Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultrafast and scalable laser liquid synthesis of tin oxide nanotubes and its application in lithium ion batteries†
Zhikun
Liu‡
a,
Zeyuan
Cao‡
c,
Biwei
Deng
a,
Yuefeng
Wang
b,
Jiayi
Shao
b,
Prashant
Kumar
a,
C. Richard
Liu
a,
Bingqing
Wei
*c and
Gary J.
Cheng
*ab
aIndustrial Engineering, Purdue University, West Lafayette, Indiana 47907, USA. E-mail: gjcheng@purdue.edu; weib@udel.edu
bBirck Nanotechnology Centre, Purdue University, West Lafayette, Indiana 47907, USA
cDepartment of Mechanical Engineering, University of Delaware, Newark, DE 19716, USA
First published on 21st February 2014
Abstract
Laser-induced photo-chemical synthesis of SnO2 nanotubes has been demonstrated by employing a nanoporous polycarbonate membrane as a template. The SnO2 nanotube diameter can be controlled by the nanoporous template while the nanotube length can be tuned by laser parameters and reaction duration. The microstructure characterization of the nanotubes indicates that they consist of mesoporous structures with sub 5 nm size nanocrystals connected by the twinning structure. The application of SnO2 nanotubes as an anode material in lithium ion batteries has also been explored, and they exhibited high capacity and excellent cyclic stability. The laser based emerging technique for scalable production of crystalline metal oxide nanotubes in a matter of seconds is remarkable. The compliance of the laser based technique with the existing technologies would lead to mass production of novel nanomaterials that would be suitable for several emerging applications.
Production of nanomaterials on a large scale and at a fast pace is a current demand as nanotechnology based industries are flourishing and adding new production lines. For better device functionality, one needs to have nanostructures with better spatial uniformity in size, high crystallinity and integration. Most of the presently available techniques employed for the synthesis of nanowires involve multiple steps and are time consuming. Therefore, novel approaches to satisfy the needs of emerging nano-devices are being developed and so is the scalable production of the device components.
Lithium ion batteries1–6 are attracting more attention from industry because of their high energy density and compactness. Tin oxide (SnO2) nanotubes, as a promising anode material for lithium ion batteries,7–10 can be synthesized via either chemical or physical approaches.11–18 However, the dimension control, purity and scalability are still issues yet to be addressed. Precision nanomanufacturing of tin oxide nanotubes would therefore be a crucial step towards their energy applications. Lasers are capable of delivering high energy density in an instantaneous manner and have earlier been exploited for the pursuit of material synthesis,19–28 which makes them an appropriate and suitable choice for on-demand, ultrafast production of nanoarchitectures such as nanowires, nanotubes, nanodots, etc. Laser processing has brought many discoveries and novel approaches in material synthesis with added advantages such as scalability and a fast processing speed.
We hereby demonstrate a laser based approach for the ultrafast photo-chemical synthesis of tin oxide nanotubes by employing a polycarbonate membrane as a template (Fig. 1(a)). It is worth noting that the present technique is not only limited to tin oxide but is also applicable to many materials, and therefore, this emerging flexible technique would pave the way to nanomanufacture nanostructures of many functional material systems. As an example, the electrochemical performance of the SnO2 nanotubes synthesized by the laser based photochemical technique has also been investigated, and they exhibit an excellent cyclic stability with high rate capability and a high capacity close to the theoretical value.
The lucrative aspect of the present technique is that it provides the freedom to write lines and patterns of vertically aligned nanowires, simply by irradiating the laser along a desired path onto the polycarbonate membrane template. Such a degree of freedom and scalability would be beneficial for patterned nanowire growth which is usually desired for battery devices. Fig. 1(b) shows one of such laser written tracks. Fig. 1(c) and (d) are FESEM images of the microstructures inside and outside the laser written track, respectively. In brief, Fig. 1(c) shows a dense, uniform, and vertically aligned SnO2 nanotube array on the sputtered gold thin film. This image was captured after the polycarbonate nanoporous template was dissolved in the dichloromethane solvent. The height and diameter of the nanotubes are approximately 4 μm and 450 nm, respectively. The nanotube diameter can conveniently be controlled by the nanopore's diameter in a polycarbonate nanoporous template. The inset in Fig. 1(c) is a plot of average nanotube length vs. laser power. Laser power proves to be a good control parameter for the nanotube length.
