Open Access Article
Open Access ArticleThe acid-catalysed synthesis of 7-azaindoles from 3-alkynyl-2-aminopyridines and their antimicrobial activity†
Tlabo C.
Leboho
a,
Sandy F.
van Vuuren
b,
Joseph P.
Michael
a and
Charles B.
de Koning
*a
aMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, PO Wits 2050, Johannesburg, South Africa. E-mail: Charles.deKoning@wits.ac.za; Fax: +27 11 7176749; Tel: +27 11 7176724
bDepartment of Pharmacy and Pharmacology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
First published on 7th November 2013
Abstract
The synthesis of 7-azaindoles from 3-alkynyl-2-aminopyridines using acidic conditions, namely, a mixture of trifluoroacetic acid (TFA) and trifluoroacetic anhydride (TFAA), is described. This methodology resulted in the synthesis of fifteen 7-azaindoles, with most containing substituents at the 2- and 5-positions. The majority of these were tested for antimicrobial activity against a range of bacteria and yeasts. The 7-azaindoles displayed the best activity against the yeasts, particularly against Cryptococcus neoformans, where activities as low as 3.9 μg ml−1 were observed.
Introduction
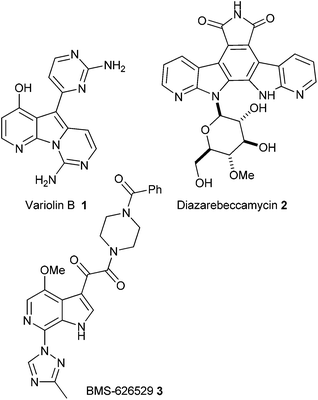
The azaindoles are a growing class of indole isosteres that possess promising biological profiles. For example, the natural product variolin B 1 (Fig. 1) isolated from an extremely rare Antarctic sponge is a promising anti-cancer agent.1Other examples include the synthetic azaindole derivative of rebeccamycin, known as diazarebeccamycin 2, which has also been shown to exhibit promising anticancer activity,2 and 6-azaindole 3 which has demonstrated activity as an inhibitor of HIV-1 attachment to CD4+ T cells.3
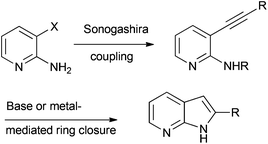
The synthesis of 7-azaindoles is often accomplished by initial Sonogashira coupling of a 3-halosubstituted-2-aminopyridine as shown in Scheme 1.4 The intermediate alkynylpyridines are then exposed to a variety of different reaction conditions and reagents to afford the desired azaindoles. Many methods have been developed for this step and include the use of cesium- or potassium-containing bases such as potassium hydride, potassium t-butoxide or cesium t-butoxide5a–d or even bases such as DBU5e and triethylamine.5f Metal-containing catalysts have also been used for this purpose and include amongst others, the use of copper,6a an indium salt6b or even gold catalysts.6c The Cacchi reaction in the presence of a palladium catalyst6d has also been successfully used to facilitate both the Sonogashira coupling reaction and the azaindole ring forming step to produce 2,3-disubstituted azaindoles directly from the aminopyridines. Even the use of microwaves in the presence of water with7a or without7b added salts has been reported.
As a result of our interest in the synthesis of indole-containing compounds and their antibacterial and antifungal activity,8 we initiated a programme directed towards the synthesis of the related compounds, azaindoles. The specific goal described in this publication was to attempt to develop versatile and cheap conditions for the synthesis of 7-azaindoles from the corresponding Sonogashira coupled 3-alkynyl-2-aminopyridine precursors. Thereafter, as azaindoles have interesting biological profiles, we wished to ascertain if the 7-azaindoles we had synthesized would display activity against a range of bacterial and yeast cell lines.
Results and discussion
Initially the required halogenated substituted pyridines were prepared or obtained from commercial sources. 5-Chloro-2-aminopyridine 4, 2-aminopyridine 5, 3-bromo-2-aminopyridine 6 and 5-methyl-2-amino-3-bromopyridine 7 were obtained from Sigma-Aldrich. 5-Chloro-2-aminopyridine 4 was treated with I2 in the presence of Ag2SO49 in ethanol to afford the 3-iodo substituted aminopyridine 8 in good yield.Finally, 5-bromo-3-iodopyridin-2-amine 10 was prepared by initially treating 2-aminopyridine 5 with NBS10 in MeCN to afford 5-bromo-2-aminopyridine 9. Then 9 was subjected to potassium iodate, I2 and a mixture of H2SO4, acetic acid and water6a to provide 5-bromo-3-iodopyridin-2-amine 10 in good yield (Scheme 2).
