FeCl3-catalyzed synthesis of functionally diverse dibenzo[b,f]oxepines and benzo[b]oxepines via alkyne–aldehyde metathesis†
Krishnendu
Bera
,
Swapnadeep
Jalal
,
Soumen
Sarkar
and
Umasish
Jana
*
Department of Chemistry, Jadavpur University, Kolkata-700 032, West Bengal, India. E-mail: jumasish2004@yahoo.co.in
First published on 28th October 2013
Abstract
An efficient synthesis of dibenzo[b,f]oxepines and benzo[b]oxepines via FeCl3-catalyzed alkyne–aldehyde metathesis reaction is described. Structurally diverse dibenzo[b,f]oxepines and benzo[b]oxepines have been achieved in good yields with high regio- and chemoselectivity under mild conditions. Notably, among the various catalysts such as Fe(III), Au(III), In(III), Zn(II), Ag(I) and triflic acid, the alkyne–aldehyde metathesis reaction of 2-(2′-phenylethynyl-phenyloxy)-benzaldehyde is only catalyzed by environmentally friendly and sustainable iron(III) chloride.
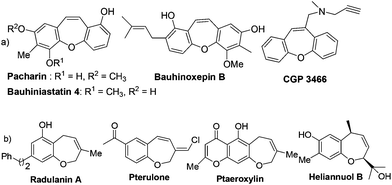
Seven-membered oxygen heterocycles are ubiquitous in natural products and show a wide spectrum of biological activity.1 In particular, the tricyclic dibenzo[b,f]oxepine unit represents an important structural motif found in many natural products and biologically relevant compounds (Fig. 1).2 The dibenzo[b,f]oxepine motif containing natural products, such as pacharin and bauhiniastatin 4 has the ability to inhibit cancer cell growth.3 Moreover, the compound containing dibenzo[b,f]oxepines such as bauhinoxepin B has also shown anti-inflammatory activities.4 Furthermore, CGP 3466 was identified as a highly potent substance preventing neuronal cell death in different in vitro and in vivo systems which also contain dibenzo[b,f]oxepines.5 In addition, these compounds are very important in medicinal chemistry due their potential pharmaceutical properties such as antipsychotic,6a–c antidepressant,6d antihypertensive,6e and anti-inflammatory.6f
On the other hand, the benzo[b]oxepine scaffold is also present in many natural products,7a–f biologically active substances7g and natural herbicides (Fig. 1).7h The biological activity exhibited by dibenzo[b,f]oxepines and benzo[b]oxepines and their derivatives makes them very attractive targets for synthetic chemists. Accordingly, the development of efficient methodologies for the construction of dibenzo[b,f]oxepines and benzo[b]oxepines motifs remains a very important challenge in organic synthesis.
However, only a few methods have been developed for the synthesis of dibenzo[b,f]oxepines.8–11 Among them, the two most common approaches for the synthesis of dibenzo[b,f]oxepines are: (a) intramolecular C–O ether bond formation of 2-styrylphenols5a,8a,b and (b) cyclodehydration or intramolecular Friedel–Crafts alkylation reactions of intermediate with preformed biaryl ether intermediates.6c,6f,8a,8c–e
The Wagner–Meerwein rearrangement of xanthenes or Mn(III) based oxidative radical rearrangement has also been developed.9 Very recently, the synthesis of dibenzo[b,f]oxepines using sequential intermolecular Heck reaction, ring closing metathesis, palladium-catalyzed etherification reactions, and a cascade formation of dibenzo[b,f]oxepines have also been reported.10 Similarly, much attention has been paid to the synthesis of functionalized benzo[b]oxepines. Among the various strategies, Rh-catalyzed olefin hydroacylations,11a Os-catalyzed hydroxylation of aromatic alkynols,11b Pd-catalyzed [5 + 2] annulation of 2-acylmethoxyarylboronic acids with allenoates or alkynes,11c Pd-catalyzed tandem alkylation/alkenylation reactions,11d and Au-catalyzed cyclizations11e,f are very important.
However, these protocols are subjected to some limitations, such as non-availability of starting materials, lack of generality, harsh reaction conditions, low chemical yields, and the use of expensive and toxic reagents. Thus, the development of a new and general strategy which enables the efficient synthesis of diversely substituted dibenzo[b,f]oxepines and benzo[b]oxepines from easily available substrates under mild conditions would be an important challenge in organic synthesis.
