Open Access Article
Open Access ArticleFacile synthesis of intense green light emitting LiGdF4:Yb,Er-based upconversion bipyramidal nanocrystals and their polymer composites†
Hyejin
Na
a,
Jong Seok
Jeong
b,
Hye Jung
Chang
c,
Hyun You
Kim
de,
Kyoungja
Woo
a,
Kipil
Lim
a,
K. Andre
Mkhoyan
b and
Ho Seong
Jang‡
*a
aMolecular Recognition Research Center, Korea Institute of Science and Technology, Hwarangno 14-gil 5, Seongbuk-gu, Seoul 136-791, Republic of Korea. E-mail: msekorea@kist.re.kr; Fax: +82-2-958-5451; Tel: +82-2-958-5263
bDepartment of Chemical Engineering and Materials Science, University of Minnesota, 421 Washington Avenue SE, Minneapolis, MN 55455, USA
cAdvanced Analysis Center, Korea Institute of Science and Technology, Hwarangno 14-gil 5, Seongbuk-gu, Seoul 136-791, Republic of Korea
dCenter for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York 11973, USA
eDepartment of Nanomaterials Engineering, Chungnam National University, 99 Daehak-ro, Yuseong-gu, Daejeon 305-764, Republic of Korea
First published on 16th April 2014
Abstract
A pathway for achieving intense green light emitting LiGdF4:Yb,Er upconversion nanophosphors (UCNPs) via Y3+ doping is demonstrated. It was revealed that Y3+ doping initiated the formation of a tetragonal phase and affected the particle size. Single tetragonal-phase LiGd0.4Y0.4F4:Yb(18%),Er(2%) (LGY0.4F:Yb,Er) UCNPs exhibited strong upconversion (UC) green luminescence and tetragonal bipyramidal morphologies. They showed 1325 and 325-fold higher photoluminescence intensity than the 0 and 80 mol% Y3+-doped LiGdF4:Yb,Er UCNPs, respectively. Additionally the particle size (edge length) of LiGdF4:Yb,Er-based upconversion tetragonal bipyramids (UCTBs) was controlled from 60.5 nm to an ultrasmall size of 9.3 nm with varying Y3+ doping concentration. In an LGY0.4F:Yb,Er UCTB, uniform distribution of all constituent elements was directly confirmed by using high-angle annular dark-field scanning transmission electron microscopy and energy-filtered transmission electron microscopy (EFTEM) image analyses. In particular, existence of activator Er3+ ions with extremely small quantity was clearly seen over a particle on the EFTEM image. Moreover, the LGY0.4F:Yb,Er UCTBs were successfully incorporated into the polydimethylsiloxane (PDMS) polymer and the highly transparent UCTB–PDMS composites showed bright green light under the excitation of 980 nm infrared light.
1. Introduction
Lanthanide doping into inorganic crystals imparts these materials with new functionalities such as upconversion (UC),1–3 downconversion/downshifting,4–7 dual-mode luminescence,8–10 enhanced computed tomography (CT) contrast11 and magnetism.12,13 In particular, lanthanide ion-doped (Ln3+-doped) upconversion nanophosphors (UCNPs) have been in the spotlight due to their excellent chemical and optical properties. The UCNPs have many advantages over conventional fluorescent organic dyes and quantum dots, such as high photostability (without photobleaching), long life time (from μs to ms) due to the luminescence attributed to parity-forbidden f–f transition, large anti-Stokes shifts, non-toxicity (no Cd or Pb), and a lack of photoblinking.14 Moreover, the UCNPs exhibit an infrared (IR) to visible light conversion efficiency an order of magnitude higher than the two-photon absorption process. This high conversion efficiency of the UCNPs is due to the existence of a real, intermediate energy level between the ground and excited states.14,15 Given this high UC efficiency, commercially available, inexpensive continuous-wave diode lasers can be used as an excitation light source. Recently, sub-10 nm, ultrasmall UCNPs were successfully synthesized with excellent luminescent properties that could allow further bioimaging applications.16,17To date, Ln3+-doped fluoride-based UCNPs have been extensively studied, because fluoride materials have low phonon energies and show high optical transparency in the visible region due to their large band-gap energy.18,19 In particular, NaLnF4 (Ln = Gd, La, Lu, and Y)-based UCNPs have attracted great attention.17,20–25 For example, β-NaYF4 is known as the most efficient host material for blue and green upconversion luminescence.26 On the other hand, LiGdF4 is an outstanding host for downconversion luminescence with a visible quantum efficiency approaching 190%.27 However, although LiYF4:Yb,Tm/Er and LiYF4:Er UCNPs have been reported,28–31 few reports describe LiGdF4-based UCNPs possibly due to the difficulty in synthesizing LiGdF4 nanocrystals with a single tetragonal phase.32 Because lanthanide doping simultaneously affects both the size and phase of the UCNPs,33 we used Y doping as the synthesis pathway for the fabrication of single-phase LiGdF4 UCNPs. In this article, we report on the facile synthesis of highly bright Y3+-doped LiGdF4:Yb,Er UCNPs with a single tetragonal phase. The Li(Gd,Y)F4:Yb,Er showed intense green UC luminescence with higher efficiency than β-NaYF4:Yb,Er UCNPs at 150 W cm−2 power density and a tunable size from several tens of nanometers to sub-10 nm via Y3+ doping. In addition, the applicability of the Li(Gd,Y)F4:Yb,Er UCNPs to transparent display devices was examined through fabrication of transparent polymer composites.
