hz, NMe2), 3.68 (3H, s, CO2Me), 5.84 (1H, d, J = 16.2 Hz, CH), 7.21 (2H, d, J = 8.2 Hz, Ph), 7.25 (1H, d, J = 16.2 Hz, CH), 7.56 (2H, d, J = 8.0 Hz, Ph), 7.70 (2H, d, J = 8.0 Hz, Ph), 8.26 (2H, d, J = 7.9 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.5, 35.9 (d, 2JPC = 5.2 Hz), 51.5, 65.1 (d, 1JPC = 190.0 Hz), 116.8, 122.8, 123.3 (d, 3JPC = 10.6 Hz), 127.6, 127.9 (d, 3JPC = 14.2 Hz), 128.8, 131.1, 135.9, 136.7, 139.5, 143.9, 146.6, 166.8 (d, 2JPC = 21.5 Hz), 167.7 ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 50.8 ppm. FTIR (KBr):![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1658, 1723 cm−1. λmax(CHCl3): 452 nm. HRMS (ESI+): calcd for C27H34N5O7PS [M+] 603.1917; found 603.1916.
= 1658, 1723 cm−1. λmax(CHCl3): 452 nm. HRMS (ESI+): calcd for C27H34N5O7PS [M+] 603.1917; found 603.1916.
(E)-Methyl 3-(2-(3-nitrophenyl)-5-oxo-1-tosyl-4-(triphenylphosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4g)
Orange solid. M.p. 96–99 °C. Rf = 0.13 (hexanes–EA, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 59% (0.0621 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.48 (3H, s, Me), 3.38 (3H, s, CO2Me), 5.01 (1H, d, J = 16.1 Hz, CH), 6.34 (1H, d, J = 16.1 Hz, CH), 7.29 (2H, d, J = 8.2 Hz, Ph), 7.41–7.77 (19H, m, Ph), 8.12 (1H, dd, J = 1.3, 8.2 Hz, Ph), 8.19 (1H, t, J = 1.8 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.6, 50.9, 60.0 (d, 1JPC = 129.8 Hz), 120.9, 122.4 (d, 2JPC = 11.3 Hz), 122.7 (d, 3JPC = 12.1 Hz), 122.8 (d, 1JPC = 92.8 Hz), 125.2, 127.9, 128.3, 129.0 (d, 3JPC = 12.8 Hz), 129.1, 131.9, 133.0 (d, 4JPC = 3.0 Hz), 133.8 (d, 2JPC = 10.6 Hz), 134.8, 135.9, 136.3, 137.0, 143.8, 147.4, 165.9, 166.4 (d, 2JPC = 15.9 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 12.8 ppm. FTIR (KBr):
1). Isolated yield 59% (0.0621 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.48 (3H, s, Me), 3.38 (3H, s, CO2Me), 5.01 (1H, d, J = 16.1 Hz, CH), 6.34 (1H, d, J = 16.1 Hz, CH), 7.29 (2H, d, J = 8.2 Hz, Ph), 7.41–7.77 (19H, m, Ph), 8.12 (1H, dd, J = 1.3, 8.2 Hz, Ph), 8.19 (1H, t, J = 1.8 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.6, 50.9, 60.0 (d, 1JPC = 129.8 Hz), 120.9, 122.4 (d, 2JPC = 11.3 Hz), 122.7 (d, 3JPC = 12.1 Hz), 122.8 (d, 1JPC = 92.8 Hz), 125.2, 127.9, 128.3, 129.0 (d, 3JPC = 12.8 Hz), 129.1, 131.9, 133.0 (d, 4JPC = 3.0 Hz), 133.8 (d, 2JPC = 10.6 Hz), 134.8, 135.9, 136.3, 137.0, 143.8, 147.4, 165.9, 166.4 (d, 2JPC = 15.9 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 12.8 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1639, 1722 cm−1. λmax(CHCl3): 385 nm. HRMS (ESI+): calcd for C39H31N2O7PS [M+] 702.1590; found 702.1581.
= 1639, 1722 cm−1. λmax(CHCl3): 385 nm. HRMS (ESI+): calcd for C39H31N2O7PS [M+] 702.1590; found 702.1581.
