Ratiometric fluorescent pH probes based on aggregation-induced emission-active salicylaldehyde azines†
Xiaofeng
Ma
,
Jinghui
Cheng
,
Jiaoyan
Liu
,
Xiangge
Zhou
and
Haifeng
Xiang
*
College of Chemistry, Sichuan University, Chengdu, 610041, China. E-mail: xianghaifeng@scu.edu.cn; Fax: +86 28-8541-2291
First published on 31st October 2014
Abstract
A series of luminescent salicylaldehyde azines (SAs) containing different electron-accepting substituents (–NO2, –F, and –Cl), electron-donating substituents (–OMe and –NEt2), and a π-extended system (naphthalene ring) are prepared for the application of fluorescent pH probes. These SAs inheriting the aggregation-induced emission (AIE) features display strong blue, green, and red fluorescence with large Stokes shifts in water and solid medium. Combining the advantages of AIE and the chemical reactivity of phenol towards OH−/H+, most of the SAs can be used as ratiometric fluorescent pH probes with a broad pH range (2–14) in water and solid medium (test paper). Moreover, the inherent relationship between their chemical structures and AIE properties/pKa values (7.5–13.3) is studied, which provides unequivocal insights into the design of AIE-active dyes and their applications.
Introduction
Protons are some of the most important targets of interest, because many biological and geochemical processes occurring in freshwater, seawater, and marine sediments involve strong pH changes. Accordingly, the recognition and detection of pH values have been an especially active research area. The most traditional pH measurement is the glass electrode, however, it has some obvious disadvantages including single point measurements and bulky and invasive properties for bio-applications.1 Recently, fluorescent pH probes have attracted considerable interest, because fluorescence detection allows the noninvasive measurement of biological objects, parallel monitoring of multiple samples, and imaging.2 The most common fluorescence detection method is the measurement of fluorescence intensity, but, in most cases, its determination accuracy is frequently compromised by ambient or scattered light, instrumental fluctuation, and background fluorescence. Therefore, ratiometric fluorescence measurement is more desired.3 Moreover, it usually provides the perceived colour change, which would be useful for rapid visual sensing. In general, most of the reported ratiometric fluorescent pH probes employ a mixture of different fluorescent dyes4 or a single fluorescent dye containing different multiple pH-sensitive segments.1,5 Both of them might have some shortcomings, such as unequal stability, reliability, and photobleaching of the different dyes or segments. Another simpler and more efficient method is to design and synthesize a single fluorescent dye that has only one kind of pH-sensitive segment that can emit light at different wavelengths after deprotonation or protonation. Up to now, this kind of probe is much scarcer,6 because a fluorescent dye containing only one kind of pH-sensitive segment usually would tend to quench or enhance fluorescence, without ratiometric fluorescence after deprotonation or protonation.For pH detection applications, the preferred work conditions are aqueous media, but it is incongruous that most organic fluorescent dyes are hydrophobic aromatics that are barely soluble in water. Introduction of hydrophilic groups would improve their aqueous solubility, meanwhile, the resulting amphiphilic dyes would be ready to aggregate in water and lead to fluorescence quenching by the aggregation-caused quenching (ACQ) effect. Tang et al. recently discovered an exactly opposite phenomenon of aggregation-induced emission (AIE).7 Since then a large number of AIE-active dyes have been developed and used in organic light-emitting diodes, bio/chemosensors, bioimaging, and so on.8
Our previous work focused on the synthesis, photophysical properties, and sensing applications of salicylaldehyde-based Salen Schiff bases (Scheme 1).9 Most of these Salen Schiff bases usually exhibit much stronger fluorescence in organic solution than in solid medium or water, indicating the possible existence of ACQ. On the other hand, salicylaldehyde azines (SAs) (Scheme 1) explored by Tong et al. have similar synthetic processes and chemical structures to Salen Schiff bases but exhibit AIE effects.10 Unlike the most common AIE-active dyes of tetraphenylethene (TPE), SAs have some unique properties: (1) two salicylaldimine moieties are connected by a rotatable N–N single bond rather than the C–C bond; (2) the rotations are restricted in the N–N bond only by intramolecular hydrogen bonds;10a (3) the intramolecular hydrogen bonds lead to keto and enol tautomers for SAs through an ultrafast excited-state intramolecular-proton transfer (ESIPT) process.11 Furthermore, SAs have been used as fluorescent probes for the detection of Zn2+,12 Co2+,12b Cu2+, Fe3+,13 F−,14 CN−,15 protein,16 and heparin.17 At present, there are only limited examples of the AIE-active dyes for fluorescent pH sensing applications in the literature.5a,18 In this work, we have systematically investigated the fluorescent pH sensing properties of a series of AIE-active SAs containing different electron-accepting (EA) substituents (–NO2, –F, and –Cl), electron-donating (ED) substituents (–OMe and –NEt2), and a π-extended system (naphthalene ring) (Scheme 1), which provide a new paradigm in the design of ratiometric fluorescent pH probes. The photophysical properties of most SAs have not been examined and 3-F has not been reported.