On the other hand, one must establish a compositional map of these UCNPs and the locations of the activator Ln3+ ions within the particles because the luminescence of the UCNPs is strongly affected by surface defects.34,35 Although the dopant distribution in the Ln3+-doped NaGdF4 has been analyzed via synchrotron X-ray photoelectron spectroscopy (XPS), determining the precise elemental distribution at the single-nanoparticle level remains difficult because the XPS technique is an ensemble measurement.36 Thus, transmission electron microscopy (TEM) combined with energy dispersive X-ray spectroscopy (EDS) and electron energy-loss spectroscopy (EELS) analysis is necessary to identify the compositional distributions within a nanoparticle. Here, for the first time, we successfully synthesized highly bright, single-phase LiGdF4-based UC tetragonal bipyramids (UCTBs) via Y doping. We also provide direct identification of the elemental distribution of each constituent in the UCTBs by applying energy-filtered TEM (EFTEM) and high-angle annular dark-field scanning transmission electron microscopy (HAADF STEM).
2. Experimental
2.1. Materials
LiOH·H2O (99.995%), GdCl3·6H2O (99%), YCl3·6H2O (99.99%), YbCl3·6H2O (99.9%), ErCl3·6H2O (99.9%), NH4F (99.99+%), oleic acid (OA, technical grade 90%), and 1-octadecene (ODE, technical grade 90%) were purchased from Aldrich and they were used without further purification. Sodium oleate (>97%) was obtained from TCI.2.2. Synthesis of the Li(Gd,Y)F4:Yb,Er upconversion nanophosphors
First, the lanthanide oleates [Ln(oleate)3, Ln = Gd, Y, Yb, and Er] were prepared by adopting the synthesis reported by Hyeon's group.37 Then, 1 mmol of Ln(oleate)3 complexes, in which the quantities of Yb and Er were fixed at 0.18 and 0.02 mmol, respectively, were loaded into a three-necked flask containing a solvent mixture of 10.5 ml OA and 10.5 ml ODE. The mixture was heated to 150 °C for 40 min to yield a transparent solution. After the reaction mixture cooled to 50 °C, a methanol (MeOH) solution (10 ml) containing LiOH·H2O (2.5 mmol) and NH4F (4 mmol) was added to the reaction flask and then stirred for 40 min. After the MeOH was removed, the solution was heated to 320 °C for 90 min under an Ar atmosphere. The as-synthesized nanophosphors were washed several times with ethanol and dispersed in chloroform.2.3. Preparation of the UCTB–PDMS composites
To prepare the UCTB–polydimethylsiloxane (PDMS) composites, 0.4 ml of UCTB solution (approximately 1 wt%) was thoroughly mixed with 10 ml of SYLGARD silicone elastomer 184 followed by the addition of a curing agent (1 ml). Finally, the UCTB–PDMS composites (approximately 0.04 wt% UCTB) were aged overnight and then heat-treated at 80 °C for 1 h.2.4. Characterization
The absorption and transmittance spectra were recorded using a Perkin-Elmer Lambda25 UV/VIS spectrophotometer. The absorption spectrum of the UCNP chloroform solution (∼1 wt%) in a quartz cuvette (1 cm × 1 cm, Hellma QS cell) was recorded under the conditions of 240 nm min−1 of scan speed and 1 nm of slit width. The crystal structures of the as-synthesized nanophosphors were determined using a Bruker D8 ADVANCE diffractometer with Cu Kα radiation at 40 kV and 40 mA. The photoluminescence (PL) spectra were collected using a Hitachi F-7000 spectrophotometer. The scanning electron microscopy (SEM) and TEM images were obtained on an FEI Nova nanoSEM operating at 10 kV and a Tecnai G2 F20 operating at 200 kV, respectively. The EFTEM images were obtained on an FEI Titan 80/300 operating at 300 kV equipped with a GIF Quantum®ERS (Gatan, Inc., USA). The high-resolution STEM (HR-STEM) images were obtained using an FEI Tecnai G2 F30 S-TEM operating at 300 kV.2.5. Density functional theory (DFT) calculation
We performed spin-polarized density functional theory (DFT) calculation on a plane-wave basis with the Vienna Ab initio Simulation Package (VASP) code38 and the Perdew–Burke–Ernzerhof (PBE) sol39 GGA functional. Valence electrons were described by plane waves up to an energy cutoff of 400 eV and the core electrons were described within the projector augmented wave framework.40 We used a 6 × 6 × 3 k-points grid sampling of the Brillouin zone for unit cell calculations. Final convergence criteria for the electronic wave function and geometry were 10−4 eV and 0.01 eV Å−1, respectively. The Gaussian smearing method with a width of 0.05 eV was used to improve convergence with respect to states near the Fermi level.3. Results and discussion