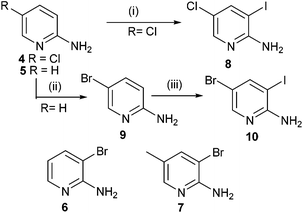 | ||
| Scheme 2 Reagents and conditions: (i) I2, Ag2SO4, EtOH, rt, 86%; (ii) NBS, MeCN, rt, 98%; (iii) I2, KIO3, H2SO4, AcOH, H2O, 80 °C, 90%. | ||
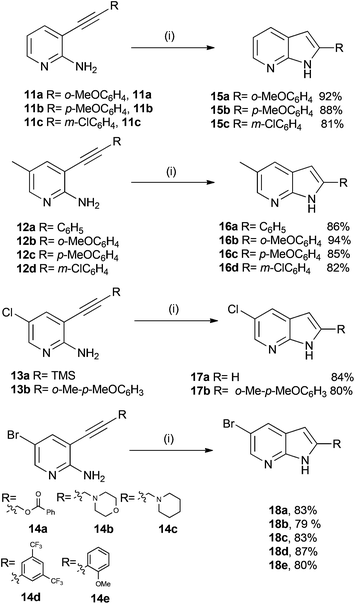
We were now in a position to attempt the Sonogashira coupling reaction, for which we believed that coupling would take place at the more reactive iodine substituent in substrates 8 and 10, or at the bromine in the case of aminopyridines 6 and 7. The 2-amino-3-halogen containing pyridines were treated with catalytic tetrakis(triphenylphosphine)palladium(0), copper(I) iodide, triethylamine as a base and a range of acetylenes to afford the desired compounds (11–14) in good to excellent yield as shown in Scheme 3.
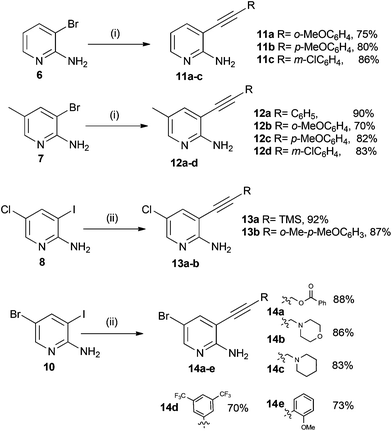 | ||
Scheme 3
Reagents and conditions: (i) RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, Et3N, CuI, cat. Pd(PPh3)4, THF, 70 °C; (ii) RC CH, Et3N, CuI, cat. Pd(PPh3)4, THF, 70 °C; (ii) RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH Et3N, CuI, cat. Pd(PPh3)4, THF, rt. CH Et3N, CuI, cat. Pd(PPh3)4, THF, rt. | ||
The stage was now set to attempt the formation of the pyrrole ring in order to assemble the 7-azaindole nucleus. For this step we wished to investigate if it was possible to use acidic conditions to affect the ring closure. A variety of acids (HCl, H2SO4, AcOH and trifluoroacetic acid (TFA)) were initially used. The use of TFA seemed to be promising as a small amount of azaindole was forming but the conversion rate was estimated to be only 10%. Since there are examples in the literature4 of the corresponding amide (rather than amine) undergoing ring closure to afford azaindoles, we thought that the addition of trifluoroacetic anhydride (TFAA) to the reaction mixture containing TFA might improve the rate, conversion and yield of the desired azaindoles. After much experimentation we found that in the presence of 1 equivalents of TFA and 1.3 equivalents of TFAA in MeCN as a solvent and heating to reflux for 8 h, the substrate afforded the desired azaindoles (15–18) in generally excellent yields (Scheme 4).
