Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of a superatom monolayer using gas-phase-synthesized Ta@Si16 nanocluster ions†
Masato
Nakaya
ab,
Takeshi
Iwasa
ab,
Hironori
Tsunoyama
ab,
Toyoaki
Eguchi
ab and
Atsushi
Nakajima
*abc
aNakajima Designer Nanocluster Assembly Project, ERATO, JST, KSP, 3-2-1 Sakado, Takatsu-ku, Kawasaki 213-0012, Japan. E-mail: nakajima@chem.keio.ac.jp
bDepartment of Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan
cKeio Institute of Pure and Applied Sciences (KiPAS), Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan
First published on 7th October 2014
Abstract
The controlled assembly of superatomic nanocluster ions synthesized in the gas phase is a key technology for constructing a novel series of functional nanomaterials. However, it is generally difficult to immobilize them onto a conductive surface while maintaining their original properties owing to undesirable modifications of their geometry and charge state. In this study, it has been shown that this difficulty can be overcome by controlling the donor–acceptor interaction between nanoclusters and surfaces. Cations of Ta-atom-encapsulated Si16 cage nanoclusters (Ta@Si16) behaving as rare-gas-like superatoms are synthesized in the gas phase and deposited on conductive surfaces terminated with acceptor-like C60 and donor-like α-sexithiophene (6T) molecules. Scanning tunneling microscopy and spectroscopy have demonstrated that Ta@Si16 cations can be densely immobilized onto C60-terminated surfaces while retaining their cage shape and positive charge, which is realized by creating binary charge transfer complexes (Ta@Si16+–C60−) on the surfaces. The Ta@Si16 nanoclusters exhibit excellent thermal stability on C60-teminated surfaces similar to those in the gas phase, whereas the nanoclusters destabilize at room temperature on 6T-terminated surfaces owing to the loss of electronic closure via a change in the charge state.
1 Introduction
The ability to create solid-state materials from atomic elements using a bottom-up method such as epitaxial growth, alloying, or chemical synthesis plays a crucial role in today's science and engineering. The controlled assembly of nanoclusters1–9 is expected to be a novel material-processing methodology providing hierarchical nanostructures with tailored dimensionality and functionalities such as ultrathin heterojunctions exhibiting electrical rectification, photoelectric conversion, ferroelectricity, and high catalytic reactivity. Gas-phase-synthesized Si16 cages encapsulating a single metal atom (M@Si16) are potential building blocks for such nanocluster assemblies, because their physicochemical properties are tunable while retaining the symmetrical cage shape by changing the type of metal atom and the charge state.10–16 For instance, experimental and theoretical studies on M@Si16 nanoclusters in the gas phase have revealed that neutral Si16 cages including group-4 metals, such as Ti@Si16 and Zr@Si16, behave as rare-gas-like superatoms because of their geometric and electronic shell closure,10–15,17,18 whereas halogen- and alkali-like superatom characteristics emerge upon encapsulating metal atoms from group 3 (e.g. Sc@Si16 and Lu@Si16) and group 5 (e.g. V@Si16 and Ta@Si16), respectively.13,14,18 Since their halogen- and alkali-like nature originates from the electronic open shell owing to the deficit and surplus of a single valence electron, they complete electron shells by adding and removing one electron, respectively.13–18 These rare-gas-like M@Si16 anions and cations are selectively synthesized in the gas phase while exhibiting chemical and thermal stability.13,14 Although this feature is advantageous for hierarchical nanostructuring on solid surfaces without losing the identity of each nanocluster building block, there is no knowledge about the properties, stability, and geometry of M@Si16 ions on solid surfaces.The surface immobilization of M@Si16 ions without changing their original geometry and charge state is the key to M@Si16-based nanostructuring. So far, the guiding principle for nondestructive nanocluster immobilization is a soft landing,19–24 namely the deposition of cluster ions with sufficiently lower kinetic energy (Ek) than their interatomic binding energy. However, most nanocluster ions are neutralized on conductive substrates in the conventional soft landing, which undesirably modifies their original properties. Our strategy toward overcoming this issue is to immobilize rare-gas-like M@Si16 cations and anions on conductive surfaces functionalized with acceptor- and donor-like species, by which the positive and negative charges in the nanoclusters are expected to be retained by a donor–acceptor interaction,25,26 respectively. In this study, we demonstrate by scanning tunneling microscopy and spectroscopy (STM/STS) that monolayers of Ta@Si16 cations are formed by densely immobilizing Ta@Si16 cations on conductive surfaces terminated with acceptor-like C60 molecules, in which each Ta@Si16 nanocluster covalently connects to a single C60 molecule while maintaining the cage shape and positive charge by forming binary charge transfer complexes (Ta@Si16+–C60−). In contrast, Ta@Si16 cations destabilize on metallic surfaces terminated with donor-like α-sexithiophene (referred to as 6T) molecules owing to the loss of electronic closure via a change in the charge state.