Nanotubes fabricated with the present technique have typically thin walls. The TEM image in Fig. 2(a) showing nanotubes with a wall thickness of about 14 nm confirms this. In order to investigate the nanotube formation and to develop a deeper understanding, we have examined the bottom part of the nanotube formed, which would reveal the early stage of growth features of the nanotubes. The selected area electron diffraction (SAED) pattern from the SnO2 nanotubes exhibits diffraction rings corresponding to [110], [101] and [301] crystallographic planes as shown in Fig. 2(a). Fig. 2(b)–(f) show the details of the SnO2 nanotube formation. As is apparent from the HRTEM image of the bottom of the nanotubes, growth of SnO2 nanotubes begins with ultrafine, 2 nm diameter SnO2 nanoparticles (see Fig. 2(b)). Fig. 2(c) depicts the formation of mesopores (2–5 nm in diameter). The image in Fig. 2(d) reveals that the nanoparticles are connected by the twinning structure, which is a typical microstructure at the early stage of nanoparticle coalescence.
When a laser pulse is absorbed by the Au back plate, the irradiated area becomes the heating source for the solution. Since heating at the bottom of the solution generates fluid convection within the channel, the heat transfer of the solution is governed by conduction and convection. Comsol Multiphysics simulation has been carried out which is capable of coupling both heat transfer and laminar flow in solution (conjugate heat transfer mode). Fig. 2(e) is a simulation result showing temperature modulation by a laser pulse at the location 1 μm above the back plate within the channel. The temperature gradually rises up to 390 K within the pulse duration of 400 ns. After the laser pulse is off, the conduction and convection of the fluid cool down the temperature within 200 ns. The cooling rate after the laser pulse is switched off is estimated to be at the scale of 5 × 108 K s−1, which is a very rapid cooling rate.
The synthesis of SnO2 nanotubes in the present technique can easily be understood in terms of laser-induced photo-chemical decomposition of precursors and consequent crystallization. The heating of the solution leads to stronger ionization of water. The exponential increase of concentration of OH− results in the supersaturation of Sn(OH)4. SnO2 is then formed by laser-induced dehydration of Sn(OH)4. The formation of nanocrystalline SnO2 has been confirmed from the diffraction pattern in Fig. 2(a) and the high resolution TEM image in Fig. 2(d).
| Sn4+ + 4(OH)− → Sn(OH)4 | (1) |
 | (2) |
Nucleation and growth occur within a nanosecond time span as the La Mer model29 for the laser-induced crystallization in such a photo-chemical reaction, as demonstrated in Fig. 2(f). It is shown in Fig. 2(f) that nucleation can be controlled by the nitric acid concentration. For example, for a solution containing 180 mM nitric acid, fine nanoparticles of 2 nm diameter formed, while nanoparticles of 3 nm diameter formed when the nitric acid concentration is reduced to 50 mM.