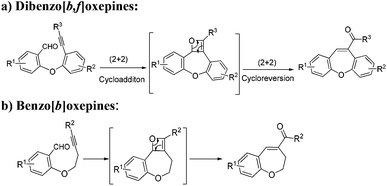
Recently, the intramolecular alkyne–carbonyl metathesis reaction has received much attention, since valuable functionalized hetero- and carbocycles can be readily formed under mild conditions.12 Moreover, such reactions are highly efficient and atom-economical by nature, unfortunately, and have been less explored. This reaction normally proceeds through a [2 + 2] cycloaddition and cycloreversion processes by the activation of the carbonyl group by formation of σ-complex or activation of alkyne by formation of π-complex or activation of both simultaneously depending on the catalyst. Generally, this reaction is initiated either by Brønsted acids or Lewis acids such as TfOH, HBF4, BF3·OEt2, In(OTf)3, AgSbF6, AuCl3, and a combination of AuCl3/AgSbF6 acting as a catalyst for this process. Notably, this reaction has been mostly employed for the synthesis of five- or six- membered heterocycles or carbocycles. However, synthesis of seven-membered heterocycle using this reaction has not been reported yet.
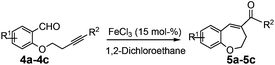
During our ongoing interest in the area of development of iron(III)-catalyzed new reactions, very recently, our group has employed intramolecular alkyne–carbonyl metathesis strategy in developing an alternative process for the efficient construction of carbo- and heterocycle catalysed by iron(III) chloride. We have observed that in contrast to other catalysts, iron(III) chloride works under mild conditions and more efficiently and without any additives.12a,c Furthermore, iron(III) chloride is inexpensive and environmentally friendly so it is highly desirable in organic synthesis. Keeping in mind these facts, we envisioned that the alkyne–carbonyl metathesis strategy could also be applied to the synthesis of functionalised seven-membered oxygen heterocycles such as dibenzo[b,f]oxepines and benzo[b]oxepines from easily available starting materials (Scheme 1).
Herein, we report an efficient and general synthetic route to the easy access to the library of functionalized dibenzo[b,f]oxepines and benzo[b]oxepines derivatives. To the best of our knowledge this is the first report of the construction of seven-membered oxygen heterocycles employing alkyne–carbonyl metathesis.
Results and discussion
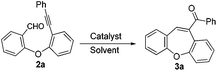
The required substrates for the synthesis of dibenzo[b,f]oxepines 3a–3j can easily and efficiently be prepared by a two-step transformation in good yield. This involves an aromatic nucleophilic substitution of o-fluorobenzaldehyde derivatives with a suitably substituted ortho-halo phenol derivatives followed by palladium catalyzed Sonogashira coupling with substituted phenyl acetylene derivatives (Scheme 2). After having a series of 2-(2′-phenylethynyl-phenyloxy)-benzaldehydes, we began our investigation using 2a to optimize the reaction condition under various catalytic conditions. The results are shown in Table 1.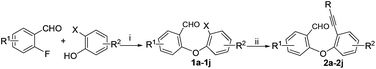 | ||
| Scheme 2 Substrates preparation for the dibenzo[b,f]oxepines synthesis. Reaction conditions: (i) K2CO3, DMF, 100 °C; (ii) Pd(PPh3)4, CuI, Et3N, reflux. | ||
| Entry | Catalyst (mol%) | Temperature | Yield (%) |
|---|---|---|---|
| a Conditions: substrate 2a (0.5 mmol) and 1,2-dichloroethane (3 mL), 12 h. | |||
| 1 | FeCl3 (5) | r.t. | n.r. |
| 2 | FeCl3 (5) | 60 °C | n.r. |
| 3 | FeCl3 (5) | Reflux | 35 |
| 4 | FeCl3 (10) | Reflux | 56 |
| 5 | FeCl3 (15) | Reflux | 77 |
| 6 | FeBr3 (15) | Reflux | n.r. |
| 7 | NaAuCl4 (15) | Reflux | n.r. |
| 8 | InCl3 (15) | Reflux | n.r. |
| 9 | AgOTf (15) | Reflux | n.r. |
| 10 | AgOTf (30) | Reflux | n.r. |
| 11 | TfOH (15) | Reflux | n.r. |
As part of our interest in the area of iron-catalyzed reactions, we first examined the alkyne–carbonyl metathesis reaction of 2a in the presence of environmentally friendly and sustainable FeCl3. It was observed that the reaction did not initiate at room temperature or even heating at 60 °C in the presence of FeCl3 (5 mol%) in 1,2-dichloroethane. However, to our delight the reaction initiated when the reaction mixture was heated to reflux for 12 h, the desired dibenzo[b,f]oxepines 3a was isolated 35% (Table 1, entry 3). The yield of the product was increased to 56% on increasing the amount of catalyst to 10 mol%. Gratifyingly, the desired dibenzo[b,f]oxepine 3a was obtained cleanly in 77% yield in the presence of 15 mol% of anhydrous FeCl3 (Table 1, entry 5). Further increasing the amount of catalyst to 20 mol% did not improve the yields. However, FeBr3 did not initiate this reaction under similar reaction conditions. We were then interested in checking other commonly used catalysts for alkyne–carbonyl metathesis such as Au(III), In(III), Ag(I) and triflic acid. Similarly, ZnI2 is also known as an efficient Lewis acid and has been used for various organic transformations.13 However, it was also ineffective for the present transformation.