2 Materials and methods
All experiments were carried out under vacuum conditions. Highly oriented pyrolytic graphite (HOPG), Si(111)7 × 7, and Si(111)√3 × √3R30°-Ag [referred to as Si(111)√3-Ag] were prepared as substrates. These substrates were functionalized with monolayered films of C60 and 6T molecules by respectively depositing these molecules at room temperature (RT) under ultrahigh vacuum (UHV). HOPG surfaces were cleaned by thermal annealing at 770 K in UHV prior to the deposition of molecules. Si(111)√3-Ag surfaces were prepared by depositing 1 ML (7.83 × 106 atoms μm−2) of Ag atoms on a Si(111)7 × 7 surface at 873 K. C60 molecules were deposited at RT by the thermal evaporation of C60 powder (purity: 99.95%) from a Ta crucible while maintaining a deposition rate of 0.03 ML min−1, where 1 ML of C60 corresponds to 1.15 × 106 molecules μm−2. 6T molecules were also deposited at RT by the thermal evaporation of 6T powder from a Ta crucible while maintaining a deposition rate of 0.015 ML min−1, where 1 ML of 6T molecules corresponds to 0.62 × 106 molecules μm−2.TanSim nanoclusters with various charge states were produced in gas aggregation apparatus with a direct-current magnetron sputtering source27,28 from a Ta–Si mixed target. Ta@Si16 cations were selectively created by the fine-tuning of synthesis conditions, similar to previous studies using the laser vaporization method.13,14 The Ta@Si16 cations were mass-selected from the cationic TanSim species using a quadrupole mass spectrometer and were deposited onto substrates at ∼90 K with a typical deposition rate of ∼2.6 × 103 ions μm−2 min−1. Ek for the Ta@Si16 cations was controlled to as low as possible by applying an appropriate positive voltage to the substrate during the deposition. The samples were transferred into an analysis chamber while maintaining the vacuum condition and were evaluated by STM/STS at RT under UHV.