(E)-Methyl 3-(2-(3-nitrophenyl)-5-oxo-1-tosyl-4-(tri-p-tolylphosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4h)
Orange solid. M.p. 116–119 °C. Rf = 0.32 (hexanes–EA, 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 54% (0.0603 g). 1H NMR (500 MHz, CDCl3, 25 °C): δ = 2.36 (9H, s, Me), 2.45 (3H, s, Me), 3.37 (3H, s, CO2Me), 4.96 (1H, d, J = 16.0 Hz, CH), 6.33 (1H, d, J = 16.0 Hz, CH), 7.19 (6H, dd, J = 2.0, 8.5 Hz, Ph), 7.25 (2H, d, J = 7.0 Hz, Ph), 7.34 (6H, dd, J = 8.0, 13.0 Hz, Ph), 7.44 (1H, t, J = 8.0 Hz, Ph), 7.69 (1H, d, J = 7.5 Hz, Ph), 7.71 (2H, d, J = 8.5 Hz, Ph), 8.07 (1H, dt, J = 1.5, 8.0 Hz, Ph), 8.13 (1H, s, Ph) ppm. 13C NMR (125 MHz, CDCl3, 25 °C): δ = 21.5, 21.6, 51.0, 61.0 (d, 1JPC = 129.8 Hz), 119.7 (d 1JPC = 95.8 Hz), 120.8, 121.9, 122.4 (d, 2JPC = 12.2 Hz), 122.9 (d, 3JPC = 11.1 Hz), 125.1, 127.9, 128.2, 129.0, 129.7 (d, 3JPC = 13.3 Hz), 133.7 (d, 2JPC = 11.2 Hz), 135.0, 136.1, 136.5, 137.0, 143.6, 143.8 (d, 4JPC = 2.3 Hz), 147.4, 165.9, 166.4 (d, 2JPC = 16.5 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 11.90 ppm. FTIR (KBr):
1). Isolated yield 54% (0.0603 g). 1H NMR (500 MHz, CDCl3, 25 °C): δ = 2.36 (9H, s, Me), 2.45 (3H, s, Me), 3.37 (3H, s, CO2Me), 4.96 (1H, d, J = 16.0 Hz, CH), 6.33 (1H, d, J = 16.0 Hz, CH), 7.19 (6H, dd, J = 2.0, 8.5 Hz, Ph), 7.25 (2H, d, J = 7.0 Hz, Ph), 7.34 (6H, dd, J = 8.0, 13.0 Hz, Ph), 7.44 (1H, t, J = 8.0 Hz, Ph), 7.69 (1H, d, J = 7.5 Hz, Ph), 7.71 (2H, d, J = 8.5 Hz, Ph), 8.07 (1H, dt, J = 1.5, 8.0 Hz, Ph), 8.13 (1H, s, Ph) ppm. 13C NMR (125 MHz, CDCl3, 25 °C): δ = 21.5, 21.6, 51.0, 61.0 (d, 1JPC = 129.8 Hz), 119.7 (d 1JPC = 95.8 Hz), 120.8, 121.9, 122.4 (d, 2JPC = 12.2 Hz), 122.9 (d, 3JPC = 11.1 Hz), 125.1, 127.9, 128.2, 129.0, 129.7 (d, 3JPC = 13.3 Hz), 133.7 (d, 2JPC = 11.2 Hz), 135.0, 136.1, 136.5, 137.0, 143.6, 143.8 (d, 4JPC = 2.3 Hz), 147.4, 165.9, 166.4 (d, 2JPC = 16.5 Hz) ppm. 31P NMR (202 MHz, CDCl3, 25 °C): δ = 11.90 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1637, 1723 cm−1. λmax(CHCl3): 400 nm. HRMS (ESI+): calcd for C42H37N2O7PS [M+] 744.2059; found 744.2052.
= 1637, 1723 cm−1. λmax(CHCl3): 400 nm. HRMS (ESI+): calcd for C42H37N2O7PS [M+] 744.2059; found 744.2052.
(E)-Methyl 3-(4-(diphenyl(p-tolyl)phosphoranylidene)-2-(3-nitrophenyl)-5-oxo-1-tosyl-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4i)
Orange solid. M.p. 96–98 °C. Rf = 0.25 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Isolated yield 62% (0.0666 g). 1H NMR (400 MHz, CDCl3, 25 °C): δ = 2.41 (3H, s, Me), 2.49 (3H, s, Me), 3.40 (3H, s, CO2Me), 4.98 (1H, d, J = 16.0 Hz, CH), 6.33 (1H, d, J = 16.0 Hz, CH), 7.23–7.26 (2H, m, Ph), 7.36–7.46 (7H, m, Ph), 7.49–7.57 (8H, m, Ph), 7.72–7.77 (3H, m, Ph), 8.10–8.15 (2H, m, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.6 (d, 5JPC = 1.6 Hz), 21.6, 51.0, 60.3 (d, 1JPC = 129.8 Hz), 119.1 (d, 1JPC = 95.1 Hz), 121.5 (d, 1JPC = 92.1 Hz), 122.5, 122.65, 122.70 (d, 3JPC = 12.1 Hz), 123.7, 125.2, 128.0, 128.3, 129.0 (d, 3JPC = 12.8 Hz), 129.1, 129.9 (d, 3JPC = 12.8 Hz), 133.0 (d, 4JPC = 3.0 Hz), 133.7 (d, 2JPC = 10.6 Hz), 133.8 (d, 2JPC = 10.6 Hz), 134.9, 136.0, 136.4, 137.0, 143.8, 144.1 (d, 4JPC = 3.1 Hz), 147.4, 165.9, 166.5 (d, 2JPC = 16.0 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.7 ppm. FTIR (KBr):
2). Isolated yield 62% (0.0666 g). 1H NMR (400 MHz, CDCl3, 25 °C): δ = 2.41 (3H, s, Me), 2.49 (3H, s, Me), 3.40 (3H, s, CO2Me), 4.98 (1H, d, J = 16.0 Hz, CH), 6.33 (1H, d, J = 16.0 Hz, CH), 7.23–7.26 (2H, m, Ph), 7.36–7.46 (7H, m, Ph), 7.49–7.57 (8H, m, Ph), 7.72–7.77 (3H, m, Ph), 8.10–8.15 (2H, m, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.6 (d, 5JPC = 1.6 Hz), 21.6, 51.0, 60.3 (d, 1JPC = 129.8 Hz), 119.1 (d, 1JPC = 95.1 Hz), 121.5 (d, 1JPC = 92.1 Hz), 122.5, 122.65, 122.70 (d, 3JPC = 12.1 Hz), 123.7, 125.2, 128.0, 128.3, 129.0 (d, 3JPC = 12.8 Hz), 129.1, 129.9 (d, 3JPC = 12.8 Hz), 133.0 (d, 4JPC = 3.0 Hz), 133.7 (d, 2JPC = 10.6 Hz), 133.8 (d, 2JPC = 10.6 Hz), 134.9, 136.0, 136.4, 137.0, 143.8, 144.1 (d, 4JPC = 3.1 Hz), 147.4, 165.9, 166.5 (d, 2JPC = 16.0 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.7 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1632, 1726 cm−1. λmax(CHCl3): 385 nm. HRMS (ESI+): calcd for C40H33N2O7PS [M+] 716.1746; found 716.1732.