Such laser-induced crystallization of monodispersed ultrafine nanoparticles is very interesting from the crystallization point of view, as the time needed for the crystallization process is only a few hundred nanoseconds. According to La Mer's model, it is necessary to distinguish the nucleation stage and the growth process in timescale in order to achieve a nanocrystal size distribution close to a monodisperse level. As the concentration of hydroxide ions gradually increases above the equilibrium solubility, the nucleation of Sn(OH)4 bursts out when the supersaturation achieves a definite value. Let Cnucleation be the critical concentration of the OH− to trigger nucleation. As soon as the laser is switched off, within a short duration, the nucleation process commences and the temperature quickly drops. At the concentration below Cnucleation, nuclei formation would not be favored. Now, if the duration of nucleation is minimal, monodispersed nanoparticles can be achieved.30
After Region II, the crystal growth would proceed until the concentration of growth species has reduced to the equilibrium concentration Cgrowth. Further reduction of the OH− ions into Region IV brings the particle growth to a halt. The duration of time between the commencement of nucleation and the finish point for crystal growth is denoted as τ. The duration τ of the photochemical process in the present technique is estimated to be at the scale of 10−7 s. A smaller τ value generally favors ultrafine monodispersed nanocrystal growth. In addition, usually the fast cooling rate favors monodispersity in nanoparticle size, even under a wide range of activation energy of crystallization. The cooling rate in the present case of laser-induced photo-chemical transformation is estimated to be at the scale of 5 × 108 K s−1. Also, it should be noted that majority of the laser energy is utilized in photochemical reactions and therefore, residual laser power is not able to give rise to a thermal rise. In addition, an aqueous background does not allow laser pulses to create abnormal thermal spike and helps maintain a low background temperature. This is in contrast to usual laser crystallization techniques where the material undergoes a thermal spike and a moderate cooling rate, which give rise to larger crystals.31
Thus, the present photo-chemical technique which yields monodisperse ultrafine nanocrystals relies on the deterministic factors: (a) adequate concentration of growth species, (b) marginalized OH− ion concentration, (c) low background temperature due to liquid ambient, and (d) supercooling. All of these deterministic factors lead to spontaneous crystal growth with negligible duration and yield monodisperse ultrafine nanocrystals when a particular set of the above factors are created. If the reaction is forced to halt at the moment close to the commencement, one would achieve such monodisperse ultrafine nanocrystals. It has to be noted that the size of the SnO2 nanocrystals achieved in the present work is one of the thinnest nanosized-SnO2 ever synthesized. From materials point of view, such ultrafine quantum confined semiconducting nanodots (quantum dots) would exhibit fascinating and unusual physical behavior.
The present technique to photo-chemically synthesize inorganic nanotubes, employing a nanoporous cylindrical template, presents a novel approach towards fabrication of nanotubes in a scalable manner. The formation of a tubular structure (see Fig. 3(a)) instead of the solid wire structure can be understood by the fluid convection initiated by bubbling. To study the mechanism of the SnO2 nanotube formation, a control experiment has been carried out by heating the entire solution uniformly at 95 °C on a hotplate for 10 min. The SEM image shows very short gel-like columns instead of tubes as shown in Fig. 3(c). The inset of Fig. 3(c) is the diffraction pattern of the short columns. The absence of the ring patterns suggests that the column is composed of amorphous Sn(OH)4. Laser induced local heating thus plays two crucial roles in nanotube formation. The first is the laser-induced convection for the formation of the desired tubular structure and the second is the laser dehydration of Sn(OH)4. As discussed earlier, laser local heating generates temperature gradient in the solution. Hotter solution at the bottom of the channel will float up and is replaced by the cold solution. Fluid convection by the buoyancy difference is simulated using the Comsol Multiphysics and shown in Fig. 3(b) and (d). During the laser pulse, the hot fluid flows upward at the center of the channel while the solution is static at the interface to the polycarbonate membrane because of the non slippery boundary conditions. The convection at the center of the channel will prevent the formation of the columnar structure and retain the tubular structure during the growth. The tubular structure formed by laser-induced photo-chemical synthesis is shown in Fig. 3(a). The relative rigid network of the SnO2 nanocrystals prevents the collapse of the thin-wall tubular structures after the polycarbonate template is dissolved. As shown in Fig. 1(b), we have demonstrated that laser can be employed to write patterns consisting of segments, which are constituted by vertically aligned freestanding SnO2 nanotubes. Such patterned features can find incredible importance in device design and manufacturing.