3 Results and discussion
Fig. 1a and b respectively show wide- and molecular-scale STM images of a C60/HOPG surface obtained after depositing a small amount of Ta@Si16 cations with an Ek of ∼0.01 eV per atom. Dot-shaped structures were uniformly created on the surface. The surface was densely covered with dots by continuously depositing cations for a long period (Fig. 1c). Our results indicate that each dot corresponds to an individual Ta@Si16 nanocluster, as discussed below. In the histogram of dot heights (hd) measured for the high-density dots (Fig. 1f), a peak appears at a hd of ∼0.8 nm. This value is close to the theoretical size of isolated Ta@Si16 cations, which lies in the range of 0.89–0.95 nm for the isomers with C3v and D4d symmetry (Fig. 1e). Although the values of hd shown in Fig. 1 were measured from STM height profiles obtained at a tip bias voltage (Vtip) of −2.3 V, the typical hd of ∼0.8 nm does not change with the value of the negative Vtip (see section 1 in the ESI†). The dots larger than the theoretical cluster size are considered to originate from the direct adsorption of Ta@Si16 cations onto preexisting nanoclusters. On the other hand, the formation of smaller dots with hd < 0.8 nm is attributed to the diversity in the adsorption sites of Ta@Si16 nanoclusters. In a simple model using hard spheres with diameters of 1 nm for C60 molecules and 0.95 nm for Ta@Si16 nanoclusters, the apparent heights of Ta@Si16 nanoclusters adsorbed on the hollow and bridge sites in the C60 film are ∼0.19 and ∼0.14 nm lower than those on the atop sites, respectively. In fact, there is a small peak at hd ∼ 0.6 nm in the height histogram (Fig. 1f). Note that even smaller dots with hd < 0.45 nm were dominantly created by depositing Ta@Si16 cations with an intentionally larger Ek of ∼1.25 eV per atom (Fig. S3a and S3b†), which is considered to come from the deformation and/or fragmentation of Ta@Si16 nanoclusters on the surface, because such small values of hd cannot be explained by the adsorption of Ta@Si16 nanoclusters. These small dots are minor products in the current deposition, as shown in Fig. 1f. These results suggest that the Ta@Si16 cations are immobilized without marked disintegration, which is further supported by evaluating the thermal stability and electronic structure of the dots.Fig. 1d shows an STM image obtained after annealing the surface shown in Fig. 1c at 493 K. No marked changes in the density and spatial distribution of the dots were induced by annealing. In addition, there was no obvious change in the dot height distribution after the annealing, as confirmed from the height histograms measured before and after the annealing (Fig. 1f and 1g, respectively). To examine the thermal stability at higher temperatures, Ta@Si16 cations were deposited onto a C60-terminated Si(111)7 × 7 surface. This surface has high thermal durability owing to the covalent bonding between C60 molecules and the Si(111)7 × 7 surface,29 while C60 molecules start to desorb from HOPG surfaces at ∼510 K. Surprisingly, the dots remained after high-temperature annealing at 773 K (Fig. 1h and i).
The high thermal stability of the adsorbates on C60 films is consistent with the robust cage structure of Ta@Si16 cations with an interatomic binding energy of ∼4.45 eV.16 In addition, the present results strongly indicate that the thermally activated diffusion and desorption of the adsorbate are inhibited by the strong adsorbate-C60 interaction compared with pure van der Waals forces. This consideration provides us with a reasonable explanation for the reduced typical dot height (∼0.8 nm) compared with the theoretical size of isolated Ta@Si16 cations (0.89–0.95 nm). The reduction of ∼0.1 nm is similar to the difference between the van der Waals radius of Si atoms (0.211 nm) and the covalent radius in the Si–C bond (e.g. 0.094 nm in SiC crystals). In other words, a binary complex is locally created by the covalent bonding between the Ta@Si16 nanocluster and the C60 molecule. Fig. 2 shows three theoretical motifs of neutral Ta@Si–C60 complexes (see section 3 in the ESI†). Ta@Si16 nanoclusters are interconnected with each C60 molecule via one or two covalent bonds without impairing the cage shape. The theoretical height differences between Ta@Si16 nanoclusters and C60 molecules are within the range of 0.76–0.85 nm, in good agreement with the typical dot height. Further evidence for such one-to-one covalent connection between Ta@Si16 nanoclusters and C60 molecules is given as follows. It has been found that Ta@Si16 nanoclusters change their adsorption position from the bridge or hollow sites to atop sites when C60 films sparsely covered with Ta@Si16 nanoclusters (e.g., Fig. 1a) are thermally annealed. Although the one-to-one covalent connection allows the Ta@Si16 nanocluster to locally change their position among the neighboring adsorption sites via thermally activated precessional motion, it is considered to hardly occur for Ta@Si16 nanoclusters covalently connected to multiple C60 molecules at bridge and atop sites; in other words, our result suggests that Ta@Si16–(C60)2 and Ta@Si16–(C60)3 are minor products.