= 1632, 1726 cm−1. λmax(CHCl3): 385 nm. HRMS (ESI+): calcd for C40H33N2O7PS [M+] 716.1746; found 716.1732.
(E)-Methyl 3-(2-(3-nitrophenyl)-5-oxo-1-tosyl-4-(tris(4-fluorophenyl)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4j)
Yellow solid. M.p. 104–107 °C. Rf = 0.28 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 61% (0.0692 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.50 (3H, s, Me), 3.45 (3H, s, CO2Me), 4.99 (1H, d, J = 16.1 Hz, CH), 6.26 (1H, d, J = 16.1 Hz, CH), 7.17 (6H, td, J = 2.2, 8.7 Hz, Ph), 7.30 (2H, d, J = 8.3 Hz, Ph), 7.47–7.57 (7H, m, Ph), 7.71–7.77 (3H, m, Ph), 8.14–8.17 (2H, m, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 22.1, 51.7, 60.6 (d, 1JPC = 132.2 Hz), 117.5 (dd, 3JPC = 14.3 Hz, 2JFC = 21.9 Hz), 118.9 (dd, 4JFC = 3.5 Hz, 1JPC = 97.4 Hz), 121.7, 121.9 (d, 2JPC = 12.2 Hz), 123.0, 123.8 (d, 3JPC = 12.5 Hz), 125.7, 128.6, 129.0, 129.7, 135.0, 136.4, 136.5, 136.9 (dd, 3JFC = 9.2 Hz, 2JPC = 12.4 Hz), 137.6, 144.6, 148.0, 166.3 (dd, 4JPC = 3.3 Hz, 1JFC = 257.9 Hz), 166.3, 166.8 (d, 2JPC = 16.2 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 10.77 ppm. FTIR (KBr):
1). Isolated yield 61% (0.0692 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.50 (3H, s, Me), 3.45 (3H, s, CO2Me), 4.99 (1H, d, J = 16.1 Hz, CH), 6.26 (1H, d, J = 16.1 Hz, CH), 7.17 (6H, td, J = 2.2, 8.7 Hz, Ph), 7.30 (2H, d, J = 8.3 Hz, Ph), 7.47–7.57 (7H, m, Ph), 7.71–7.77 (3H, m, Ph), 8.14–8.17 (2H, m, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 22.1, 51.7, 60.6 (d, 1JPC = 132.2 Hz), 117.5 (dd, 3JPC = 14.3 Hz, 2JFC = 21.9 Hz), 118.9 (dd, 4JFC = 3.5 Hz, 1JPC = 97.4 Hz), 121.7, 121.9 (d, 2JPC = 12.2 Hz), 123.0, 123.8 (d, 3JPC = 12.5 Hz), 125.7, 128.6, 129.0, 129.7, 135.0, 136.4, 136.5, 136.9 (dd, 3JFC = 9.2 Hz, 2JPC = 12.4 Hz), 137.6, 144.6, 148.0, 166.3 (dd, 4JPC = 3.3 Hz, 1JFC = 257.9 Hz), 166.3, 166.8 (d, 2JPC = 16.2 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 10.77 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1640, 1722 cm−1. λmax(CHCl3): 376 nm. HRMS (ESI+): calcd for C39H28F3N2O7PS [M+] 756.1307; found 756.1312.
= 1640, 1722 cm−1. λmax(CHCl3): 376 nm. HRMS (ESI+): calcd for C39H28F3N2O7PS [M+] 756.1307; found 756.1312.
(E)-Methyl 3-(2-(4-chloro-3-nitrophenyl)-5-oxo-1-tosyl-4-(tri-p-tolylphosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4k)
Orange solid. M.p. 102–104 °C. Rf = 0.15 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 51% (0.0595 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.39 (9H, s, Me), 2.49 (3H, s, Me), 3.44 (3H, s, CO2Me), 5.03 (1H, d, J = 16.2 Hz, CH), 6.34 (1H, d, J = 15.9 Hz, CH), 7.20–7.38 (14H, m, Ph), 7.45 (1H, d, J = 8.4 Hz, Ph), 7.53 (1H, dd, J = 8.0, 1.8 Hz, Ph), 7.74 (2H, d, J = 8.1 Hz, Ph), 7.82 (1H, d, J = 1.5 Hz, Ph) ppm. 13C NMR (100 MHz, CDCl3, 25 °C): δ = 21.55 (d, 5JPC = 1.1 Hz), 21.64, 51.1, 61.5 (d, 1JPC = 129.0 Hz), 119.6 (d, 1JPC = 94.9 Hz), 120.9 (d, 2JPC = 11.8 Hz), 121.4, 123.9 (d, 3JPC = 11.7 Hz), 125.2, 127.2, 128.0, 129.1, 129.8 (d, 3JPC = 12.9 Hz), 130.8, 133.4, 133.7 (d, 2JPC = 10.6 Hz), 135.4, 135.9, 136.4, 143.8, 143.9 (d, 4JPC = 2.6 Hz), 146.7, 165.8, 166.5 (d, 2JPC = 15.9 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.1 ppm. FTIR (KBr):
1). Isolated yield 51% (0.0595 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.39 (9H, s, Me), 2.49 (3H, s, Me), 3.44 (3H, s, CO2Me), 5.03 (1H, d, J = 16.2 Hz, CH), 6.34 (1H, d, J = 15.9 Hz, CH), 7.20–7.38 (14H, m, Ph), 7.45 (1H, d, J = 8.4 Hz, Ph), 7.53 (1H, dd, J = 8.0, 1.8 Hz, Ph), 7.74 (2H, d, J = 8.1 Hz, Ph), 7.82 (1H, d, J = 1.5 Hz, Ph) ppm. 13C NMR (100 MHz, CDCl3, 25 °C): δ = 21.55 (d, 5JPC = 1.1 Hz), 21.64, 51.1, 61.5 (d, 1JPC = 129.0 Hz), 119.6 (d, 1JPC = 94.9 Hz), 120.9 (d, 2JPC = 11.8 Hz), 121.4, 123.9 (d, 3JPC = 11.7 Hz), 125.2, 127.2, 128.0, 129.1, 129.8 (d, 3JPC = 12.9 Hz), 130.8, 133.4, 133.7 (d, 2JPC = 10.6 Hz), 135.4, 135.9, 136.4, 143.8, 143.9 (d, 4JPC = 2.6 Hz), 146.7, 165.8, 166.5 (d, 2JPC = 15.9 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.1 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1643, 1723 cm−1. λmax(CHCl3): 398 nm. HRMS (ESI+): calcd for C42H36ClN2O7PS [M+] 778.1669; found 778.1670.