As one final example we wished to take advantage of the different reactivity of the halogens on substrate 10 to establish if it was possible to introduce two different alkyne substituents using different temperatures. Hence as shown in Scheme 5, 10 was treated under Sonogashira reaction conditions with phenylacetylene initially at room temperature. After TLC showed that the starting material had all been consumed, the solvent was evaporated under reduced pressure and the crude material was then subjected to the same palladium catalyst using trimethylsilylacetylene as the alkyne coupling partner. After the reaction was deemed to be complete by TLC, work-up of the reaction and analysis of the product by NMR spectroscopy showed that two different alkyne substituents were present on the 3,5-substituted aminopyridine 19. Presumably, this is due to the different reactivities of the iodine and bromine substituents in the two Sonogashira reactions. Exposure of 19 to our acid mediated reaction conditions for azaindole formation resulted in the formation of the desired azaindole 20 in excellent yield.
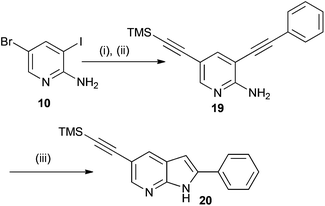 | ||
Scheme 5
Reagents and conditions: (i) Ph-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, Et3N, CuI, Pd(PPh3)4, THF, rt; (ii) TMS-C CH, Et3N, CuI, Pd(PPh3)4, THF, rt; (ii) TMS-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, Et3N, CuI, Pd(PPh3)4, THF, 70 °C. 85% over two steps; (iii) TFA, TFAA, MeCN, 100 °C, 91%. CH, Et3N, CuI, Pd(PPh3)4, THF, 70 °C. 85% over two steps; (iii) TFA, TFAA, MeCN, 100 °C, 91%. | ||
As we now had a series of substituted azaindoles on hand they were quantitatively evaluated for antimicrobial activity (Table 1). While compound 16b showed promising antibacterial activity (7.8 μg ml−1) against Pseudomonas aeruginosa, many of the azaindoles showed very good and selective anti-yeast activity, particularly against Cryptococcus neoformans, where all compounds showed noteworthy inhibitory values of between 3.9–15.6 μg ml−1. Previous studies on the antimicrobial effects of other azaindole derivatives include the quantitative study by Kumaran et al.,11 and the promising antifungal effects found by Lebouvier.12 Previous antimicrobial studies on azaindoles for activity against Cryptococcus neoformans is, however, lacking and the promising activity noted here warrants further examination, particularly, given that increased reports of infections from Cryptococcus neoformans show that this represents a major health threat for HIV infected patients.13,14 Newer antifungals to combat this opportunistic pathogen will certainly be beneficial in future treatments, particularly with emerging trends of resistance evident.
| Compound | Pseudomonas aeruginosa | Escherichia coli | Klebsiella pneumoniae | Staphylococcus aureus | Enterococcus faecalis | Cryptococcus neoformans | Candida tropicalis | Candida albicans |
|---|---|---|---|---|---|---|---|---|
| NCTC 9027 | ATCC 8739 | ATCC 8739 | ATCC25923 | ATCC 29212 | ATCC 90112 | ATCC 201380 | ATCC 10231 | |
| a Control for bacteria, ciprofloxacin; for the yeasts, amphotericin. | ||||||||
| 15a | 31.3 | 62.5 | 31.3 | 31.3 | 93.8 | 4.0 | 5.9 | 7.8 |
| 15b | 31.3 | 62.5 | 31.3 | 31.3 | 125.0 | 7.8 | 31.3 | 15.6 |
| 15c | 31.3 | 31.3 | 23.5 | 31.3 | 62.5 | 15.6 | 31.3 | 31.3 |
| 16a | 31.3 | 31.3 | 31.3 | 62.5 | 250.0 | 7.8 | 31.3 | 15.6 |
| 16b | 7.8 | 62.5 | 31.3 | 31.3 | 125.0 | 3.9 | 31.3 | 31.3 |
| 16c | 31.3 | 46.9 | 31.3 | 31.3 | 62.5 | 7.8 | 15.6 | 31.3 |
| 17a | 31.3 | 62.5 | 31.3 | 62.5 | 125.0 | 15.6 | 15.6 | 31.3 |
| 17b | 31.3 | 31.3 | 31.3 | 250.0 | 125.0 | 15.6 | 15.6 | 250.0 |
| 18a | 31.3 | 31.3 | 46.9 | 31.3 | 250.0 | 7.8 | 15.6 | 23.5 |
| 18b | 31.3 | 31.3 | 31.3 | 62.5 | 62.5 | 8.0 | 31.3 | 31.3 |
| 18c | 31.3 | 31.3 | 31.3 | 62.5 | 62.5 | 15.6 | 31.3 | 31.3 |
| 18d | 62.5 | 31.3 | 62.5 | 31.3 | 250.0 | 15.6 | 31.3 | 15.6 |
| 18e | 62.5 | 31.3 | 31.3 | 62.5 | 250.0 | 15.6 | 31.3 | 15.6 |
| 20 | 46.9 | 250.0 | 31.3 | 250.0 | 125.0 | 3.9 | 31.3 | 31.3 |
| Controla | 0.3 | 0.6 | 0.6 | 0.6 | 0.3 | 0.8 | 2.5 | 2.5 |
In summary, to the best of our knowledge this is the first report outlining the synthesis of azaindoles from 3-alkynyl-2-aminopyridines under acidic conditions. The azaindoles synthesized display significant anti-yeast activity.