The stability of the deposited Ta@Si16 cations strongly depends on the surface. Fig. 3a shows an STM image of a 6T-terminated Si(111)√3-Ag surface obtained after the initial deposition of Ta@Si16 cations. Small dots with a hd of ∼0.3 nm are created, as shown in the STM image and the dot-height histogram (Fig. 3c). This value does not sensitively fluctuate with the value of Vtip (see section 1 in the ESI†) and is close to the size of a single Si atom covalently bound with the surface, suggesting that the deposited Ta@Si16 cations disintegrate into atoms and react with the 6T-teminated surface. Similar disintegration has been reported for Ag309 nanoclusters immobilized onto C60 monolayers formed on Au(111) surfaces, which is induced by the attractive force acting between the Ag309 nanoclusters and the substrate.23 On the other hand, islands of dots with a disordered arrangement are formed by depositing Ta@Si16 cations on HOPG surfaces (Fig. 3b). The height histogram of the dots/HOPG has the main peak at a hd of 1.0–1.2 nm (Fig. 3d), suggesting that the marked disintegration of Ta@Si16 nanoclusters, as observed on 6T/Si(111)√3-Ag, does not occur. However, the disordered arrangement of dots/HOPG is not improved by annealing, at least up to 493 K, also suggesting that the Ta@Si16 nanoclusters aggregate via a strong interaction such as covalent Si–Si bonding. These results strongly indicate that the chemical reactivity of Ta@Si16 cations is much greater on the 6T/Si(111)√3-Ag and HOPG surfaces than in the gas phase13,14 and on C60-terminated surfaces. The thermal and chemical stability of rare-gas-like M@Si16 ions in the gas phase are interlinked with their simultaneous shell closure in the geometric and electronic structures.10–18 Considering that the geometrical destruction of nanoclusters upon their collision with surfaces hardly occurs in the soft-landing scheme, particularly for the present deposition with a small Ek of ∼0.01 eV per atom, the observed destabilization of Ta@Si16 cations may be triggered by the loss of electronic closure via a change in the charge state. Paradoxically, the excellent thermal stability of Ta@Si16/C60 implies that Ta@Si16 cations were immobilized onto C60-terminated surfaces while retaining their charge state, which is supported by the following STS results.
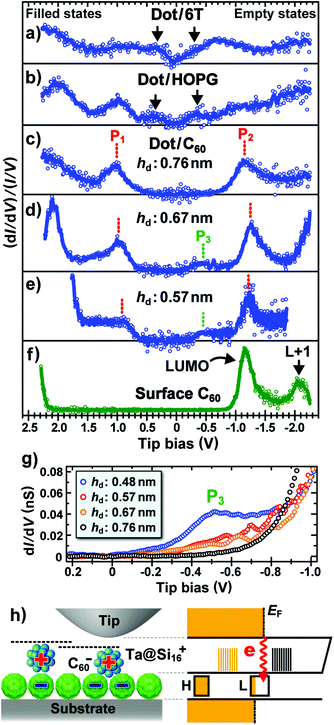
Fig. 4a and b show normalized dI/dV spectra recorded on dot/6T and dot/HOPG surfaces, such as those shown in Fig. 3a and 3b, respectively. Positive and negative Vtip correspond to the filled and empty states of samples, respectively. In these spectra, peaks of the electronic density of states (DOS) appear near the Fermi level (EF; Vtip = 0 V), as indicated by black arrows. The peak separations of ∼0.6 eV for dot/6T and ∼0.7 eV for dot/HOPG are clearly smaller than the theoretical energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of isolated Ta@Si16 cations (2.44 and 1.76 eV for C3v and D4d isomers,16 respectively). In contrast, the spectrum recorded on dot/C60 with a hd of 0.76 nm shows a large energy gap of ∼2.1 eV between DOS peaks P1 and P2 (Fig. 4c), which is close to the theoretical HOMO–LUMO gap of the isolated Ta@Si16 cations. Note that HOMO–LUMO gaps in dI/dV spectra are sometimes measured to be larger than the actual values, because Coulomb repulsion and attraction via electron and hole injections sometimes locally increase and decrease the potential energy of samples, respectively.30,31 However, such an increase in the energy gap is greatly suppressed in STS measurements of adsorbates strongly bound with a surface, such as Ta@Si16 nanoclusters deposited on 6T- and C60-terminated surfaces. Actually, a small energy gap of ∼0.6 eV is observed for dot/6T as shown in Fig. 4a. Furthermore, the difference in the energy gaps shown in Fig. 4a–c is qualitatively explained by the geometry and static charge state of Ta@Si16 nanoclusters on surfaces without considering the energy-gap modification in the STS measurements, as discussed next.