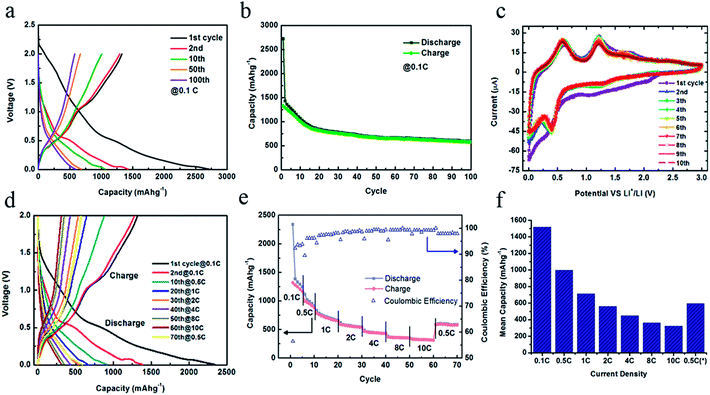
The large surface area inherited from the colloid of SnO2 particles can facilitate the lithium reaction and, therefore, SnO2 nanotubes would act as a nice platform for lithiation. Furthermore, the tubular structures could serve as channels for lithium ion transport to shorten the Li+ diffusion length efficiently and to enhance the charge transfer process along the axial direction. To explore the seemingly advantageous electrochemical aspect of the SnO2 nanotubes, electrochemical measurements were performed. Fig. 4(a) presents the galvanostatic discharge–charge profiles of the SnO2 nanotubes at the rate of 0.1 C for the selected cycles: the 1st, 2nd, 10th, 20th, and 100th cycle. The initial capacity of 2750 mA h g−1 is quite impressive in comparison to the recent literature on lithium ion batteries made up of SnO2/Sn systems32 and SnO2 nanotubes.33 It is also noted from Fig. 4(a) that the capacity sharply drops to 1400 mA h g−1, which is only half of the initial capacity after the first discharge. The irreversible loss of capacity is attributed to the formation of the solid electrolyte interface (SEI) and the decomposition of SnO2 to Sn during the initial cycle. In the beginning the curves retain a similar shape with two distinct short plateaus at around 0.5 and 0.05 V corresponding to phase transition processes during Li+ insertion, which would be clarified in the following cyclic voltammetry (CV) analysis. Whereas, a variation occurs with the cycles: the plateau gradually disappears and the curves become a line with a steepened slope. It is indicative of a typical solid solution reaction by Li+ insertion.
The cycling performance of 100 cycles at 0.1 C (Fig. 4(b)) shows that the capacity decreases linearly with a slope of 30 mA h g−1 per cycle in the first 20 cycles except initial discharge and evolves in a steady state since the 20th cycle. The capacity retains about 600 mA h g−1 at the end of the 100th cycle while it was 818 mA h g−1 at the 20th cycle. The capacity degradation rate is about 2.75 mA h g−1 per cycle during the last eighty cycles. It is evident that the capacity and cyclic stability are improved exceptionally well as compared with the commercial SnO2 nanopowders that usually fade quickly within 50 cycles. The electrochemical impedance spectra (EIS) in Fig. S1 (ESI)† for the four respective cycles (1st, 5th, 25th and 100th cycle) also show similar Nyquist plots comprised of a depressed semicircle at a high frequency attributed to the charge-transfer process at the electrolyte/electrode interface and a linear tail named the Warburg component at low frequencies corresponding to the Li+ diffusion in the solid electrodes. Their knee frequency (intersection between the semicircle and Warburg tail) is at around 15 Hz close to each other and the similar charge-transfer resistances deduced from the radius of the semicircle reveal similar kinetics.
The CV curves in consecutive ten cycles are presented in Fig. 4(c). There are two well-defined cathodic peaks at around 0.05 V and 0.5 V for all the curves except for a broad peak in the range of 0.5–2 V during the first cycle, which comes from the reduction of SnO2 by Li+ to Sn and formation of Li2O in eqn (3). This reaction accounts for the irreversible loss of capacity shown in Fig. 4(b). The peaks at 0.5 V and 0.05 V correspond to the formation of a series of Li–Sn alloys, LixSn as described in eqn (4).
| SnO2 + 4Li+ + 4e− → Sn + 2Li2O | (3) |
| Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4) | (4) |
In the anodic sweep, the two peaks at 0.55 V and 1.25 V are ascribed to the reversed dealloying processes of eqn (4) mainly in two steps. All the four features related to the lithium insertion/extraction in eqn (4) are well overlapped with cycles, indicating the excellent reversible capacity of lithiation. This is in good agreement with the previous results of capacity retention.