Experimental
1H NMR and 13C NMR spectra were recorded either on a Bruker AVANCE 300 spectrometer or a Bruker AVANCE III 500 spectrometer. All chemical shift values are reported in parts per million referenced against TMS which is given an assignment of zero parts per million. Coupling constants (J-values) are given in hertz (Hz). All mass spectroscopy data were collected on a Waters Acquity UPLC system coupled to a Waters HDMS G1 QTOF mass spectrometer. UPLC settings: analytical column: BEH C18 150 × 2.1 mm; column temperature: 60 °C; mobile phase: 90% water (0.1% formic acid): 5% acetonitrile; flow rate: 0.4 ml min−1. MS settings: mode: VTOF; ionisation: ESIPos and ESINeg; scan range: 100–1000 Da; scan speed: 0.1 second; run time: 10 min. Infrared spectra were recorded on a Bruker Tensor 27 standard system spectrometer with diamond ATR attachment. Macherey-Nagel Kieselgel 60 (particle size 0.063–0.200 mm) was used for conventional silica gel column chromatography with various EtOAc and hexane mixtures as the mobile phase. TLC was performed on aluminum-backed Macherey-Nagel Alugram Sil G/UV254 plates pre-coated with 0.25 mm silica gel 60. The following acetylenes are commercially available: phenylacetylene, trimethylsilylacetylene, 2-ethynylanisole, 4-ethynylanisole, 1-ethynyl-4-methoxy-2-methylbenzene, 1-ethynyl-3,5-bis(trifluoromethyl)benzene and propargylbenzoate. The remaining two acetylenes 1-(prop-2-ynyl)piperidine and 4-(prop-2-ynyl)morpholine were prepared according to ref. 15 and 16.5-Chloro-3-iodo-2-aminopyridine 8
To a solution of 2-amino-5-chloropyridine 4 (0.700 g, 5.45 mmol) in ethanol was added silver sulfate (1.699 g, 5.45 mmol) in one portion followed by the addition of iodine (1.383 g, 5.45 mmol) portionwise. The heterogeneous mixture was stirred at rt until determined complete by TLC. After the reaction was complete, the mixture was filtered through celite or silica gel and the ethanol removed under reduced pressure. The resulting crude material was re-dissolved in dichloromethane and washed with a saturated solution of aqueous sodium thiosulfate. The separated dichloromethane layer was removed under reduced pressure and the resulting solid was purified by silica gel chromatography to give 2-amino-5-chloro-3-iodopyridine 89 (1.189 g 86%). 1H NMR (300 MHz, CDCl3) δ 7.98 (d, J = 2.2, 1H), 7.84 (d, J = 2.2, 1H), 5.06 (s, 2H).5-Bromo-2-aminopyridine 9
2-Aminopyridine 5 (2.600 g, 0.0276 mol) was dissolved in dry MeCN (100 ml), treated with N-bromosuccinimide (5.219 g, 0.0293 mol) and stirred for 2 h at rt. The solvent was removed and the resulting cream white solid purified by flash chromatography to give 2-amino-5-bromopyridine 910 as a white solid (4.692 g, 98%). 1H NMR (300 MHz, CDCl3) δ 8.08 (s, 1H), 7.48 (dd, J = 8.7, 2.4, 1H), 6.41 (d, J = 8.7, 1H), 4.44 (s, 2H); 13C NMR (75 MHz, CDCl3) δ 157.36, 148.88, 140.49, 110.42, 108.56.5-Bromo-3-iodo-2-aminopyridine 10
A mixture of 2-amino-5-bromopyridine 9 (45.00 g, 0.260 mol), iodic acid (11.86 g, 0.0676 mol), iodine (26.40 g, 0.104 mol), sulfuric acid (4.50 ml), acetic acid (150 ml) and water (30 ml) was heated to 80 °C for 8 h. After this time, the reaction mixture was concentrated under vacuum and then made basic with an aqueous 12 M sodium hydroxide solution. The basic solution was extracted with dichloromethane, washed with a saturated solution of sodium thiosulfate and then brine, dried over MgSO4 and then the organic solvent was removed under vaccum. The resulting cream white solid was purified by flash chromatography to give 2-amino-5-bromo-3-iodopyridine 106a as a white solid (70.11 g, 90%). 1H NMR (300 MHz, CDCl3) δ 8.05 (s, 1H), 7.95 (s, 1H), 5.03 (s, 2H); 13C NMR (75 MHz, CDCl3) δ 156.75, 148.72, 148.57, 107.58, 77.93.General procedure for the Sonogashira reaction to synthesize 11a–c and 12a–d