The large HOMO–LUMO gap of rare-gas-like M@Si16 ions originates from both the highly symmetrical coordination of Si atoms around the central metal atom and the arrangement of valence electrons with jellium-like electronic shells.10–18 In contrast, the energy gap of M@Si16 ions markedly decreases upon deformation into low-symmetry isomers15,16 and their interconnection via covalent Si–Si bonds.32 On the other hand, it is also predicted that the electronic structure of M@Si16 ions is modified by simply changing their charge state.11,12 For instance, when a single extra electron is statically injected into rare-gas-like neutral Ti@Si16 nanoclusters, an occupied molecular orbital appears near EF after the splitting of the original electronic state.11 Although similar phenomena should occur in neutralized Ta@Si16 cations, the STS spectrum of Ta@Si16/C60 exhibits a large energy gap between the two DOS peaks that appear at much higher and lower energies than EF, suggesting that the deposited Ta@Si16 cations retain not only their cage shape but also their cationic state on C60 films. Here, since we have observed that a positive current was the output from the substrates during the deposition of Ta@Si16 cations, it is considered that the cation is first neutralized immediately after adsorption by the injection of an electron from the substrate and then cationized again by the donation of an electron into the underlying C60 molecule. This is supported by the results of a theoretical calculation: Ta@Si16 nanoclusters and C60 molecules in the complexes shown in Fig. 2 tend to be positively and negatively charged via spontaneous polarization, respectively (Table S1†).
Actually, the electron transfer from Ta@Si16 nanoclusters to C60 molecules in the Ta@Si16/C60 system is observed in the following results. Fig. 4d and 4e show the normalized dI/dV spectra measured on slightly shorter dots with a hd of 0.67 and 0.57 nm, respectively. Comparing Fig. 4c–e, the positions of peaks P1 and P2 are constant regardless of the value of hd, which is consistent with the preceding consideration that the variation of hd in the range of 0.55–0.85 nm is not due to the deformation of the Ta@Si16 nanoclusters themselves but due to their position on the C60 film. In contrast, an additional peak P3 with low intensity is observed near EF for the shorter dots. A possible origin of P3 is the molecular orbital of the underlying C60 molecule, which can be measured using STS by directly injecting tunneling electrons into the C60 molecule within the energy gap of the Ta@Si16 nanocluster, as schematically shown in Fig. 4h. However, its contribution to the tunneling conductance should be smaller for taller dots because of the increased tip–C60 distance. This feature is indeed observed in the non-normalized dI/dV spectra focused on P3 (Fig. 4g), in which the intensity of P3 decreases with increasing hd. Here, on C60 molecules located sufficiently far from dots, the LUMO appears at 1.2 eV above EF (Fig. 4f), indicating their charge neutrality.33,34 In contrast, the LUMO of C60 molecules is known to be markedly lowered and appear slightly above EF by donating electrons from chemically doped alkali-metal atoms35 or from metallic surfaces.30,33 The present STS results suggest that similar charge transfer occurs in the Ta@Si16/C60 system immediately after the neutralization of Ta@Si16 cations, which is consistent with the fact that neutral Si16 cages encapsulating a group-5 metal atom (e.g. V@Si16 and Ta@Si16) exhibit alkali-like characteristics.13–15
4 Conclusions
In summary, we have demonstrated that the successful immobilization of gas-phase-synthesized Ta@Si16 cations onto C60-terminated surfaces via a donor–acceptor interaction while maintaining their cage shape and positive charge. This has enabled us to form a heterojunction exhibiting spontaneous polarization from two types of monolayers of superatomic Ta@Si16 cations and C60 anions. Such an ultrathin heterojunction would be useful for the charge separation layers in nanoscale devices such as capacitors and photovoltaic cells. Furthermore, the present results also suggest that the controlled immobilization of nanocluster ions exhibiting various charge states would be possible by controlling the donor–acceptor interaction between the nanoclusters and the surface, which is expected to play a key role in the design of high-performance catalysts, because it is known that the charge state of supported nanoclusters is a key factor in promoting their catalytic reactivity.36 A novel avenue for developing nanocluster-based materials science and technology is thus open to us.Notes and references
- S. N. Khanna and P. Jena, Phys. Rev. Lett., 1992, 69, 1664–1667 CrossRef CAS.