Results and discussion
Synthesis and characterization
The general method of preparation of SAs is quite straightforward and consists of the condensation reaction of primary hydrazine with 2 equiv. of the salicylaldehyde precursor in ethanol under refluxing conditions, according to the previous report.19 Most SAs have good solubility in organic solvents including DMSO and MeCN but poor solubility in water.Photophysical and AIE properties
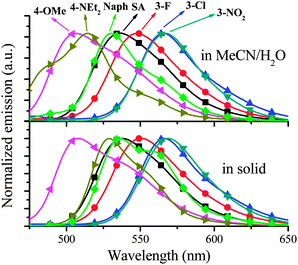
Among these SAs, the photophysical properties of SA,10a4-NEt2,20 and Naph12b have been examined and reported. As shown in Fig. 1 and Table 1, except 3-OMe and 4-NEt2, all other SAs exhibit obvious AIE effects hence the fluorescence in organic solvent is much weaker than that in solid medium or water. For example, the absorption and emission spectra of SA are depicted in Fig. 2. At room temperature, the dilute solution of SA in MeCN emits very weak fluorescence (Fig. 2a) with a large Stokes shift and low quantum yield (Φ of 0.002, which might be contributed to the rotation of the N–N single bond and the ESIPT process).11 This ESIPT process is further confirmed by the pronounced solvatochromic effect (Fig. S1 in the ESI†). However, if water was added to MeCN, the aggregate form of SA will be generated to eliminate the above effects, which results in AIE with a much higher Φ of 0.11 at a volume fraction (f) of water of 92% (Fig. 2b). Moreover, absorption spectrum of SA in MeCN–water (f = 92%) shows an obvious tail in the visible region, indicating the formation of aggregate nanoparticles.10a | ||
| Fig. 1 Photographs (top: under sunlight; bottom: under 360 nm UV light) of the SAs in solution (left: MeCN; right: MeCN–water) and solid medium. | ||
| Medium | λ abs/nm (ε/dm3 mol−1 cm−1) | λ em/nm | Φ | pKa | f/% | |
|---|---|---|---|---|---|---|
| a Nonemissive. b From ref. 20a. | ||||||
| SA | MeCN | 292 (2.53 × 104); 354 (2.15 × 104) | 509 | 0.002 | ||
| Water | 296; 400 | 536 | 0.11 | 9.3 | 92 | |
| Solid | 537 | |||||
| 3-NO2 | MeCN | 270 (2.19 × 104); 364 (1.29 × 104); 504 (9.50 × 103) | 543 | 0.004 | ||
| Water | 395 | 565 | 0.04 | 7.5 | 90 | |
| Solid | 566 | |||||
| 3-F | MeCN | 300 (3.76 × 104); 350 (2.11 × 104) | 518 | 0.002 | ||
| Water | 402 | 547 | 0.05 | 8.2 | 92 | |
| Solid | 550 | |||||
| 3-Cl | MeCN | 302 (3.01 × 104); 354 (1.97 × 104) | 513 | 0.003 | ||
| Water | 302; 398 | 564 | 0.13 | 8.5 | 94 | |
| Solid | 565 | |||||
| 3-OMe | MeCN | 302 (3.01 × 104); 354 (1.97 × 104) | 406 | 0.004 | ||
| Water | 316; 408 | —a | —a | —a | — | |
| Solid | —a | |||||
| 4-OMe | MeCN | 308 (1.55 × 104); 362 (4.71 × 104) | 488 | 0.003 | ||
| Water | 300; 340 | 507 | 0.16 | 9.5 | 92 | |
| Solid | 508 | |||||
| 4-NEt2 | MeCN | 416 (8.07 × 104); 432 (7.59 × 104) | 480 | 0.13 | ||
| Water | 408; 426; 456 | 513 | 0.10 | 13.0 | 90 | |
| Solid | 529 | 0.30b | ||||
| Naph | MeCN | 330 (1.51 × 104); 406 (3.26 × 104); 424 (2.74 × 104) | 510 | 0.004 | ||
| Water | 334; 428; 462 | 530 | 0.18 | 13.3 | 80 | |
| Solid | 536 | |||||
The absorption and emission spectra of other SAs in MeCN are shown in Fig. S2 and S3 (ESI†). Since, except 4-NEt2, all other SAs in MeCN have very weak fluorescence (Table 1), the discussion will be focused on their AIE characteristics. As shown in Fig. 3, for aggregate SAs in MeCN–water and solid medium, different substituents have different effects on the emission peak (λem). Compared with the simplest SA, 4-OMe, 4-NEt2, and Naph containing ED substituents or a π-extended system show blue-shifted emission, in contrast, 3-F, 3-Cl, and 3-NO2 containing EA substituents display red-shifted emission. This substituent effect is a simple and useful tool to achieve red, green, and blue emission (Fig. 1).