= 1643, 1723 cm−1. λmax(CHCl3): 398 nm. HRMS (ESI+): calcd for C42H36ClN2O7PS [M+] 778.1669; found 778.1670.
(E)-Methyl 3-(2-(4-chloro-3-nitrophenyl)-5-oxo-1-tosyl-4-(tris(4-chlorophenyl)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4l)
Yellow solid. M.p. 182–185 °C. Rf = 0.30 (hexanes–EA, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 56% (0.0704 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.51 (3H, s, Me), 3.51 (3H, s, CO2Me), 5.04 (1H, d, J = 15.9 Hz, CH), 6.32 (1H, d, J = 16.2 Hz, CH), 7.30 (2H, d, J = 8.1 Hz, Ph), 7.37–7.45 (12H, m, Ph), 7.51–7.52 (2H, m, Ph), 7.74 (2H, d, J = 8.1 Hz, Ph), 7.81 (1H, d, J = 1.5 Hz, Ph) ppm. 13C NMR (100 MHz, CDCl3, 25 °C): δ = 21.7, 51.6, 59.7 (d, 1JPC = 130.9 Hz), 120.6 (d, 1JPC = 95.2 Hz), 121.9, 122.1, 122.2 (d, 3JPC = 12.6 Hz), 126.1, 127.3, 128.2, 129.3, 129.9 (d, 3JPC = 13.3 Hz), 131.1, 132.8, 135.0 (d, 2JPC = 11.8 Hz), 135.5, 135.8, 135.9, 140.7 (d, 4JPC = 3.4 Hz), 144.3, 146.8, 165.6, 166.5 (d, 2JPC = 16.3 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.4 ppm. FTIR (KBr):
1). Isolated yield 56% (0.0704 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.51 (3H, s, Me), 3.51 (3H, s, CO2Me), 5.04 (1H, d, J = 15.9 Hz, CH), 6.32 (1H, d, J = 16.2 Hz, CH), 7.30 (2H, d, J = 8.1 Hz, Ph), 7.37–7.45 (12H, m, Ph), 7.51–7.52 (2H, m, Ph), 7.74 (2H, d, J = 8.1 Hz, Ph), 7.81 (1H, d, J = 1.5 Hz, Ph) ppm. 13C NMR (100 MHz, CDCl3, 25 °C): δ = 21.7, 51.6, 59.7 (d, 1JPC = 130.9 Hz), 120.6 (d, 1JPC = 95.2 Hz), 121.9, 122.1, 122.2 (d, 3JPC = 12.6 Hz), 126.1, 127.3, 128.2, 129.3, 129.9 (d, 3JPC = 13.3 Hz), 131.1, 132.8, 135.0 (d, 2JPC = 11.8 Hz), 135.5, 135.8, 135.9, 140.7 (d, 4JPC = 3.4 Hz), 144.3, 146.8, 165.6, 166.5 (d, 2JPC = 16.3 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.4 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1640, 1721 m−1. λmax(CHCl3): 380 nm. HRMS (ESI+): calcd for C39H27Cl4N2O7PS [M+] 838.0031; found 838.0030.
= 1640, 1721 m−1. λmax(CHCl3): 380 nm. HRMS (ESI+): calcd for C39H27Cl4N2O7PS [M+] 838.0031; found 838.0030.