- S. B. Darling, N. A. Yufa, A. L. Cisse, S. D. Bader and S. J. Sibener, Adv. Mater., 2005, 17, 2446–2450 CrossRef CAS.
- Md. A. Alam, Y.-S. Kim, S. Ogawa, A. Tsuda, N. Ishii and T. Aida, Angew. Chem., Int. Ed., 2008, 47, 2070–2073 CrossRef CAS PubMed.
- S. A. Claridge, A. W. Castleman Jr., S. N. Khanna, C. B. Murray, A. Sen and P. S. Weiss, ACS Nano, 2009, 3, 244–255 CrossRef CAS PubMed.
- A. W. Castleman Jr. and S. N. Khanna, J. Phys. Chem. C, 2009, 113, 2664–2675 Search PubMed.
- T. Osuga, T. Murase and M. Fujita, Angew. Chem., Int. Ed., 2012, 51, 12199–12201 CrossRef CAS PubMed.
- X. Roy, C.-H. Lee, A. C. Crowther, C. L. Schenck, T. Besara, R. A. Lalancette, T. Siegrist, P. W. Stephens, L. E. Brus, P. Kim, M. L. Steigerwald and C. Nuckolls, Science, 2013, 341, 157–160 CrossRef CAS PubMed.
- Y. H. Jang, K. Chung, L. N. Quan, B. Špačková, H. Šípová, S. Moon, W. J. Cho, H.-Y. Shin, Y. J. Jang, J.-E. Lee, S. T. Kochuveedu, M. J. Yoon, J. Kim, S. Yoon, J. K. Kim, D. Kim, J. Homola and D. H. Kim, Nanoscale, 2013, 5, 12261–12271 RSC.
- J. M. Pettibone, W. A. Osborn, K. Rykaczewski, A. A. Talin, J. E. Bonevich, J. W. Hudgens and M. D. Allendorf, Nanoscale, 2013, 5, 6558–6566 RSC.
- V. Kumar and Y. Kawazoe, Phys. Rev. Lett., 2001, 87, 045503 CrossRef CAS.
- H. Kawamura, V. Kumar and Y. Kawazoe, Phys. Rev. B: Condens. Matter, 2005, 71, 075423 CrossRef.
- J. U. Reveles and S. N. Khanna, Phys. Rev. B: Condens. Matter, 2006, 74, 035435 CrossRef.
- K. Koyasu, M. Akutsu, M. Mitsui and A. Nakajima, J. Am. Chem. Soc., 2005, 127, 4998–4999 CrossRef CAS PubMed.
- J. Atobe, K. Koyasu, S. Furuse and A. Nakajima, Phys. Chem. Chem. Phys., 2012, 14, 9403–9410 RSC.
- M. B. Torres, E. M. Fernández and L. C. Balbás, Phys. Rev. B: Condens. Matter, 2007, 75, 205425 CrossRef.
- H. Cantera-López, L. C. Balbás and G. Borstel, Phys. Rev. B: Condens. Matter, 2011, 83, 075434 CrossRef.
- J. T. Lau, K. Hirsch, Ph. Klar, A. Langenberg, F. Lofink, R. Richter, J. Rittmann, M. Vogel, V. Zamudio-Bayer, T. Möller and B. von Issendorff, Phys. Rev. A, 2009, 79, 053201 CrossRef.