To our surprise none of the catalysts worked for this transformation and the starting material remained intact even after prolonged heating. Although the reason is not very clear, however, moderately strong Lewis acid is probably very important for this transformation. Moreover, few solvents such as MeCN, MeNO2, THF and toluene were also studied to improve the yield, unfortunately no product was obtained. These results proved that 15 mol% anhydrous FeCl3 in the presence of 1,2-dichloroethane exhibited higher catalytic activity for this particular transformation.
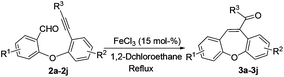
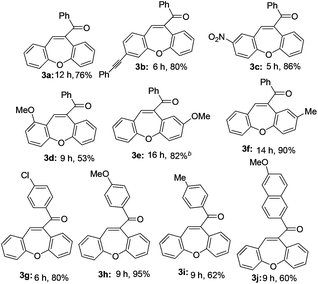
The optimized reaction conditions were then applied to the construction of wide varieties of functionalized dibenzo[b,f]oxepines derivatives (Table 2). This transformation was found to be very general, and a series of substituted dibenzo[b,f]oxepines (3a–3j) were synthesized in good to excellent yields. The reaction was not significantly affected by the variation of substituents on either of the aromatic rings. Both electron-withdrawing (3c) and electron-donating (3d–3f and 3h–3i) groups were well-tolerated on both of the aromatic rings and provided good yield of the desired product. The reaction was highly regioselective with respect to alkynes because the alkyne unit which was suitably placed for cycloaddition with aldehyde only reacted regioselectively in high yield, and the other alkyne unit remained intact under the reaction conditions (3b). Both electron-donating and electron-withdrawing substituents on the aryl group at the alkyne terminus were also reacted with almost equal efficiency (3e–3g). The product 3e was formed very slowly in the presence of only FeCl3 (15 mol%), but the reaction was accelerated in combination with AgOTf (30 mol%) and gave the desired product in 82% yield. Interestingly, AgOTf alone did not work, probably FeCl3 is being activated by the complexation of silver salt through the coordination of chloride. It is noteworthy that chlorine-substituted dibenzo[b,f]oxepine 3g can be further employed in various cross-coupling reactions that could be useful for their potential application in medicinal chemistry.
Thus, this reaction can be used to synthesize functionalized dibenzo[b,f]oxepine 3a–3j derivatives with substituents at various positions with excellent functional-group compatibility. Unfortunately, less reactive substrates i.e. alkyl substituted alkyne did not work in contrast to our previous report.12a
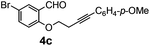
In order to extend the scope of our present method, we also applied this methodology to the synthesis of functionalized benzo[b]oxepine 5a–5c. The required starting materials 4a–4c could easily be obtained from derivatives of salicylaldehyde by simple alkylation with tosyl derivative of aryl substituted homopropargyl alcohol in the presence of K2CO3 and acetonitrile under reflux (Scheme 3).