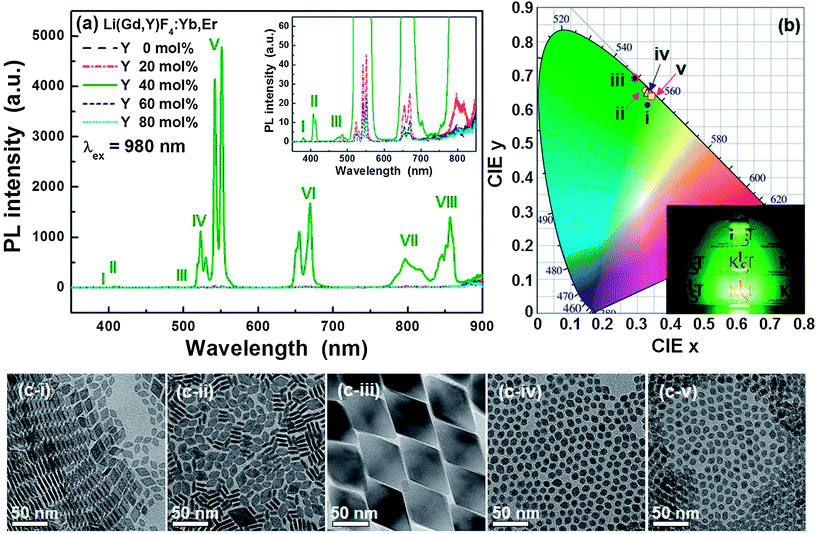
All UCNP solutions, which are highly transparent, exhibited green luminescence under IR illumination (Fig. S1†). Fig. 1a presents the PL spectra of the Li(Gd,Y)F4:Yb,Er UCNPs under 980 nm IR excitation (the Yb and Er concentrations were 18 and 2 mol%, respectively). The PL peaks are ascribed to the 4f–4f electronic transitions of the Er3+ ions via a Yb3+ → Er3+ energy transfer following IR light absorption by the Yb3+ which has an absorption band at approximately 960 nm (Fig. S2†). The emission peaks in the green and red spectral regions result from the two-photon upconversion process, as evidenced by the light power-dependent PL intensity (see Fig. S3†). As the Y3+ concentration increased to 40 mol% in the host lattice, the PL intensity increased. At higher Y3+ concentrations, however, the PL intensity decreased significantly. For example, 40 mol% Y3+-doped LiGdF4:Yb,Er (LGY0.4F:Yb,Er) exhibited an approximately 1325 and 325-fold higher PL intensity than the 0 and 80 mol% Y3+-doped LiGdF4:Yb,Er UCNPs, respectively. Given the strong PL intensity ascribed to the 4S3/2 → 4I15/2 transition, which peaks at 550 nm, the LGY0.4F:Yb,Er UCNPs emit a bright green light, as depicted in Fig. 1b inset. Previously, Chen et al. obtained the quantum yield (QY) of LiYF4:Er UCNPs by comparing with β-NaYF4:Yb,Er UCNPs.30 When the PL intensity of the LGY0.4F:Yb,Er UCNPs was compared with that of β-NaYF4:Yb,Er UCNPs, the LGY0.4F:Yb,Er showed higher PL intensity (Fig. S5†). Its QY was calculated to be 0.16% by using the QY (0.1%) of β-NaYF4:Yb,Er with 30 nm size.41 Although the relative QY of the LGY0.4F:Yb,Er UCNPs was obtained in the current study, absolute QY of the LGY0.4F:Yb,Er UCNPs will be further studied by using an integrating sphere. When we calculated the intensity ratio of green to red emission (Rg/r) of the Li(Gd,Y)F4:Yb,Er UCNPs, the Rg/r value increased with increasing Y3+ concentration under the conditions of 0 and 20 mol% Y3+ doping. In that case, the particle size increased with increasing Y3+ concentration. On the other hand, the Rg/r value decreased when the particle size decreased with increasing Y3+ concentration (Y3+ concentration = 40, 60, and 80 mol%). As shown in Fig. S6,† LGY0.4F:Yb,Er showed the highest Rg/r value of 2.85. As a consequence, the Rg/r value was dependent on the particle size and it decreased with decreasing particle size. When the particle size decreases, quenching of UC luminescence induced by surface defects and ligands becomes more important and it modifies the relative population among various excited states through phonon-assisted nonradiative relaxations.42,43 However, although the edge size of the 20 mol% Y3+-doped Li(Gd,Y)F4:Yb,Er was larger than that of 60 mol% Y3+-doped Li(Gd,Y)4:Yb,Er, its Rg/r value was smaller than that of the 60 mol% Y3+-doped Li(Gd,Y)F4:Yb,Er due to the different crystal structure (see Fig. S6†). The Commission Internationale de l'Eclairage (CIE) color coordinates (x, y) of the LGY0.4F:Yb,Er UCNPs are (0.2929, 0.6917). The LGY0.4F:Yb,Er UCNPs exhibit a very high color purity of 98.9%, which approaches that of monochromatic light due to the sharp emission peak and high green-to-red ratio in the PL intensity.As indicated in Fig. 1c, the LGY0.4F:Yb,Er UCNPs are much larger than the other Li(Gd,Y)F4:Yb,Er UCNPs. In addition, the X-ray diffraction (XRD) patterns presented in Fig. S7† reveal the formation of an orthorhombic GdF3 phase under doping conditions between 0 and 20 mol% Y3+. The crystal structures were also verified from the UCNP lattice spacings measured via high-resolution TEM (HR-TEM) (Fig. S8†). A tetragonal LiGdF4 phase (the LiYF4 phase for 80 mol% Y3+ doping) was formed under doping conditions between 40 and 80 mol% Y3+. It was reported that a single LiGdF4 tetragonal phase is hardly synthesized and instead, GdF3 orthorhombic phase is apt to be formed.32 However, as the Gd3+ ions were replaced by Y3+ ions (>20 mol%), a tetragonal phase was formed without changing the reaction temperature and/or time, which may be attributed to a decrease in the energetic barrier for the formation of a LiGdF4 phase due to lanthanide doping.33 Large size and formation of a single tetragonal phase may be attributed to strong UC luminescence of LGY0.4F:Yb,Er UCNPs. It is believed that the size effect is more dominant on the luminescence than the phase effect, judging from similar brightness of small UCNPs corresponding to Fig. 1c-i, ii, iv, and v, as shown in Fig. 1 and S1b.