(E)-Methyl 3-(2-(4-chloro-3-nitrophenyl)-5-oxo-1-tosyl-4-(tris(4-fluorophenyl)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4m)
Yellow solid. M.p. 124–126 °C. Rf = 0.33 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 52% (0.0616 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.50 (3H, s, Me), 3.49 (3H, s, CO2Me), 5.06 (1H, d, J = 16.1 Hz, CH), 6.27 (1H, d, J = 16.1 Hz, CH), 7.16 (6H, td, J = 2.2, 7.2 Hz, Ph), 7.30 (2H, d, J = 8.1 Hz, Ph), 7.32–7.29 (8H, m, Ph), 7.74 (2H, d, J = 8.3 Hz, Ph), 7.82 (1H, d, J = 1.7 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.6, 51.3, 60.7 (d, 1JPC = 132.1 Hz), 117.0 (dd, 3JPC = 14.3, 2JFC = 21.9 Hz), 118.2 (dd, 4JFC = 3.4, 1JPC = 97.6 Hz), 121.7, 121.8 (d, 2JPC = 12.5 Hz), 122.3 (d, 3JPC = 12.1 Hz), 125.9, 127.3, 128.0, 129.2, 131.1, 132.8, 132.9, 135.5, 135.8, 136.4 (dd, 3JFC = 9.2, 2JPC = 12.3 Hz), 144.2, 146.8, 165.7, 165.8 (dd, 4JPC = 3.3, 1JFC = 257.9 Hz), 166.4 (d, 2JPC = 16.2 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 10.6 ppm. FTIR (KBr):
1). Isolated yield 52% (0.0616 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.50 (3H, s, Me), 3.49 (3H, s, CO2Me), 5.06 (1H, d, J = 16.1 Hz, CH), 6.27 (1H, d, J = 16.1 Hz, CH), 7.16 (6H, td, J = 2.2, 7.2 Hz, Ph), 7.30 (2H, d, J = 8.1 Hz, Ph), 7.32–7.29 (8H, m, Ph), 7.74 (2H, d, J = 8.3 Hz, Ph), 7.82 (1H, d, J = 1.7 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.6, 51.3, 60.7 (d, 1JPC = 132.1 Hz), 117.0 (dd, 3JPC = 14.3, 2JFC = 21.9 Hz), 118.2 (dd, 4JFC = 3.4, 1JPC = 97.6 Hz), 121.7, 121.8 (d, 2JPC = 12.5 Hz), 122.3 (d, 3JPC = 12.1 Hz), 125.9, 127.3, 128.0, 129.2, 131.1, 132.8, 132.9, 135.5, 135.8, 136.4 (dd, 3JFC = 9.2, 2JPC = 12.3 Hz), 144.2, 146.8, 165.7, 165.8 (dd, 4JPC = 3.3, 1JFC = 257.9 Hz), 166.4 (d, 2JPC = 16.2 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 10.6 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1640, 1720 cm−1. λmax(CHCl3): 363 nm. HRMS (ESI+): calcd for C39H27ClF3N2O7PS [M+] 790.0917; found 790.0904.
= 1640, 1720 cm−1. λmax(CHCl3): 363 nm. HRMS (ESI+): calcd for C39H27ClF3N2O7PS [M+] 790.0917; found 790.0904.
(E)-Methyl 3-(2-(4-cyanophenyl)-5-oxo-1-tosyl-4-(tri-p-tolylphosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4n)
Yellow solid. M.p. 130–132 °C. Rf = 0.16 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 71% (0.0771 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.39 (9H, s, Me), 2.49 (3H, s, Me), 3.44 (3H, s, CO2Me), 5.00 (1H, d, J = 16.1 Hz, CH), 6.40 (1H, d, J = 16.1 Hz, CH), 7.20–7.36 (14H, m, Ph), 7.48 (2H, d, J = 8.2 Hz, Ph), 7.57 (2H, d, J = 7.8 Hz, Ph), 7.72 (2H, d, J = 7.8 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.4, 21.5, 50.9, 61.5 (d, 1JPC = 128.7 Hz), 109.7, 119.0, 119.4 (d, 1JPC = 95.2 Hz), 121.1, 122.9 (d, 2JPC = 12.1 Hz), 123.8 (d, 3JPC = 10.1 Hz), 127.8, 128.8, 129.6 (d, 3JPC = 13.2 Hz), 130.8, 130.9, 133.5 (d, 2JPC = 10.9 Hz), 135.5, 136.5, 138.0, 143.5, 143.7 (d, 4JPC = 2.9 Hz), 165.8, 166.7 (d, 2JPC = 16.0 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 10.8 ppm. FTIR (KBr):
1). Isolated yield 71% (0.0771 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.39 (9H, s, Me), 2.49 (3H, s, Me), 3.44 (3H, s, CO2Me), 5.00 (1H, d, J = 16.1 Hz, CH), 6.40 (1H, d, J = 16.1 Hz, CH), 7.20–7.36 (14H, m, Ph), 7.48 (2H, d, J = 8.2 Hz, Ph), 7.57 (2H, d, J = 7.8 Hz, Ph), 7.72 (2H, d, J = 7.8 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.4, 21.5, 50.9, 61.5 (d, 1JPC = 128.7 Hz), 109.7, 119.0, 119.4 (d, 1JPC = 95.2 Hz), 121.1, 122.9 (d, 2JPC = 12.1 Hz), 123.8 (d, 3JPC = 10.1 Hz), 127.8, 128.8, 129.6 (d, 3JPC = 13.2 Hz), 130.8, 130.9, 133.5 (d, 2JPC = 10.9 Hz), 135.5, 136.5, 138.0, 143.5, 143.7 (d, 4JPC = 2.9 Hz), 165.8, 166.7 (d, 2JPC = 16.0 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 10.8 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1648, 1717 cm−1. λmax(CHCl3): 363 nm. HRMS (ESI+): calcd for C43H37N2O5PS [M+] 724.2160; found 724.2291.
= 1648, 1717 cm−1. λmax(CHCl3): 363 nm. HRMS (ESI+): calcd for C43H37N2O5PS [M+] 724.2160; found 724.2291.