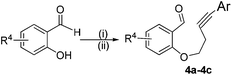 | ||
| Scheme 3 Substrates preparation for the synthesis of benzo[b]oxepines. Reaction conditions: (i) homopropargyl bromide; K2CO3; CH3CN; reflux. (ii) Ar–I, Pd(PPh3)4, Et3N, RT. | ||
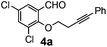
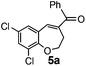
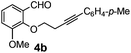
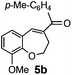
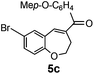
After having the required substrates 4a–4c, a few functionalized benzo[b]oxepines 5a–5c have been synthesized in moderate to good yields using this transformation under the same optimized reaction conditions. The results are presented in Table 3. The electronic effect of substituents on the benzene ring of 4a–4c has little influence in this process. Both electron-donating (Table 3, entry 2) and electron-withdrawing (Table 3, entries 1 and 3) groups containing compounds were tested, and they were smoothly converted to the desired benzo[b]oxepine derivatives in good yield. It is noteworthy that halide substituted benzo[b]oxepines 5a and 5c would be very useful for further synthetic transformation through the cross-coupling reaction to obtain a library of structurally diverse substrates for their potential application in medicinal chemistry. However, alkyl substituted alkyne did not work.
| Entry | Substrates | Products | Time (h) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: substrate (0.5 mmol), FeCl3 (0.075 mmol), and 1,2-dichloroethane (3 mL). | ||||
| 1 |
|
|
10 | 70 |
| 2 |
|
|
20 | 58 |
| 3 |
|
|
12 | 67 |
Conclusions
In conclusion, we have demonstrated a new and general synthetic strategy for the construction of functionalized seven-membered oxygen heterocycles, dibenzo[b,f]oxepines and benzo[b]oxepines in good yields involving iron(III)-catalyzed intramolecular alkyne–aldehyde metathesis reaction. The strategy is very simple and atom-economical and exhibits a broad substrates scope. In addition, the easy availability of the precursors, the high efficiency and the use of inexpensive and environmentally friendly catalyst make this methodology very attractive. Considering these, the present device of seven-membered oxygen heterocycles may find use in the synthesis of many biologically useful molecules.Acknowledgements
K. B., S. J. and S. S. are thankful to the CSIR, New Delhi, India, for their fellowships.Notes and references
- N. L. Snyder, H. M. Haines and M. W. Peczuh, Tetrahedron, 2006, 62, 9301–9320 CrossRef CAS PubMed.
- (a) A. S. R. Anjaneyulu, A. V. R. Reddy, D. S. K. Reddy, R. S. Ward, D. Adhikesavalu and T. S. Cameron, Tetrahedron, 1984, 40, 4245 CrossRef CAS; (b) M. F. Comber and M. V. Sargent, J. Chem. Soc., Perkin Trans. 1, 1990, 1371 RSC; (c) T.-X. Qian and L.-N. Li, Phytochemistry, 1992, 31, 1068 CAS; (d) M.-I. Chung, H.-H. Ko, M.-H. Yen, C.-N. Lin, S.-Z. Yang, L.-T. Tsao and J.-P. Wang, Helv. Chim. Acta, 2000, 83, 1200 CrossRef CAS; (e) H.-H. Ko and C.-N. Lin, Helv. Chim. Acta, 2000, 83, 3000 CrossRef CAS; (f) G. R. Pettit, A. Numata, C. Iwamoto, Y. Usami, T. Yamada, H. Ohishi and G. M. Cragg, J. Nat. Prod., 2006, 69, 323 CrossRef CAS PubMed; (g) L.-H. Mu, J.-B. Li, J.-Z. Yang and D.-M. Zhang, J. Asian Nat. Prod. Res., 2007, 9, 649 CrossRef CAS PubMed.
- (a) G. R. Pettit, A. Numata, C. Iwamoto, Y. Usami, T. Yamada, H. Ohishi and G. M. Cragg, J. Nat. Prod., 2006, 69, 323–327 CrossRef CAS PubMed; (b) A. S. R. Anjaneyula, A. V. R. Reddy, D. S. K. Reddy, T. S. Cameron and S. P. Roe, Tetrahedron, 1986, 42, 2417–2420 CrossRef.
- (a) P. Kittakoop, S. Nopichai, N. Thongon, P. Charoenchai and Y. Thebtaranonth, Helv. Chim. Acta, 2004, 87, 175–179 CrossRef CAS; (b) M.-I. Chung, H.-H. Ko, M.-H. Yen, C.-N. Lin, S.-Z. Yang, L.-T. Tsao and J.-P. Wang, Helv. Chim. Acta, 2000, 83, 1200–1204 CrossRef CAS; (c) H.-H. Ko, C.-N. Lin and S.-Z. Yang, Helv. Chim. Acta, 2000, 83, 3000–3005 CrossRef CAS; (d) H.-H. Ko, S.-Z. Yang and C.-N. Lin, Tetrahedron Lett., 2001, 42, 5269–5270 CrossRef CAS.