† However, further study is necessary to reveal the exact origin of strong UC luminescence from LGY0.4F:Yb,Er UCNPs. It is noted that the particle size could be controlled via Ln3+ ion doping for the case of the LiGdF4 tetragonal phase, whereas it was hardly controlled for the GdF3 orthorhombic phase. Thus, we can achieve intense UC luminescence by simply controlling the particle size when we synthesize a single tetragonal LiGdF4 phase. In addition, morphologies of the Li(Gd,Y)F4:Yb,Er UCNPs are affected by their phases. Minute differences may be noted in the TEM images in Fig. 1c. The UCNP morphologies in Fig. 1c-i and ii exhibit rhombic plate-like shapes, while the plate shape was not observed in Fig. 1c-iii to v. The particle shapes in Fig. 1c-iv and c-v appear octahedral or truncated octahedral. This morphological difference is believed to result from their different crystal structures. The rhombic plates depicted in Fig. 1c-i have edge lengths of 12.1 ± 1.0 nm and thicknesses of 4.0 ± 0.5 nm. The nanoplates are easily aligned into two-dimensional aggregates as shown in Fig. 1c-i and S9† because this alignment minimizes the free energy by hydrophobic interactions of the surface ligands of the nanoplates at their largest faces.44 As the quantity of Y3+ ions increased in the host lattice, the particle size decreased from 60.5 nm (edge length) for the 40 mol% Y3+ doping to 9.3 nm (edge length) for the 80 mol% Y3+ doping, which resulted in weak PL intensities for the Li(Gd,Y)F4:Yb,Er. As observed in Fig. 1c-iv and c-v, the small Li(Gd,Y)F4:Yb,Er UCNPs appear somewhat spherical with slight faceting, while the large LGY0.4F:Yb,Er UCNPs exhibit sharp facets.
We studied the trend in the change of the particle sizes of the Li(Gd,Y)F4:Yb,Er UCNPs with tetragonal structure as a function of Y3+ concentration from 40 mol% to 80 mol%. In the high concentration regime, 60 and 80 mol% Y3+, the difference in the particle size was marginally small (see Table S1† and Fig. S10†). However, there was significant increase in the particle size between 60 and 40 mol% Y3+-doped Li(Gd,Y)F4:Yb,Er UCNPs; the LGY0.4F:Yb,Er UCNPs were approximately 6 times larger than the 60 mol% Y3+-doped Li(Gd,Y)F4:Yb,Er UCNPs. Previously, Wang et al. studied the size change of NaYF4:Yb,Er UCNPs by Gd3+ doping by using density functional theory (DFT) calculation.33 They found that the Gd3+ ions donate more electron density to adjacent F− ions than Y3+ ions do leading to the increased electron polarization between cations and F− ions. Increased electrostatic repulsive force between the electron rich F− ions at the surface layer of NaYF4 and F− ions in the solution can substantially slow down the diffusion of F− ions from solution to the nanocrystal surface. They associated this with the reduced nanocrystal size.33 In contrast to the case of NaYF4, the Bader charge analysis45,46 on a unit-cell of LiYF4, LiGdF4, and Li(Gd0.5,Y0.5)F4 (LiGdYF4) shows that there is no significant charge redistribution upon Y3+ doping in the bulk level as shown in Table S2.† It may attribute to experimentally observed small change in the size of the nanocrystals for 60 and 80 mol% Y3+ doping. However, in the case of similar concentrations of Y3+ to Gd3+, upon surface formation, some of the surface-exposed F− ions adjacent to Y3+ dopants of the most stable LiGdYF4(101) surface, which is experimentally and theoretically confirmed (see below), were less-negatively charged compared to the F− ions in a bulk phase (Δe = 0.06). According to the discussion in ref. 33, we postulate that these less-negatively charged F− ions will reduce the electrostatic repulsive force upon the approach of F− ions in solution to the LiGdYF4(101) surface and thus can accelerate the formation of LiGdYF4 with large size.