(E)-Methyl 3-(2-(4-cyanophenyl)-5-oxo-1-tosyl-4-(tris(4-chlorophenyl)phosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4o)
Yellow solid. M.p. 134–136 °C. Rf = 0.44 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 54% (0.0635 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.51 (3H, s, Me), 3.50 (3H, s, CO2Me), 4.98 (1H, d, J = 16.0 Hz, CH), 6.35 (1H, d, J = 16.0 Hz, CH), 7.29 (2H, d, J = 9.1 Hz, Ph), 7.34–7.47 (14H, m, Ph), 7.61 (2H, d, J = 8.1 Hz, Ph), 7.72 (2H, d, J = 8.1 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.7, 51.5, 59.7 (d, 1JPC = 132.0 Hz), 110.7, 118.9, 120.6 (d, 1JPC = 95.6 Hz), 121.7, 121.9, 124.3 (d, 3JPC = 12.5 Hz), 128.1, 129.1, 129.8 (d, 3JPC = 13.7 Hz), 131.0, 131.3, 134.9 (d, 2JPC = 11.7 Hz), 135.7, 136.0, 137.5, 140.6 (d, 4JPC = 3.5 Hz), 144.1, 165.8, 166.8 (d, 2JPC = 16.1 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.1 ppm. FTIR (KBr):
1). Isolated yield 54% (0.0635 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 2.51 (3H, s, Me), 3.50 (3H, s, CO2Me), 4.98 (1H, d, J = 16.0 Hz, CH), 6.35 (1H, d, J = 16.0 Hz, CH), 7.29 (2H, d, J = 9.1 Hz, Ph), 7.34–7.47 (14H, m, Ph), 7.61 (2H, d, J = 8.1 Hz, Ph), 7.72 (2H, d, J = 8.1 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.7, 51.5, 59.7 (d, 1JPC = 132.0 Hz), 110.7, 118.9, 120.6 (d, 1JPC = 95.6 Hz), 121.7, 121.9, 124.3 (d, 3JPC = 12.5 Hz), 128.1, 129.1, 129.8 (d, 3JPC = 13.7 Hz), 131.0, 131.3, 134.9 (d, 2JPC = 11.7 Hz), 135.7, 136.0, 137.5, 140.6 (d, 4JPC = 3.5 Hz), 144.1, 165.8, 166.8 (d, 2JPC = 16.1 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 11.1 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1640, 1716 cm−1. λmax(CHCl3): 359 nm. HRMS (ESI+): calcd for C40H28Cl3N2O5PS [M+] 784.0522; found 784.0474.
= 1640, 1716 cm−1. λmax(CHCl3): 359 nm. HRMS (ESI+): calcd for C40H28Cl3N2O5PS [M+] 784.0522; found 784.0474.
(E)-Methyl 3-(2-(4-cyanophenyl)-5-oxo-1-tosyl-4-(tricyclohexylphosphoranylidene)-4,5-dihydro-1H-pyrrol-3-yl)acrylate (4p)
Yellow solid. M.p. 115–117 °C. Rf = 0.08 (hexanes–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Isolated yield 22% (0.0231 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 1.11–1.35 (18H, br, CH2), 1.62–1.67 (12H, br, CH2), 2.37 (3H, s, Me), 2.72–2.76 (3H, br, CH), 3.67 (3H, s, CO2Me), 5.49 (1H, d, J = 15.9 Hz, CH), 7.22–7.25 (3H, m, Ph), 7.50 (2H, d, J = 8.4 Hz, Ph), 7.59 (2H, d, J = 8.1 Hz, Ph), 7.70 (2H, d, J = 8.1 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.5, 25.7, 26.9 (d, 3JPC = 15.8 Hz), 27.0 (d, 2JPC = 5.3 Hz), 30.8 (d, 1JPC = 46.8 Hz), 51.6, 57.5 (d, 1JPC = 105.7 Hz), 109.8, 113.8, 119.2, 120.1 (d, 2JPC = 10.4 Hz), 123.0, 123.8 (d, 3JPC = 9.4 Hz), 127.8, 128.7, 131.0, 131.2, 135.7, 138.1, 143.5, 166.6, 166.9 (d, 2JPC = 15.1 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 27.6 ppm. FTIR (KBr):
1). Isolated yield 22% (0.0231 g). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 1.11–1.35 (18H, br, CH2), 1.62–1.67 (12H, br, CH2), 2.37 (3H, s, Me), 2.72–2.76 (3H, br, CH), 3.67 (3H, s, CO2Me), 5.49 (1H, d, J = 15.9 Hz, CH), 7.22–7.25 (3H, m, Ph), 7.50 (2H, d, J = 8.4 Hz, Ph), 7.59 (2H, d, J = 8.1 Hz, Ph), 7.70 (2H, d, J = 8.1 Hz, Ph) ppm. 13C NMR (75 MHz, CDCl3, 25 °C): δ = 21.5, 25.7, 26.9 (d, 3JPC = 15.8 Hz), 27.0 (d, 2JPC = 5.3 Hz), 30.8 (d, 1JPC = 46.8 Hz), 51.6, 57.5 (d, 1JPC = 105.7 Hz), 109.8, 113.8, 119.2, 120.1 (d, 2JPC = 10.4 Hz), 123.0, 123.8 (d, 3JPC = 9.4 Hz), 127.8, 128.7, 131.0, 131.2, 135.7, 138.1, 143.5, 166.6, 166.9 (d, 2JPC = 15.1 Hz) ppm. 31P NMR (242 MHz, CDCl3, 25 °C): δ = 27.6 ppm. FTIR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1629, 1720 cm−1. λmax(CHCl3): 413 nm. HRMS (ESI+): calcd for C40H49N2O5PS [M+] 700.3099; found 700.3090.
= 1629, 1720 cm−1. λmax(CHCl3): 413 nm. HRMS (ESI+): calcd for C40H49N2O5PS [M+] 700.3099; found 700.3090.