We also evaluated the electrochemical performance of the SnO2 nanotubes in terms of rate capability by means of galvanostatic discharge–charge cycling tests at a variety of high rates up to 10 C. The discharge–charge curves (Fig. 4(d)) show that the tendency of variation with cycles becoming a smooth slope is facilitated under the large current densities. Neither plateaus nor inflection on the curve could be observed after the 40th cycle at 4 C. The overall cycling performance at different rates (figure (e)) has a similar trend as was found for the experiment at a constant rate of 0.1 C. When raising the minimum rate of 0.1 C to the maximum 10 C and back to 0.5 C, the corresponding Coulombic efficiency varies from 93% to 99% and returns back to 98%.
The histogram (Fig. 4(f)) visually exhibits the comparison of the average capacity at various rates. The mean value of the capacities in the first five cycles at 0.1 C is 1500 mA h g−1 which is the highest of all. The values for 0.5 C, 1 C, 2 C, 4 C, 8 C and 10 C were observed to be 998 mA h g−1, 715 mA h g−1, 560 mA h g−1, 448 mA h g−1, 361 mA h g−1, and 324 mA h g−1, respectively. The capacity of 324 mA h g−1 at 10 C, which was performed within 6 min. is an encouraging result. In the last 10 cycles back to the low rate of 0.5 C, the capacity recovers to about 600 mA h g−1 on average, further confirming the excellent cyclic stability of the SnO2 nanotubes.
Since the structural integrity throughout the cycling tests ensures the good cyclability, the morphology variation of the SnO2 nanotube electrodes over continuous discharge–charge cycles at 0.1 C has been studied by means of SEM. Although the nanotubes experience deformation obviously with enlarged sizes and curled edges, only a small bunch of tubes are fully collapsed as marked in Fig. S2(a);† and tubes are rarely found cracked (Fig. S2(b)†). Many initially isolated and vertically standing SnO2 nanotubes are aggregated to bundles by the SEI layers wrapped along the tube walls as highlighted in Fig. S2(d).† From the top view of the nanotubes (Fig. S2(c) and (e)),† it is easy to find that the ultrafine nanoparticles are still intact together to constitute the tube walls after cycling. The hollow tubes are filled with the Li–Sn alloy during lithium insertion, which could be seen from the interior of the nanotubes cracked along the generating line of the cylinder in Fig. S2(f).†
In summary, the laser-induced photo-chemical technique to synthesize SnO2 nanotubes has been demonstrated. The diameter of the nanotubes can be tuned with nanoporous template dimensions and the length of the nanotube can be controlled by laser pulse irradiation duration and laser power. The growth of SnO2 nanotubes is catalyzed by laser heated hot zones on a gold back plate. Laser triggered photo-chemical reactions of precursors in an aqueous environment, which yields fast cooling and results in the formation of ultrafine SnO2 nanocrystals. These ultrafine SnO2 nanocrystals assemble themselves in rings and thus in turn nanotubes are built up by vertical alignment of such ultrafine nanocrystals. Heat convection plays an important role by creating favorable experimental conditions. This emerging photo-chemical synthesis of nanotubes can conveniently be generalized to other material systems and nanostructures. The patterning capability of such a laser-based photochemical fabrication technique has also been demonstrated. Instantaneous synthesis of inorganic nanotubes in a scalable manner with the achievement of high quality material crystallinity makes this novel technique very unique. Lithium ion batteries were fabricated using the SnO2 nanotubes synthesized by this emerging technique and electrochemical performance was observed to be better than other SnO2 based nanosystems.