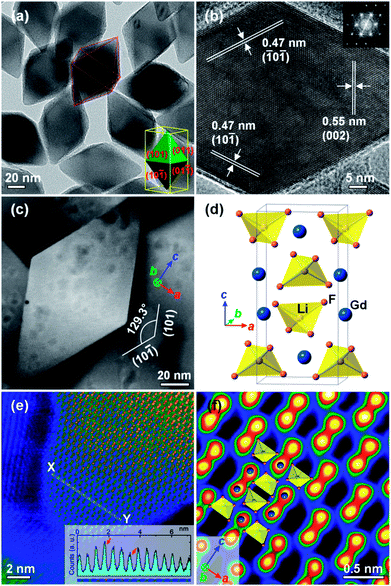
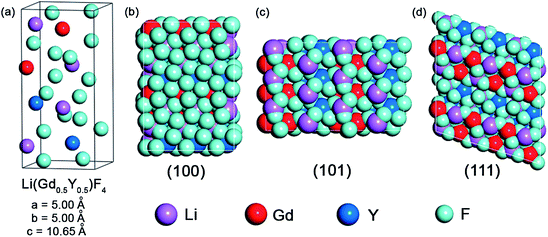
Previous reports indicate that LiYF4-based nanocrystals are plate shaped.28,32 Although a TEM image of the LGY0.4F:Yb,Er observed along a particular crystallographic orientation (Fig. 1c-iii) demonstrates a plate-like shape, the actual morphology of the LGY0.4F:Yb,Er is octahedral as indicated in Fig. 2. Fig. 2a presents a TEM image of the LGY0.4F:Yb,Er particles that were not aligned parallel to the TEM gird. No plate-shaped particles were observed in our TEM images of the Li(Gd,Y)F4:Yb,Er UCNPs for 40, 60, and 80 mol% Y3+ doping. As demonstrated in the TEM images in Fig. 2a, the central portions of the LGY0.4F:Yb,Er UCNPs are much darker than their edges (vice versa in the HAADF STEM images, Fig. S11†). This severe contrast in the UCNPs indicates that the UCNPs are thicker in their centers than at the edges. The STEM and SEM studies also revealed that LGY0.4F:Yb,Er exhibits a tetragonal bipyramidal morphology (Fig. S11 and S12†). The HR-TEM and HAADF HR-STEM analyses of the LGY0.4F:Yb,Er UCTBs shown in Fig. 2 proved that the particles were bound by {101} planes. The angles between two adjacent planes were measured to be 50.7 and 129.3° which are in agreement with the angles between the (101) and (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) planes of tetragonal LiGdF4. The fast Fourier transform (FFT) pattern presented in the inset of Fig. 2b can be indexed to be the zone axis along the [010] direction of the tetragonal LiGdF4 structure. The results indicate that LGY0.4F:Yb,Er UCTBs have a single crystalline phase with high crystallinity. To investigate the origin of the formation of the tetragonal bipyramidal morphology, the surface energies of the low-index planes of Li(Gd,Y)F4 – (100), (101) and (111) – were calculated using density functional theory. The unit cell of LiGdF4 was initially optimized and two ions of Gd3+ were substituted with Y3+ ions. The unit cell of Li(Gd0.5,Y0.5)F4 with the most stable configuration of Gd3+ and Y3+ ions is presented in Fig. 3a. The morphology of each plane and their surface energies are presented in Fig. 3b–d and Table 1, respectively. The calculations confirm that the (101) surface is thermodynamically favored. The LGY0.4F:Yb,Er UCTBs are faceted by eight equivalent {101} planes in a tetragonal structure, which induces a tetragonal bipyramidal morphology due to a longer lattice parameter along the c-axis. The lattice parameters, a = b = 5.177 Å and c = 10.773 Å, were calculated from the high resolution XRD pattern. All facets are atomically flat, and the two apices along the c-axis are blunt (Fig. S13†). The tips are blunt in the cross-sectional region of the approximately 20 × 20 unit cell (uc)2 in the ab plane (Fig. S13†).
) planes of tetragonal LiGdF4. The fast Fourier transform (FFT) pattern presented in the inset of Fig. 2b can be indexed to be the zone axis along the [010] direction of the tetragonal LiGdF4 structure. The results indicate that LGY0.4F:Yb,Er UCTBs have a single crystalline phase with high crystallinity. To investigate the origin of the formation of the tetragonal bipyramidal morphology, the surface energies of the low-index planes of Li(Gd,Y)F4 – (100), (101) and (111) – were calculated using density functional theory. The unit cell of LiGdF4 was initially optimized and two ions of Gd3+ were substituted with Y3+ ions. The unit cell of Li(Gd0.5,Y0.5)F4 with the most stable configuration of Gd3+ and Y3+ ions is presented in Fig. 3a. The morphology of each plane and their surface energies are presented in Fig. 3b–d and Table 1, respectively. The calculations confirm that the (101) surface is thermodynamically favored. The LGY0.4F:Yb,Er UCTBs are faceted by eight equivalent {101} planes in a tetragonal structure, which induces a tetragonal bipyramidal morphology due to a longer lattice parameter along the c-axis. The lattice parameters, a = b = 5.177 Å and c = 10.773 Å, were calculated from the high resolution XRD pattern. All facets are atomically flat, and the two apices along the c-axis are blunt (Fig. S13†). The tips are blunt in the cross-sectional region of the approximately 20 × 20 unit cell (uc)2 in the ab plane (Fig. S13†).
| Surface index | (100) | (101) | (111) |
|---|---|---|---|
| Surface energy (eV Å−2) | 0.418 | 0.051 | 0.098 |
| Surface energy (J m−2) | 6.67 | 0.82 | 1.57 |
The Z-contrast of the atomic columns was examined in the HAADF STEM images (Fig. 2e). The potential contrast variation by electron beam damage, which was observed in highly exposed areas (Fig. 2c and S14, S15†), can largely be prevented by acquiring fresh STEM images. The intensity profile across XY indicates considerable variation: a higher intensity can support the existence of dopants such as Er and Yb in the Gd sites, and a lower intensity can indicate Y in Gd sites as designated by the arrows (see also Fig. S14†). The lanthanide elements are clearly observed in the low-pass filtered HAADF HR-STEM image (Fig. 2f), and their arrangement is consistent with a projection of the LiGdF4 unit cell in the [010] direction. The LiGdF4 has a scheelite structure (I41/a, Z = 4), and the Li+ and Gd3+ ions are four-fold and eight-fold coordinated by the F− ions, respectively (Fig. 2d).47,48 It should be noted that the Li and F atoms are not visible in the HAADF STEM image in Fig. 2f because they are very light in comparison with the Y3+ and Ln3+ ions.