Acknowledgements
We thank the National Science Council for supporting this research financially (NSC 102-2113-M-009-011).References
- (a) Z. Chen and J. Wu, Org. Lett., 2010, 12, 4856 CrossRef CAS PubMed; (b) H.-R. Pan, Y.-J. Li, C.-X. Yan, J. Xing and Y. Cheng, J. Org. Chem., 2010, 75, 6644 CrossRef CAS PubMed; (c) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899 CrossRef CAS PubMed; (d) B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef PubMed; (e) N. R. Candeias, F. Montalbano, P. M. S. D. Cal and P. M. P. Gois, Chem. Rev., 2010, 110, 6169 CrossRef CAS PubMed; (f) A. V. Ivachtchenko, Y. A. Ivanenkov, V. M. Kysil, M. Y. Krasavin and A. P. Ilyin, Russ. Chem. Rev., 2010, 79, 787 CrossRef CAS; (g) H. Zhou, W. Wang, O. Khorev, Y. Zhang, Z. Miao, T. Meng and J. Shen, Eur. J. Org. Chem., 2012, 5585 CrossRef CAS; (h) B. O. A. Tasch, E. Merkul and T. J. J. Müller, Eur. J. Org. Chem., 2011, 4532 CrossRef CAS; (i) N. George, M. Bekkaye, G. Masson and J. Zhu, Eur. J. Org. Chem., 2011, 3695 CrossRef CAS; (j) M. Li, F.-M. Gong, L.-R. Wen and Z.-R. Li, Eur. J. Org. Chem., 2011, 3482 CrossRef CAS; (k) J. Sun, Q. Wu, E.-Y. Xia and C.-G. Yan, Eur. J. Org. Chem., 2012, 2981 Search PubMed; (l) S. T. Staben and N. Blaquiere, Angew. Chem., Int. Ed., 2010, 49, 325 CrossRef CAS PubMed; (m) J. Li, N. Wang, C. Li and X. Jia, Chem.–Eur. J., 2012, 18, 9645 CrossRef CAS PubMed; (n) T. Wennekes, K. M. Bonger, K. Vogel, R. J. B. H. N. van den Berg, A. Strijland, W. E. Donker-Koopman, J. M. F. G. Aerts, G. A. van der Marel and H. S. Overkleeft, Eur. J. Org. Chem., 2012, 6420 CrossRef CAS; (o) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed; (p) S. Brauch, S. S. van Berkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948 RSC; (q) C. M. Marson, Chem. Soc. Rev., 2012, 41, 7712 RSC; (r) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC.
- (a) O. Diels and K. Alder, Leibigs Ann. Chem., 1932, 498, 16 CrossRef; (b) R. M. Acheson, Adv. Heterocycl. Chem., 1963, 1, 125 CrossRef CAS; (c) V. Nair, A. R. Sreekanth, A. T. Biju and N. P. Rath, Tetrahedron Lett., 2003, 44, 729 CrossRef CAS; (d) V. Nair, A. R. Sreekanth, N. Abhilash, M. M. Bhadbhade and R. C. Gonnade, Org. Lett., 2002, 4, 3575 CrossRef PubMed; (e) J.-B. Gualtierotti, X. Schumacher, P. Fontaine, G. Masson, Q. Wang and J. Zhu, Chem.–Eur. J., 2012, 18, 14812 CrossRef CAS PubMed.
- (a) I. Ugi, R. Meyr, U. Fitzer and C. Steinbrücker, Angew. Chem., 1959, 71, 386 Search PubMed; (b) R. Zhou, J. Wang, H. Song and Z. He, Org. Lett., 2011, 13, 580 CrossRef CAS PubMed; (c) J. R. Harris, M. T. Haynes II, A. M. Thomas and K. A. Woerpel, J. Org. Chem., 2010, 75, 5083 CrossRef CAS PubMed; (d) X.-Y. Guan, Y. Wei and M. Shi, Org. Lett., 2010, 12, 5024 CrossRef CAS PubMed; (e) T. Wang and S. Ye, Org. Lett., 2010, 12, 4168 CrossRef CAS PubMed; (f) S. Zheng and X. Lu, Org. Lett., 2009, 11, 3978 CrossRef CAS PubMed; (g) X. Lu, Y. Du and C. Lu, Pure Appl. Chem., 2005, 77, 1985 CrossRef CAS; (h) C.-W. Cho, J.-R. Kong and M. J. Krische, Org. Lett., 2004, 6, 1337 CrossRef CAS PubMed; (i) C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS; (j) V. Nair, J. S. Nair, A. U. Vinod and N. P. Rath, J. Chem. Soc., Perkin Trans. 1, 1997, 3129 RSC; (k) V. Nair, J. S. Nair and A. U. Vinod, Synthesis, 2000, 1713 CrossRef CAS; (l) P. Xie, E. Li, J. Zheng, Y. Huang and R. Chen, Adv. Synth. Catal., 2013, 355, 161 CrossRef CAS; (m) Z. Shi, Q. Tong, W. W. Y. Leong and G. Zhong, Chem.–Eur. J., 2012, 18, 9802 CrossRef CAS PubMed; (n) J. Tian and Z. He, Chem. Commun., 2013, 49, 2058 RSC; (o) X. Li, F. Wang, N. Dong and J.-P. Cheng, Org. Biomol. Chem., 2013, 11, 1451 RSC; (p) L. Yang, P. Xie, E. Li, X. Li, Y. Huang and R. Chen, Org. Biomol. Chem., 2012, 10, 7628 RSC; (q) D. Wang, Y.-L. Yang, J.-J. Jiang and M. Shi, Org. Biomol. Chem., 2012, 10, 7158 RSC; (r) X.-N. Zhang, H.-P. Deng, L. Huang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 8664 RSC.
- (a) V. Nair and A. U. Vinod, Chem. Commun., 2000, 1019 RSC; (b) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899 CrossRef CAS PubMed; (c) S. S. van Berkel, B. G. M. Bögels, M. A. Wijdeven, B. Westermann and F. P. J. T. Rutjes, Eur. J. Org. Chem., 2012, 3543 CrossRef CAS; (d) I. Ugi and B. Werner, The Four-Component Reaction and Other Multicomponent Reactions of the Isocyanides, in Methods and Reagents for Green Chemistry: An Introduction, ed. P. Tundo, A. Perosa and F. Zecchini, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2007 DOI:10.1002/9780470124086.ch1; (e) K. Khoury, M. K. Sinha, T. Nagashima, E. Herdtweck and A. Domling, Angew. Chem., Int. Ed., 2012, 51, 10280 CrossRef CAS PubMed; (f) S. K. Guchhait and C. Madaan, Org. Biomol. Chem., 2010, 8, 3631 RSC.