To a flame-dried round-bottom flask under a nitrogen or argon atmosphere containing 2-amino-3-bromopyridine derivative (6 and 7) was added CuI (2 mol%) and Pd(PPh3)4 (2 mol%) in one portion followed by the addition of the degassed alkyne solution in THF (20 ml). Triethylamine (5 ml) was then added and the reaction mixture was stirred at 70 °C until no starting material was present as monitored by thin layer chromatography (TLC). After this time, the reaction was quenched with a saturated aqueous ammonium chloride and was then extracted with either dichloromethane or ethyl acetate. The combined organic extracts were dried over magnesium sulfate, filtered through either silica or celite and the excess solvent was removed on a rotary evaporator, followed by purification using flash chromatography (30% EtOAc–hexane) to yield the desired products 11a–c and 12a–d.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1580 (C
N), 1580 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1181 (C–O). HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1032.
C), 1181 (C–O). HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1032.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1558 (C
N), 1558 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1144 (C–O). HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1039.
C), 1144 (C–O). HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1039.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1565 (C
N), 1565 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1198 (C–O); HRMS (ES+) Calculated for C13H10N2Cl [M + H]+: 229.0533, found 229.0542.
C), 1198 (C–O); HRMS (ES+) Calculated for C13H10N2Cl [M + H]+: 229.0533, found 229.0542.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1594 (C
N), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1179 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 239.1198.
C), 1179 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 239.1198.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1564 (C
N), 1564 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1170 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 239.1185.
C), 1170 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 239.1185.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1590 (C
N), 1590 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1176 (C–O); HRMS (ES+) Calculated for C14H12N2Cl [M + H]+: 243.0689, found 243.0705.
C), 1176 (C–O); HRMS (ES+) Calculated for C14H12N2Cl [M + H]+: 243.0689, found 243.0705.
General procedure for the Sonogashira reaction to synthesize 13a–b and 14a–e
To a flame-dried round-bottom flask under a nitrogen or argon atmosphere containing 2-amino-3-iodopyridine derivative 9 and 10 was added CuI (2 mol%) and Pd(PPh3)4 (2 mol%) in one portion followed by the addition of the degassed alkyne solution in THF (20 ml). Triethylamine (5 ml) was then added and the reaction mixture was stirred at rt, until no starting material was present as monitored by thin layer chromatography (TLC). After this time, the reaction was quenched with a saturated aqueous ammonium chloride and was extracted with either dichloromethane or ethyl acetate. The combined organic extracts were dried over magnesium sulfate, filtered through either silica or celite and the excess solvent was removed on a rotary evaporator, followed by purification using flash chromatography (30% EtOAc–hexane) to yield the desired products 12a–b and 14a–e.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1558 (C
N), 1558 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1164 (C–O); HRMS (ES+) Calculated for C15H14N2ClO [M + H]+: 273.0795, found 273.0801.