The elemental distribution within a nanoparticle was examined using an EELS-based EFTEM technique. For the materials containing Li, such as our LGY0.4F:Yb,Er UCTBs, EELS analysis is a powerful tool because, unlike EDS, it can detect light elements such as Li. Additionally, EFTEM provides rapid elemental mapping for electron-beam-sensitive materials. Fig. 4 presents an elastic TEM image and thickness map of the LGY0.4F:Yb,Er UCTBs and the corresponding elemental maps. The core-loss EELS spectra of the Li–K, Gd–M4,5, Y–L2,3, F–K, Yb–M4,5, and Er–M4,5 maps obtained from the LGY0.4F:Yb,Er UCTBs are presented in Fig. S16.† Acquiring the high energy-loss spectra in TEM (above approximately 1500 eV) is challenging due to the small signal-to-noise ratio. Surprisingly, however, the core-loss edges such as the Gd–M4,5 (1185 eV), Er–M4,5 (1409 eV), Yb–M4,5 (1528 eV) and even the Y–L2,3 (2080 eV) edges were successfully acquired with the enhanced signal-to-noise ratio and collection efficiency of the GIF Quantum®ERS (Gatan, Inc., Pleasanton, CA, USA). As a result, EFTEM maps from all elements including lanthanide elements could be successfully obtained as shown in Fig. 4. One thing to note is that the sharp features observed in the image of Fig. 4a are not observed in the Gd, Y, and Yb EFTEM images of Fig. 4c which appear more rounded due to low signal intensity at thin regions such as the apex and the edge of this bipyramidal nanocrystal (see the thickness map of Fig. 4b), particularly for high energy-loss peaks (above 1000 eV). Except for this smoothing effect, however, one can notice that all elements are uniformly distributed over a single UCTB. Even the quantity of the activator Er3+ ion is very small and existence of Er3+ ions over a particle is clearly seen.
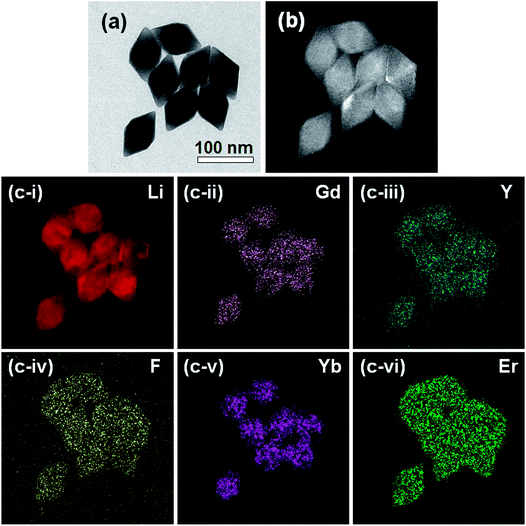 | ||
| Fig. 4 (a) Elastic TEM image and (b) thickness map of LGY0.4F:Yb,Er UCTBs. (c) EFTEM elemental maps for (i) Li, (ii) Gd, (iii) Y, (iv) F, (v) Yb, and (vi) Er are shown. | ||
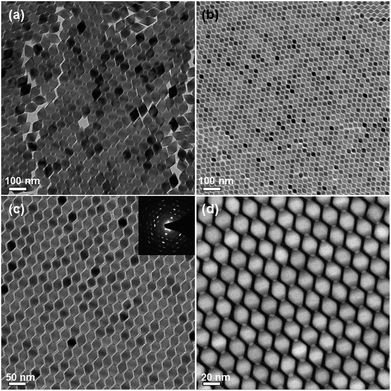
The as-synthesized LGY0.4F:Yb,Er UCTBs were highly uniform in size and shape (60.5 ± 1.6 nm × 55.3 ± 1.4 nm), which allows for two-dimensional (2D) ordered arrangement of LGY0.4F:Yb,Er UCTBs (Fig. 5a and S11b†). When the size of the UCTBs decreased due to slight increase of Y3+ concentration in the host lattice, an even higher ordered 2D superlattice could be obtained (Fig. 5b–d). The Li(Gd0.35Y0.45)F4:Yb,Er (LGY0.45F:Yb,Er) also exhibits a single tetragonal phase with high crystallinity (Fig. S17†). The slow evaporation of the solvent allows the LGY0.45F:Yb,Er UCTBs to assemble into 2D monolayers in which the {101} planes of the UCTBs are parallel to the TEM grid. The spotty selected area electron diffraction (SAED) pattern supports a highly ordered UCTB assembly. The HAADF STEM image of Fig. 5d confirms that the smaller LGY0.45F:Yb,Er constituting the 2D superlattice also has a bipyramidal shape. The bright contrast at the apices results from the overlap of apices of adjacent particles.