Experimental section
A focused laser beam (Q-switch long-pulse Nd-YAG fiber laser of wavelength 532 nm and pulse width 200 ns) of size 1 mm has been irradiated on a transparent solution (25 mM SnCl4 and 180 mM HNO3) lying above a nanoporous membrane [a polycarbonate nanoporous template with a 400 nm pore size (Whatman® polycarbonate membrane filter, 7 μm thick)], which was back-coated by a 50 nm gold thin film. The experimental set-up for the present approach is shown in Fig. 1(a). The gold thin film absorbs the laser energy, heats up a certain amount of chemical solution and thereby initiates the chemical reaction needed for the growth of the SnO2 nanotubes. The laser can cover millions of nanopores in a single scan. When the growth of nanotubes finished, a 1 μm thick layer of copper was electrodeposited on the top of the Au thin film to provide the required mechanical support. The polycarbonate nanoporous template is then dissolved in dichloroethane and vertically aligned SnO2 nanotubes are obtained. Field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) were employed for characterizing the nanoshapes formed. Comsol Multiphysics simulation has been carried out to investigate and understand the nanotube growth process. To be specific, two different simulations have been performed namely, (a) thermal profiling of the photo-chemical process in time scale, which has yielded SnO2 nanotubes and (b) convection of heat inside the interior space in the nanoporous template, with and without flow. The electrochemical measurements were performed in a coin cell configuration consisting of the as-prepared SnO2 nanotubes without any post-treatment as the working electrode and metallic Li as the counter and reference electrode. Celgard 2500 were chosen as separators and 1 M LiPF6 dissolved in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ethylene carbonate (EC)
1 v/v ethylene carbonate (EC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diethyl carbonate (DEC) was used as the electrolyte obtained from Ferro Co. Cyclic voltammetry (CV) measurements and electrochemical impedance spectra (EIS) were collected by using a PARSTAT 2273 (Princeton Applied Research) potentiostat/galvanostat. CV was carried out at a scan rate of 0.1 mV s−1 in the potential window of 0–3 V to identify the electrochemical reactions with lithium during cycling. EIS was performed with a 10 mV AC signal from 100 kHz to 10 mHz. The galvanostatic discharge–charge tests were carried out using BT-4 4-channel battery test equipment (Arbin Instrument, Ltd.).
diethyl carbonate (DEC) was used as the electrolyte obtained from Ferro Co. Cyclic voltammetry (CV) measurements and electrochemical impedance spectra (EIS) were collected by using a PARSTAT 2273 (Princeton Applied Research) potentiostat/galvanostat. CV was carried out at a scan rate of 0.1 mV s−1 in the potential window of 0–3 V to identify the electrochemical reactions with lithium during cycling. EIS was performed with a 10 mV AC signal from 100 kHz to 10 mHz. The galvanostatic discharge–charge tests were carried out using BT-4 4-channel battery test equipment (Arbin Instrument, Ltd.).
Acknowledgements
Authors would like to acknowledge financial support from the Purdue Office of Vice President for Research. BQW gratefully acknowledges the financial support from the US National Science Foundation (NSF) under the contract of 1067947.References
- J.-M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef PubMed.
- B. Scrosati, Electrochim. Acta, 2000, 45, 2461–2466 CrossRef CAS.
- N. Li, C. R. Martin and B. Scrosati, J. Power Sources, 2001, 97, 240–243 CrossRef.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J.-M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- B. Scrosati, J. Hassoun and Y.-K. Sun, Energy Environ. Sci., 2011, 4, 3287–3295 CAS.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1397 CrossRef CAS.
- F. Belliard, P. A. Connor and J. T. S. Irvine, Solid State Ionics, 2000, 135, 163–167 CrossRef CAS.
- J. Y. Huang, L. Zhong, C. M. Wang, J. P. Sullivan, W. Xu, L. Q. Zhang, S. X. Mao, N. S. Hudak, X. H. Liu, A. Subramanian, H. Fan, L. Qi, A. Kushima and J. Li, Science, 2010, 330, 1515–1520 CrossRef CAS PubMed.
- M.-S. Park, G.-X. Wang, Y.-M. Kang, D. Wexler, S.-X. Dou and H.-K. Liu, Angew. Chem., Int. Ed., 2007, 119, 764–767 CrossRef.
- Y. Wang, X. Jiang and Y. Xia, J. Am. Chem. Soc., 2003, 125, 16176–16177 CrossRef CAS PubMed.