- (a) K. Zimmermann, P. C. Waldmeier and W. G. Tatton, Pure Appl. Chem., 1999, 71, 2039–2046 CrossRef CAS; (b) K. Zimmermann, S. Roggo, E. Kragten, P. Fürst and P. Waldmeier, Bioorg. Med. Chem. Lett., 1998, 8, 1195–1200 CrossRef CAS; (c) Y. Sagot, N. Toni, D. Perrelet, S. Lurot, B. King, H. Rixner, L. Mattenberger, P. C. Waldmeier and A. C. Kato, Br. J. Pharmacol., 2000, 131, 721–728 CrossRef CAS PubMed.
- (a) J. Fernández, J. M. Alonso, J. I. Andrés, J. M. Cid, A. Díaz, L. Iturrino, P. Gil, A. Megens, V. K. Sipido and A. A. Trabanco, J. Med. Chem., 2005, 48, 1709–1712 CrossRef PubMed; (b) A. A. Trabanco, J. M. Alonso, J. I. Andrés, J. M. Cid, J. Fernández, L. Iturrino and A. Megens, Chem. Pharm. Bull., 2004, 52, 262–265 CrossRef CAS; (c) T. W. Harris, H. E. Smith, P. L. Mobley, D. H. Manier and F. Sulser, J. Med. Chem., 1982, 25, 855–858 CrossRef CAS; (d) H. H. Ong, J. A. Profitt, V. B. Anderson, T. C. Spaulding, J. C. Wilker, H. M. Geyer III and H. Kruse, J. Med. Chem., 1980, 23, 494–501 CrossRef CAS; (e) R. Kiyama, T. Honma, K. Hayashi, M. Ogawa, M. Hara, M. Fujimoto and T. Fujishita, J. Med. Chem., 1995, 38, 2728–2741 CrossRef CAS; (f) Y. Nagai, A. Irie, H. Nakamura, K. Hino, H. Uno and H. Nishimura, J. Med. Chem., 1982, 25, 1065–1070 CrossRef CAS.
- For examples, see: (a) M. Bruder, P. L. Haseler, M. Muscarella, W. Lewis and C. J. Moody, J. Org. Chem., 2010, 75, 353 CrossRef CAS PubMed; (b) F. A. Macias, J. M. G. Molinillo, R. M. Varela, A. Torres and F. R. Fronczek, J. Org. Chem., 1994, 59, 8261 CrossRef CAS; (c) M. Engler, T. Anke and O. J. Sterner, J. Antibiot., 1997, 50, 330 CrossRef CAS; (d) M. Engler, T. Anke, O. J. Sterner and U. J. Brandt, J. Antibiot., 1997, 50, 325 CrossRef CAS; (e) S. Kim, B.-N. Su, S. Riswan, L. B. S. Kardono, J. J. Afriastini, J. C. Gallucci, H. Chai, N. R. Farnsworth, G. A. Cordell, S. M. Swanson and A. D. Kinghorn, Tetrahedron Lett., 2005, 46, 9021 CrossRef CAS PubMed; (f) K. Kashima, K. Sano, Y. S. Yun, H. Ina, A. Kunugi and H. Inoue, Chem. Pharm. Bull., 2010, 58, 191 CrossRef CAS; (g) J. B. P. A. Wijnberg, A. vanVeldhuizen, H. J. Swarts, J. C. Frankland and J. A. Field, Tetrahedron Lett., 1999, 40, 5767 CrossRef CAS; (h) J. R. Vyvyan and R. E. Looper, Tetrahedron Lett., 2000, 41, 1151 CrossRef CAS.
- (a) For review, see: R. Olivera, R. SanMartin, F. Churruca and E. Domínguez, Org. Prep. Proced. Int., 2004, 36, 297–330 CrossRef CAS , and references cited therein; ; (b) H. Hoyer and M. Vogel, Monatsh. Chem., 1962, 93, 766–774 CrossRef CAS; (c) H. H. Ong, J. A. Profitt, V. B. Anderson, T. C. Spaulding, J. C. Wilker, H. M. Geyer III and H. Kruse, J. Med. Chem., 1980, 23, 2728–2741 Search PubMed; (d) R. H. F. Manske and A. E. Ledingham, J. Am. Chem. Soc., 1950, 72, 4797–4799 CrossRef CAS; (e) R. Olivera, R. SanMartin, F. Churruca and E. Domínguez, J. Org. Chem., 2002, 67, 7215–7225 CrossRef CAS PubMed.