C), 1164 (C–O); HRMS (ES+) Calculated for C15H14N2ClO [M + H]+: 273.0795, found 273.0801.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1548 (C
N), 1548 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1173 (C–O); HRMS (ES+) Calculated for C15H12N2O2Br [M + H]+: 331.0082, found 331.0080.
C), 1173 (C–O); HRMS (ES+) Calculated for C15H12N2O2Br [M + H]+: 331.0082, found 331.0080.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1572 (C
N), 1572 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1132 (C–O); HRMS (ES+) Calculated for C12H15N3BrO [M + H]+: 296.0398, found 296.0420.
C), 1132 (C–O); HRMS (ES+) Calculated for C12H15N3BrO [M + H]+: 296.0398, found 296.0420.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1558 (C
N), 1558 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C13H17N3Br [M + H]+: 294.0606, found 294.0613.
C); HRMS (ES+) Calculated for C13H17N3Br [M + H]+: 294.0606, found 294.0613.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1571 (C
N), 1571 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C15H8N2BrF6 [M + H]+: 408.9775, found 408.9778.
C); HRMS (ES+) Calculated for C15H8N2BrF6 [M + H]+: 408.9775, found 408.9778.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1571 (C
N), 1571 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1181 (C–O); HRMS (ES+) Calculated for C14H12N2BrO [M + H]+: 303.0133, found 303.0147.
C), 1181 (C–O); HRMS (ES+) Calculated for C14H12N2BrO [M + H]+: 303.0133, found 303.0147.
General method for the synthesis of azaindoles 15a–c, 16a–d, 17a–b and 18a–e
The corresponding 2-amino-3-ethynylpyridine derivatives (40–200 mg) 11a–c, 12a–d, 13a–b and 14a–e were dissolved in MeCN. To the resulting solution was added trifluoroacetic acid (1 eq.) in one portion followed by the addition of trifluoroacetic anhydride (1.3 eq.) dropwise. The resulting mixture was then heated to reflux for 8 h and was allowed to cool to rt. The solvent was then removed on a rotary evaporator and the resulting crude material was re-dissolved in dichloromethane and washed with aqueous sodium carbonate (10%). The separated and dried (MgSO4) organic phase was purified by flash silica chromatography using ethyl acetate–hexane mixture (10–50%) gave desired azaindoles 15a–c, 16a–d, 17a–d and 18a–e in good yields.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1578 (C
N), 1578 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1179 (C–O); HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1042.
C), 1179 (C–O); HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1042.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1594 (C
N), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1176 (C–O); HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1035.
C), 1176 (C–O); HRMS (ES+) Calculated for C14H13N2O [M + H]+: 225.1028, found 225.1035.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1588 (C
N), 1588 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C13H10N2Cl [M + H]+: 229.0533, found 229.0536.
C); HRMS (ES+) Calculated for C13H10N2Cl [M + H]+: 229.0533, found 229.0536.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1579 (C
N), 1579 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1178 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 269.1190.
C), 1178 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 269.1190.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1575 (C
N), 1575 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1168 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 239.1190.
C), 1168 (C–O); HRMS (ES+) Calculated for C15H15N2O [M + H]+: 239.1184, found 239.1190.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1587 (C
N), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C14H12N2Cl [M + H]+: 243.0689, found 243.0697.
C); HRMS (ES+) Calculated for C14H12N2Cl [M + H]+: 243.0689, found 243.0697.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1553 (C
N), 1553 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1571 (C
N), 1571 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1182 (C–O). HRMS (ES+) Calculated for C15H14N2ClO [M + H]+: 273.0795, found 273.0803.
C), 1182 (C–O). HRMS (ES+) Calculated for C15H14N2ClO [M + H]+: 273.0795, found 273.0803.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (C
N), 1559 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1164 (C–O); HRMS (ES+) Calculated for C15H12N2BrO2 [M + H]+: 331.0082, found 331.0100.
C), 1164 (C–O); HRMS (ES+) Calculated for C15H12N2BrO2 [M + H]+: 331.0082, found 331.0100.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1573 (C
N), 1573 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1140 (C–O); HRMS (ES+) Calculated for C12H15N3BrO [M + H]+: 296.0398, found 296.0401.