- P. Meduri, C. Pendyala, V. Kumar, G. U. Sumanasekera and M. K. Sunkara, Nano Lett., 2009, 9, 612–616 CrossRef CAS PubMed.
- J. Q. Hu, X. L. Ma, N. G. Shang, Z. Y. Xie, N. B. Wong, C. S. Lee and S. T. Lee, J. Phys. Chem. B, 2002, 106, 3823–3826 CrossRef CAS.
- Z. R. Dai, J. L. Gole, J. D. Stout and Z. L. Wang, J. Phys. Chem. B, 2002, 106, 1274–1279 CrossRef CAS.
- X. Jiang, Y. Wang, T. Herricks and Y. Xia, J. Mater. Chem., 2004, 14, 695–703 RSC.
- S. Luo, J. Fan, W. Liu, M. Zhang, Z. Song, C. Lin, X. Wu and P. K. Chu, Nanotechnology, 2006, 17, 1695–1699 CrossRef CAS.
- P. Parthangal, R. E. Cavicchi, D. C. Meier, A. Herzing and M. R. Zachariah, J. Mater. Res., 2011, 26, 430–436 CrossRef CAS.
- G. Sberveglieri, I. Concina, E. Comini, M. Falasconi, M. Ferroni and V. Sberveglieri, Vacuum, 2012, 6, 532–535 CrossRef PubMed.
- A. M. Morales and C. M. Lieber, Science, 1998, 279, 208–211 CrossRef CAS.
- D. H. Lowndes, D. B. Geohegan, A. A. Puretzky, D. P. Norton and C. M. Rouleau, Science, 1996, 273, 898–903 CAS.
- A. E. Espinal, L. Zhang, C.-H. Chen, A. Morey, Y. Nie, L. Espinal, B. O. Wells, R. Joesten, M. Aindow and S. L. Suib, Nat. Mater., 2010, 9, 54–59 CrossRef CAS PubMed.
- Z. Liu, D. Zhang, S. Han, C. Li, T. Tang, W. Jin, X. Liu, B. Lei and C. Zhou, Adv. Mater., 2003, 15, 1754–1757 CrossRef CAS.
- V. V. Osipov, Y. A. Kotov, M. G. Ivanov, O. M. Samatov, V. V. Lisenkov, V. V. Platonov, A. M. Murzakaev, A. I. Medvedev and E. I. Azarkevich, Laser Phys., 2006, 16, 116–125 CrossRef CAS.
- F. Kim, J. H. Song and P. Yang, J. Am. Chem. Soc., 2002, 124, 14316–14317 CrossRef CAS PubMed.
- M. Sakamoto, M. Fujistuka and T. Majima, J. Photochem. Photobiol., C, 2009, 10, 33–56 CrossRef CAS PubMed.
- V. S. Letokhov, Appl. Phys. B, 1988, 46, 237–251 CrossRef.
- P. Kumar, RSC Adv., 2013, 3, 11987–12002 RSC.
- S. Besner and M. Meunier, Laser Synthesis of Nanomaterials, in Laser Precision Microfabrication, ed. K. Sugioka, et al., Springer, Series in Materials Science, 135, DOI:10.1007/978-3-642-10523-4_7, 2010.
- V. K. LaMer, J. Am. Chem. Soc., 1950, 72, 4847–4854 CrossRef CAS.
- G. Cao, in Nanostructures and Nanomaterials, Synthesis, Properties and Applications, Imperial College Press, London, 2004, p. 53, ISBN 1-86094-480-9 Search PubMed.
- P. Kumar and M. G. Krishna, Phys. Status Solidi A, 2009, 207, 947–954 CrossRef.
- K. Kravchyk, L. Protesescu, M. I. Bodnarchuk, F. Krumeich, M. Yarema, M. Walter, C. Guntlin and M. V. Kovalenko, J. Am. Chem. Soc., 2013, 135, 4199–4202 CrossRef CAS PubMed.
- J. Ye, H. Zhang, R. Yang, X. Li and L. Qi, Small, 2010, 6, 296–306 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nr06444a |
| ‡ Both authors contributed equally to the paper. |
| This journal is © The Royal Society of Chemistry 2014 |