- (a) T. Storz, E. Vangrevelinghe and P. Dittmar, Synthesis, 2005, 2562–2570 CrossRef CAS PubMed; (b) B. A. Hess, A. S. Bailey and V. Boekelheide, J. Am. Chem. Soc., 1967, 89, 2746–2747 CrossRef CAS; (c) Z. Cong, T. Miki, O. Urakawa and H. Nishino, J. Org. Chem., 2009, 74, 494–501 CrossRef PubMed.
- (a) L. A. Arnold, W. Luo and R. K. Guy, Org. Lett., 2004, 6, 3005–3007 CrossRef CAS PubMed; (b) F. Royer, C. Vilain, L. T. Elkailm and L. Grimaud, Org. Lett., 2003, 5, 2007–2009 CrossRef CAS PubMed; (c) Y. L. Choi, H. S. Lim, H. J. Lim and J.-N. Heo, Org. Lett., 2012, 14, 5102–5105 CrossRef CAS PubMed.
- (a) M. M. Coulter, P. K. Dornan and V. M. Dong, J. Am. Chem. Soc., 2009, 131, 6932 CrossRef CAS PubMed; (b) A. Varela-Fernández, C. García-Yebra, J. A. Varela, M. A. Esteruelas and C. Saá, Angew. Chem., Int. Ed., 2010, 49, 4278 CrossRef PubMed; (c) G. Liu and X. Lu, Adv. Synth. Catal., 2007, 349, 2247 CrossRef CAS; (d) M. Lautens, J.-F. Paquin and S. Piguel, J. Org. Chem., 2002, 67, 3972 CrossRef CAS PubMed; (e) E. M. L. Sze, W. Rao, M. J. Koh and P. W. H. Chan, Chem.–Eur. J., 2011, 17, 1437–1441 CrossRef CAS PubMed; (f) J. Liu and Y. Liu, Org. Lett., 2012, 14, 4742–4745 CrossRef CAS PubMed.
- A few selected recent literature reports for intramolecular alkyne–carbonyl metathesis: (a) FeCl3-catalyzed reaction, see: K. Bera, S. Sarkar, S. Jalal and U. Jana, J. Org. Chem., 2012, 77, 8780–8786 CrossRef CAS PubMed; (b) HBF4-catalyzed reaction, see: L. Escalante, C. González-Rodíguez, J. A. Varela and C. Saa, Angew. Chem., Int. Ed., 2012, 51, 12316–12320 CrossRef CAS PubMed; (c) Iron-catalyzed reaction, see: K. Bera, S. Sarkar, S. Biswas, S. Maiti and U. Jana, J. Org. Chem., 2011, 76, 3539–3544 CrossRef CAS PubMed; (d) Review on Au-catalysis, see: L. Liu, B. Xu and G. B. Hammond, Beilstein J. Org. Chem., 2011, 7, 606–614 CrossRef CAS PubMed and references cited therein; ; (e) SbF5–MeOH catalyzed reaction, See: A. Saito, J. Kasai, Y. Odaira, H. Fukaya and Y. Hanzawa, J. Org. Chem., 2009, 74, 5644–5647 CrossRef CAS PubMed; (f) TfOH-catalyzed reaction, see: T. Jin, F. Yang, C. Liu and Y. Yamamoto, Chem. Commun., 2009, 3533–3535 RSC; (g) TFA-catalyzed reaction, see: C. González-Rodríguez, L. Escalante, J. A. Varela, L. Castedo and C. Saá, Org. Lett., 2009, 11, 1531 CrossRef PubMed; (h) T. Jin and Y. Yamamoto, Org. Lett., 2008, 10, 3137–3139 CrossRef CAS PubMed.
- Few selected literature for ZnI2 catalyzed reaction: (a) X.-F. Wu and H. Neumann, Adv. Synth. Catal., 2012, 354, 3141–3160 CrossRef CAS; (b) Z. Li, G. Deng and Y.-C. Li, Synlett, 2008, 3053–3057 CrossRef CAS PubMed; (c) G. Deng, Z. Li, S.-Y. Peng, L. Fang and Y.-C. Li, Tetrahedron, 2007, 63, 4630–4635 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ob41624k |
| This journal is © The Royal Society of Chemistry 2014 |