- (a) Y. C. Fan and O. Kwon, Sci. Synth.: Asymm. Organocatal., 2012, 1, 723 CAS; (b) C. Gomez, J.-F. Betzer, A. Voituriez and A. Marinetti, ChemCatChem, 2012, 5, 1055 CrossRef.
- C. E. Henry and O. Kwon, Org. Lett., 2007, 9, 3069 CrossRef CAS PubMed.
- X.-F. Zhu, J. Lan and O. Kwon, J. Am. Chem. Soc., 2003, 125, 4716 CrossRef CAS PubMed.
- X.-F. Zhu, A.-P. Schaffner, R. C. Li and O. Kwon, Org. Lett., 2005, 7, 2977 CrossRef CAS PubMed.
- J. Ma, P. Xie, C. Hu, Y. Huang and R. Chen, Chem.–Eur. J., 2011, 17, 7418 CrossRef CAS PubMed.
- J.-C. Deng and S.-C. Chuang, Org. Lett., 2011, 13, 2248 CrossRef CAS PubMed.
- (a) S.-C. Chuang, J.-C. Deng, F.-W. Chan, S.-Y. Chen, W.-J. Huang, L.-H. Lai and V. Rajeshkumar, Eur. J. Org. Chem., 2012, 2606 CrossRef CAS; (b) P.-Y. Tseng and S.-C. Chuang, Adv. Synth. Catal., 2013, 355, 2165 CrossRef CAS.
- J.-C. Deng, F.-W. Chan, C.-W. Kuo, C.-A. Cheng, C.-Y. Huang and S.-C. Chuang, Eur. J. Org. Chem., 2012, 5738 CrossRef CAS.
- A. S. Chavan, J.-C. Deng and S.-C. Chuang, Molecules, 2013, 18, 2611 CrossRef CAS PubMed.
- G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed.
- Unpublished results—using N-cinnamylidene-p-toluenesulfonamide as an aldimine substrate, we isolated isatin derivatives having α-phosphorus ylides through [2 + 2 + 2] electrocyclization and oxidation in the air.
- (a) S. Asghari, M. Tajbakhsh and V. Taghipour, Tetrahedron Lett., 2008, 49, 1824 CrossRef CAS; (b) D. Q. Tan, K. S. Martin, J. C. Fettinger and J. T. Shaw, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6781 CrossRef CAS PubMed; (c) F. Pan, J.-M. Chen, Z. Zhuang, Y.-Z. Fang, S. X.-A. Zhang and W.-W. Liao, Org. Biomol. Chem., 2012, 10, 2214 RSC; (d) M. González-López and J. T. Shaw, Chem. Rev., 2009, 109, 164 CrossRef PubMed; (e) A. Younai, G. F. Chin and J. T. Shaw, J. Org. Chem., 2010, 75, 8333 CrossRef CAS PubMed; (f) H. Zhang, Y. Leng, W. Liu and W. Duan, Synth. Commun., 2012, 42, 1115 CrossRef CAS; (g) S. Roy and O. Reiser, Angew. Chem., Int. Ed., 2012, 51, 4722 CrossRef CAS PubMed; (h) A. Alizadeh, A. Mikaeili and T. Firuzyar, Synthesis, 2012, 1380 CrossRef CAS.
- (a) R. B. Lettan II, C. C. Woodward and K. A. Scheidt, Angew. Chem., Int. Ed., 2008, 47, 2294 CrossRef PubMed; (b) A. Zamudio-Medina, M. C. García-González, J. Padilla and E. González-Zamora, Tetrahedron Lett., 2010, 51, 4837 CrossRef CAS.
- X-ray crystallographic data for compound 4a: red bricks; crystal size: 0.25 × 0.20 × 0.07 mm3; formula: C39H31N2O7PS; crystal system: monoclinic; space group P121/n1; d = 1.385 mg m−3, V = 3369.8(2) Å3; a = 10.7329(4) Å; b = 16.5196(6) Å; c = 19.3778(8) Å; β = 101.2410(10)°; R1 = 0.0365; Rw = 0.0849. CCDC-911444 contains the supplementary crystallographic data for this paper.
- X-ray crystallographic data for compound 4l: orange-red blocks; crystal size: 0.15 × 0.12 × 0.05 mm3; formula: C40H28Cl7N2O7PS; crystal system: triclinic; space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; d = 1.539 mg m−3, V = 2070.7(6) Å3; a = 10.2628(16) Å; b = 14.1040(2) Å; c = 15.1470(2) Å; α = 85.999(3)°, β = 73.595(3)°, γ = 79.998(3)°; R1 = 0.0726; Rw = 0.1825. CCDC-912012 contains the supplementary crystallographic data for this paper.
; d = 1.539 mg m−3, V = 2070.7(6) Å3; a = 10.2628(16) Å; b = 14.1040(2) Å; c = 15.1470(2) Å; α = 85.999(3)°, β = 73.595(3)°, γ = 79.998(3)°; R1 = 0.0726; Rw = 0.1825. CCDC-912012 contains the supplementary crystallographic data for this paper. - (a) A. M. Brouwer, Pure Appl. Chem., 2011, 83, 2213 CrossRef CAS; (b) A. T. R. Williams, S. A. Winfield and J. N. Miller, Analyst, 1983, 108, 1067 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 911444 and 912012. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ob41811a |
| This journal is © The Royal Society of Chemistry 2014 |