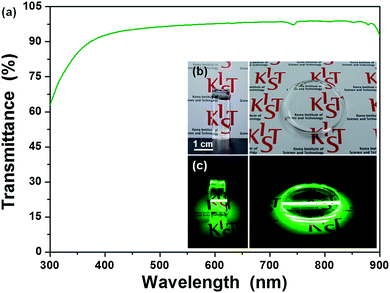
The feasibility of applying LGY0.4F:Yb,Er UCTBs to transparent volumetric three-dimensional (3D) displays was investigated by incorporating UCTBs into a polydimethylsiloxane (PDMS) polymer. Although these UCTBs have an anisotropic morphology and are larger than the previously reported NPs that were successfully incorporated into a PDMS polymer,33 they were well-dispersed in the PDMS polymer, which allowed for the fabrication of highly transparent UCTB–PDMS composites (Fig. 6). The transmittance of the UCTB–PDMS composites was found to exceed 90% in the visible spectral region (Fig. 6a). As indicated in the photographs of the LGY0.4F:Yb,Er UCTB–PDMS bar and disk in Fig. 6c, the luminescence is homogenous, bright green and sufficiently intense to render characters on the background paper legible. The high transparency and brightness of the UCTB–PDMS composites can be attributed to the strong UC luminescence from the LGY0.4F:Yb,Er UCTBs which allows small quantities of UCTBs to mix with PDMS. These results also indicate that the UCTBs have potential for applications in volumetric 3D displays.1,33
4. Conclusions
In summary, we demonstrated a pathway for achieving intense green light emitting LiGdF4:Yb,Er UCNPs via Y3+ doping. We found that Y3+ doping initiated the formation of a tetragonal phase and affected the particle size. Single tetragonal-phase Li(Gd,Y)F4:Yb,Er UCNPs with Y3+ concentrations similar to the Gd3+ concentration exhibited intense UC green luminescence and tetragonal bipyramid morphologies. Additionally, sub-10 nm ultrasmall UCTBs could be obtained via Y3+ doping. Uniform distribution of all constituent elements of LGY0.4F:Yb,Er UCTBs was directly seen via EFTEM mapping. These LGY0.4F:Yb,Er UCTBs were also successfully incorporated into and uniformly distributed throughout PDMS polymer composites. The strong UC luminescence of the LGY0.4F:Yb,Er allowed the fabrication of highly transparent and bright green light emitting PDMS composites. These Li(Gd,Y)F4:Yb,Er materials are potentially applicable in transparent volumetric 3D displays.Acknowledgements
This work was supported in part by the Dream project (2V03410) and Flagship project (2E24572) funded by the Korea Institute of Science and Technology (KIST), the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2013M3C1A3065040), the MRSEC Program of the National Science Foundation (NSF) under Award number DMR-0819885, and the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract DE-AC02-98CH10886 (H.Y.K.), and parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from the NSF through the MRSEC program. We thank Seo Kyoung Ryu and Min Kyoung Cho for their technical help for EFTEM analysis (2V02951).References
- H. Na, K. Woo, K. Lim and H. S. Jang, Nanoscale, 2013, 5, 4242 RSC.
- Z. Li and Y. Zhang, Angew. Chem., Int. Ed., 2006, 45, 7732 CrossRef CAS PubMed.
- F. Zhang, Y. Wan, T. Yu, F. Zhang, Y. Shi, S. Xie, Y. Li, L. Xu, B. Tu and D. Zhao, Angew. Chem., Int. Ed., 2007, 46, 7976 CrossRef CAS PubMed.
- H. S. Jang, H. Yang, S. W. Kim, J. Y. Han, S.-G. Lee and D. Y. Jeon, Adv. Mater., 2008, 20, 2696 CrossRef CAS.
- C. Feldmann, T. Justel, C. R. Ronda and D. U. Wiechert, J. Lumin., 2001, 92, 245 CrossRef CAS.
- H. S. Jang, H. Y. Kim, Y.-S. Kim, H. M. Lee and D. Y. Jeon, Opt. Express, 2012, 20, 2761 CrossRef CAS PubMed.
- S. Y. Kim, K. Woo, K. Lim, K. Lee and H. S. Jang, Nanoscale, 2013, 5, 9255 RSC.
- H. S. Jang, K. Woo and K. Lim, Opt. Express, 2012, 20, 17107 CrossRef CAS.
- P. Li, Q. Peng and Y. D. Li, Adv. Mater., 2009, 21, 1945 CrossRef CAS.
- Y. Liu, D. Tu, H. Zhu, R. Li, W. Luo and X. Chen, Adv. Mater., 2010, 22, 3266 CrossRef CAS PubMed.
- Y. Liu, K. Ai, J. Liu, Q. Yuan, Y. He and L. Lu, Angew. Chem., Int. Ed., 2012, 51, 1437 CrossRef CAS PubMed.
- Y. Liu, D. Wang, J. Shi, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2013, 52, 4366 CrossRef CAS PubMed.
- R. Kumar, M. Nyk, T. Y. Ohulchanskyy, C. A. Flask and P. N. Prasad, Adv. Funct. Mater., 2009, 19, 853 CrossRef CAS.