- P. Jena, J. Phys. Chem. Lett., 2013, 4, 1432–1442 CrossRef CAS.
- K. Bromann, C. Félix, H. Brune, W. Harbich, R. Monot, J. Buttet and K. Kern, Science, 1996, 274, 956–958 CrossRef CAS.
- V. N. Popok, I. Barke, E. E. B. Campbell and K.-H. Meiwes-Broer, Surf. Sci. Rep., 2011, 66, 347–377 CrossRef CAS PubMed.
- K. A. Wepasnick, X. Li, T. Mangler, S. Noessner, C. Wolke, M. Grossmann, G. Ganteföer, D. H. Fairbrother and K. H. Bowen, J. Phys. Chem. C, 2011, 115, 12299–12307 CAS.
- B. Wang, B. Yoon, M. König, Y. Fukamori, F. Esch, U. Heiz and U. Landman, Nano Lett., 2012, 12, 5907–5912 CrossRef CAS PubMed.
- S. Duffe, N. Grönhagen, L. Patryarcha, B. Sieben, C. Yin, B. von Issendorff, M. Moseler and H. Hövel, Nat. Nanotechnol., 2010, 5, 335–339 CrossRef CAS PubMed.
- M. Nakaya, T. Iwasa, H. Tsunoyama, T. Eguchi and A. Nakajima, Adv. Funct. Mater., 2014, 24, 1202–1210 CrossRef CAS.
- E. Mena-Osteritz and P. Bäuerle, Adv. Mater., 2006, 18, 447–451 CrossRef CAS.
- L. Chen, W. Chen, H. Huang, H. L. Zhang, J. Yuhara and A. T. S. Wee, Adv. Mater., 2008, 20, 484–488 CrossRef CAS.
- H. Tsunoyama, C. Zhang, H. Akatsuka, H. Sekiya, T. Nagase and A. Nakajima, Chem. Lett., 2013, 42, 857–859 CrossRef CAS.
- C. Zhang, H. Tsunoyama, H. Akatsuka, H. Sekiya, T. Nagase and A. Nakajima, J. Phys. Chem. A, 2013, 117, 10211–10217 CrossRef CAS PubMed.
- K. Sakamoto, M. Harada, D. Kondo, A. Kimura, A. Kakizaki and S. Suto, Phys. Rev. B: Condens. Matter, 1998, 58, 13951–13956 CrossRef CAS.
- X. Lu, M. Grobis, K. H. Khoo, S. G. Louie and M. F. Crommie, Phys. Rev. B: Condens. Matter, 2004, 70, 115418 CrossRef.
- I. F. Torrente, K. J. Franke and J. I. Pascual, J. Phys.: Condens. Matter, 2008, 20, 184001 CrossRef.
- M. B. Torres, E. M. Fernández and L. C. Balbás, J. Phys. Chem. C, 2011, 115, 335–350 CAS.
- M. Nakaya, Y. Kuwahara, M. Aono and T. Nakayama, Small, 2008, 4, 538–541 CrossRef CAS PubMed.
- M. Nakaya, Y. Kuwahara, M. Aono and T. Nakayama, J. Nanosci. Nanotechnol., 2011, 11, 2829–2835 CrossRef CAS PubMed.
- M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Classification and Structure of Doped Fullerenes, in Science of Fullerenes and Carbon Nanotubes, Academic Press, San Diego, CA, 1996, pp. 224–263 Search PubMed.
- B. Yoon, H. Häkkinen, U. Landman, A. S. Wörz, J.-M. Antonietti, S. Abbet, K. Judai and U. Heiz, Science, 2005, 307, 403–407 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. Effect of Vtip on the height of dots in STM images, deposition of Ta@Si16 cations with higher kinetic energy, and the theoretical optimization of geometries and electronic properties of Ta@Si16–C60 complexes. See DOI: 10.1039/c4nr04211e |
| This journal is © The Royal Society of Chemistry 2014 |