C), 1140 (C–O); HRMS (ES+) Calculated for C12H15N3BrO [M + H]+: 296.0398, found 296.0401.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1572 (C
N), 1572 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C13H13N3Br [M + H]+: 294.0606, found 294.0621.
C); HRMS (ES+) Calculated for C13H13N3Br [M + H]+: 294.0606, found 294.0621.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1576 (C
N), 1576 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C15H8N2BrF6 [M + H]+: 408.9775, found 408.9764.
C); HRMS (ES+) Calculated for C15H8N2BrF6 [M + H]+: 408.9775, found 408.9764.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1576 (C
N), 1576 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1160 (C–O); HRMS (ES+) Calculated for C14H12N2BrO [M + H]+: 303.0133, found 303.0146.
C), 1160 (C–O); HRMS (ES+) Calculated for C14H12N2BrO [M + H]+: 303.0133, found 303.0146.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1583 (C
N), 1583 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C18H18N2Si [M + H]+: 291.2238, found 291.2237.
C); HRMS (ES+) Calculated for C18H18N2Si [M + H]+: 291.2238, found 291.2237.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1586 (C
N), 1586 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); HRMS (ES+) Calculated for C18H19N2Si [M + H]+: 291.1318, found 291.1327.
C); HRMS (ES+) Calculated for C18H19N2Si [M + H]+: 291.1318, found 291.1327.
Antimicrobial activity
The substituted azaindoles were quantitatively evaluated for antimicrobial activity using the minimum inhibitory concentration (MIC) assay.18 The azaindoles were tested against a number of reference test organisms including Gram-positive (Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 25923), Gram-negative (Pseudomonas aeruginosa NCTC 9027, Escherichia coli ATCC 8739 and Klebsiella pneumoniae ATCC 13883) and yeasts (Candida tropicalis ATCC 201380, Candida albicans ATCC 10231 and Cryptococcus neoformans ATCC 90112). The positive control used for the bacteria was ciprofloxacin, while that used for the yeasts was amphotericin. The solvent (acetone) was included in the assay as a negative control. All antimicrobial assays were undertaken at least in duplicate or triplicate where necessary.Acknowledgements
This work was supported by the National Research Foundation (NRF, GUN 2053652 and IRDP of the NRF (South Africa) for financial support provided by the Research Niche Areas programme), Pretoria, and the University of the Witwatersrand (Science Faculty Research Council). We also gratefully acknowledge the NRF scarce skills programme and the CSIR scholarship scheme for generous funding to TCL. Dr R Mampa (University of the Witwatersrand) and Dr P Steenkamp (CSIR, Pretoria) are thanked for providing the NMR and HRMS MS spectroscopy services, respectively.Notes and references
- S. R. Walker, E. J. Carter, B. C. Huff and J. C. Morris, Chem. Rev., 2009, 109, 3080–3098 CrossRef CAS PubMed.
- M. Prudhomme, Eur. J. Med. Chem., 2003, 38, 123–140 CrossRef CAS PubMed.
- B. Nowicka-Sans, Y.-F. Gong, B. McAuliffe, I. Dicker, H.-T. Ho, N. Zhou, B. Eggers, P.-F. Lin, N. Ray, M. Wind-Rotolo, L. Zhu, A. Majumdar, D. Stock, M. Lataillade, G. J. Hanna, J. D. Matiskella, Y. Ueda, T. Wang, J. F. Kadow, N. A. Meanwell and M. Krystal, Antimicrob. Agents Chemother., 2012, 56, 3498–3507 CrossRef CAS PubMed.
- (a) J. Y. Mérour, S. Routier, F. Suzenet and B. Joseph, Tetrahedron, 2013, 69, 4767–4834 CrossRef; (b) J. J. Song, J. T. Reeves, F. Gallou, Z. Tan, N. K. Yee and C. H. Senanayake, Chem. Soc. Rev., 2007, 36, 1120–1132 RSC; (c) J.-Y. Mérour and B. Joseph, Curr. Org. Chem., 2001, 5, 471–506 CrossRef; (d) F. Popowycz, S. Routier, B. Joseph and J.-Y. Mérour, Tetrahedron, 2007, 63, 1031–1064 CrossRef CAS.