- S. Wu, G. Han, D. J. Milliron, S. Aloni, V. Altoe, D. V. Talapin, B. E. Cohen and P. J. Schuck, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10917 CrossRef CAS PubMed.
- M. Haase, S. Heer, K. Kömpe and H. U. Güdel, Adv. Mater., 2004, 16, 2102 CrossRef.
- N. J. J. Johnson, W. Oakden, G. J. Stanisz, R. S. Prosser and F. C. J. M. van Veggel, Chem. Mater., 2011, 23, 3714 CrossRef CAS.
- Q. Liu, Y. Sun, T. Yang, W. Feng, C. Li and F. Li, J. Am. Chem. Soc., 2011, 133, 17122 CrossRef CAS PubMed.
- F. Wang, J. Wang, J. Xu, X. Xue, H. Chen and X. Liu, Spectrosc. Lett., 2010, 43, 400 CrossRef CAS.
- E. van der Kolk, P. Dorenbos, K. Kramer, D. Biner and H. U. Gudel, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 125110 CrossRef.
- G. Chen, J. Shen, T. Y. Ohulchanskyy, N. J. Patel, A. Kutikov, Z. Li, J. Song, R. K. Pandey, H. Agren, P. N. Prasad and G. Han, ACS Nano, 2012, 6, 8280 CrossRef CAS PubMed.
- H.-X. Mai, Y.-W. Zhang, R. Si, Z.-G. Yan, L.-D. Sun, L.-P. You and C.-H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS PubMed.
- L. W. Yang, Y. Li, Y. C. Li, J. J. Li, J. H. Hao, J. X. Zhong and P. K. Chu, J. Mater. Chem., 2012, 22, 2254 RSC.
- Q. Cheng, J. Sui and W. Cai, Nanoscale, 2012, 4, 779 RSC.
- G. Chen, T. Y. Ohulchanskyy, W. C. Law, H. Agren and P. N. Prasad, Nanoscale, 2011, 3, 2003 RSC.
- R. Naccache, F. Vetrone, V. Mahalingam, L. A. Cuccia and J. A. Capobianco, Chem. Mater., 2009, 21, 717 CrossRef CAS.
- K. W. Krämer, D. Biner, G. Frei, H. U. Güdel, M. P. Hehlen and S. R. Lüthi, Chem. Mater., 2004, 16, 1244 CrossRef.
- R. T. Wegh, H. Donker, K. D. Oskam and A. Meijerink, Science, 1999, 283, 663 CrossRef CAS.
- V. Mahalingam, F. Vetrone, R. Naccache, A. Speghini and J. A. Capobianco, Adv. Mater., 2009, 21, 4025 CrossRef CAS.
- J. Wang, F. Wang, J. Xu, Y. Wang, Y. Liu, X. Chen, H. Chen and X. Liu, C. R. Chim., 2010, 13, 731 CrossRef CAS PubMed.
- G. Chen, T. Y. Ohulchanskyy, A. Kachynski, H. Agren and P. N. Prasad, ACS Nano, 2011, 5, 4981 CrossRef CAS PubMed.
- V. Mahalingam, R. Naccache, F. Vetrone and J. A. Capobianco, Chem.–Eur. J., 2009, 15, 9660 CrossRef CAS PubMed.
- Y.-P. Du, Y.-W. Zhang, L.-D. Sun and C.-H. Yan, Dalton Trans., 2009, 8574 RSC.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- F. Wang, J. Wang and X. Liu, Angew. Chem., Int. Ed., 2010, 49, 7456 CrossRef CAS PubMed.
- Q. Su, S. Han, X. Xie, H. Zhu, H. Chen, C.-K. Chen, R.-S. Liu, X. Chen, F. Wang and X. Liu, J. Am. Chem. Soc., 2012, 134, 20849 CrossRef CAS PubMed.
- C. Dong, J. Pichaandi, T. Regier and F. C. J. M. van Veggel, J. Phys. Chem. C, 2011, 115, 15950 CAS.
- J. Park, K. J. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2008, 100, 136406 CrossRef.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef.
- J.-C. Boyer and F. C. J. M. van Veggel, Nanoscale, 2010, 2, 1417 RSC.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CAS.
- X. Ye, J. E. Collins, Y. Kang, J. Chen, D. T. N. Chen, A. G. Yodh and C. B. Murray, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 22430 CrossRef CAS PubMed.
- H. Wang and T. Nann, Nanoscale Res. Lett., 2011, 6, 267 CrossRef PubMed.
- G. Henkelman, A. Arnaldsson and H. Jónsson, Comput. Mater. Sci., 2006, 36, 354 CrossRef PubMed.
- W. Tang, E. Sanville and G. Henkelman, J. Phys.: Condens. Matter, 2009, 21, 084204 CrossRef CAS PubMed.
- R. E. Thoma, G. D. Brunton, R. A. Penneman and T. K. Keenan, Inorg. Chem., 1970, 9, 1096 CrossRef CAS.
- A. V. Goryunov, A. I. Popov, N. M. Khajdukov and P. P. Fedorov, Mater. Res. Bull., 1992, 27, 213 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nr00857j |
| ‡ Present address: Center for Materials Architecturing, Korea Institute of Science and Technology, Hwarangno 14-gil 5, Seongbuk-gu, Seoul 136-791, Republic of Korea. |
| This journal is © The Royal Society of Chemistry 2014 |