- (a) M. C. de Mattos, S. Alatorre-Santamaría, V. Gotor-Fernández and V. Gotor, Synthesis, 2007, 2149–2152 Search PubMed; (b) C. Koradin, W. Dohle, A. L. Rodriguez, B. Schmid and P. Knochel, Tetrahedron, 2003, 59, 1571–1587 CrossRef CAS; (c) M. McLaughlin, M. Palucki and I. W. Davies, Org. Lett., 2006, 8, 3307–3310 CrossRef CAS PubMed; (d) A. L. Rodriguez, C. Koradin, W. Dohle and P. Knochel, Angew. Chem., Int. Ed., 2000, 39, 2488–2490 CrossRef CAS; (e) C. Harcken, Y. Ward, D. Thomson and D. Riether, Synlett, 2005, 3121–3125 CAS; (f) K. C. Majumdar and S. Mondal, Tetrahedron Lett., 2007, 48, 6951–6953 CrossRef CAS.
- (a) S. E. Pearson and S. Nandan, Synthesis, 2005, 2503–2506 CrossRef CAS; (b) N. Sakai, N. Annaka, A. Fujita, A. Sato and T. Konakahara, J. Org. Chem., 2008, 73, 4160–4165 CrossRef CAS PubMed; (c) K. C. Majumdar, S. Samanta and B. Chattopadhyay, Tetrahedron Lett., 2008, 49, 7213–7216 CrossRef CAS; (d) S. Cacchi, G. Fabrizi and L. M. Parisi, J. Comb. Chem., 2005, 7, 510–512 CrossRef CAS PubMed.
- (a) A. Carpita and A. Ribecai, Tetrahedron Lett., 2009, 50, 6877–6881 CrossRef CAS; (b) A. Carpita, A. Ribecai and P. Stabile, Tetrahedron, 2010, 66, 7169–7178 CrossRef CAS.
- T. C. Leboho, J. P. Michael, W. A. L. van Otterlo, S. F. van Vuuren and C. B. de Koning, Bioorg. Med. Chem. Lett., 2009, 19, 4948–4951 CrossRef CAS PubMed.
- L. V. Politanskaya, I. P. Chuikov, E. A. Kolodina, M. S. Shvartsberg and V. D. Shteingarts, J. Fluorine Chem., 2012, 135, 97–107 CrossRef CAS.
- K. Venkateswarlu, K. Suneel, B. Das, K. N. Reddy and T. S. Reddy, Synth. Commun., 2009, 39, 215–219 CrossRef CAS.
- K. Kumaran, M. K. S. Raja and K. R. Jaisankar, Int. J. PharmTech Res., 2012, 4, 169–175 CAS.
- N. Lebouvier, F. Pagniez, M. Duflos, P. Le Pape, Y. M. Na, G. Le Baut and M. Le Borgne, Bioorg. Med. Chem. Lett., 2007, 17, 3686–3689 CrossRef CAS PubMed.
- R. M. La Hoz and P. G. Pappas, Drugs, 2013, 73, 495–504 CrossRef CAS PubMed.
- Y. Liao, M. Chen, T. Hartmann, R. Y. Yang and W. Q. Liao, Chin. Med. J., 2013, 126, 361–368 Search PubMed.
- H.-R. Tsou, N. Mamuya, B. D. Johnson, M. F. Reich, B. C. Gruber, F. Ye, R. Nilakantan, R. Shen, C. Discafani, R. DeBlanc, R. Davis, F. E. Koehn, L. M. Greenberger, Y.-F. Wang and A. Wissner, J. Med. Chem., 2001, 44, 2719–2734 CrossRef CAS PubMed.
- G. Acquaah-Harrison, S. Zhou, J. V. Hines and S. C. Bergmeier, J. Comb. Chem., 2010, 12, 491–496 CrossRef CAS PubMed.
- B. Cash, C. Fischer, Y. Garcia, J. Jung, J. Katz, J. Kim, A. Rivkin, A. Schell, T. Siu, D. Witter and H. Zhou, Azaindoles as janus kinase inhibitors, WO2011137022 A1, 2011, 3 November 2011 Search PubMed.
- CLSI, Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard M100-S22, National Committee for Clinical Laboratory Standards, Fort Wayne, Ind, USA, 22nd edn, 2012 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra are provided. See DOI: 10.1039/c3ob41798k |
| This journal is © The Royal Society of Chemistry 2014 |