It is interesting that only the 3-OMe aggregate is nonemissive. The molecular arrangements play a key role in AIE. In order to achieve high Φ, AIE-active dyes should stack closely with a short interplanar distance (d) and weak intermolecular face-to-face π–π interactions. The former can ensure the elimination of molecular rotation, the latter would prevent the formation of an excimer and consequently enhance fluorescence.8c,20 In order to investigate the molecular arrangements, the X-ray single crystals of SA, 4-OMe and 3-OMe are depicted in Fig. 4, Fig. S4 and S5 (ESI†). All atoms in SA are located in one plane of the π-conjugated system (Fig. 4a). As expected, the intramolecular hydrogen bonds (1.90 Å) between the H atom in –OH and the N atom are found in SA. Moreover, rod-like SA molecules are cross stacking with a short d of ∼3.45 Å (Fig. 4c). The above two factors ensure the elimination of the rotation of N–N and C–C single bonds, thereby resulting in AIE. However, it should be noted that there still are weak intermolecular face-to-face π–π interactions (overlaps in π-conjugated systems) between two neighbouring SA molecules (Fig. 4b), which might be one possible reason for its moderate Φ (0.11). 4-OMe molecules have a similar d (∼3.46 Å) (Fig. 4e) but weaker face-to-face π–π interactions (Fig. 4d), and thus 4-OMe has a higher Φ (0.16) than SA. On the other hand, 3-OMe molecules have a shorter d (∼3.30 Å) (Fig. 4g) but much stronger face-to-face π–π interactions (Fig. 4f), resulting in their weak fluorescence.
Another interesting phenomenon is the fact that only 4-NEt2 displays strong fluorescence in both dilute MeCN and aggregate states. In the previous work,20b the strong fluorescence in solution was attributed to the intramolecular charge transfer (ICT) between the D–A system21 of the strong ED –NEt2 substituent and the EA salicylaldimine moiety. However, this cannot explain the fact that 4-OMe has strong ED substituents of –OMe as well, but it exhibits weak fluorescence in dilute MeCN. It was reported that 4-NEt2 powder has two distinctive crystalline lattices with different λem at 553 (d = 3.53 Å) and 529 nm (d = 6.32 Å),20a which are consistent with our results. The –NEt2 substituents in 4-NEt2 are much bulkier than the –OMe substituents in 4-OMe, which might lead to reducing self-quenching and consequently strong fluorescence in both solution and solid medium for 4-NEt2.
As shown in Table 1, Naph has the highest Φ of 0.18 in MeCN–water. Naph has a similar molecular arrangement to SA (Fig. 5). The d (∼3.10 Å) (Fig. 5c) of Naph molecules is the smallest. Moreover, unlike 3-OMe molecules, Naph molecules almost have no face-to-face π–π interactions (Fig. 5b). Therefore, in order to enhance AIE, d should be short and face-to-face π–π interactions should be weak, even though sometimes they are contradictory. The best way to solve such a problem is to adopt cross molecular stacking like Naph. The above discussion provides unequivocal insights into the inherent relationship between AIE properties and chemical structures, which is a useful tool for the design of AIE-active dyes.
 | ||
| Fig. 5 X-ray single crystal structures of Naph ((a) molecular arrangement; (b) top view; (c) side view). | ||
Fluorescent pH probes
It is well-known that phenol is a weak acid with a pKa of about 10.22 Our previous work9d demonstrated that salicylaldehyde derivatives can be used as fluorescent pH probes, however, the measurement is not ratiometric fluorescence detection but turn-on or turn-off fluorescence detection.The absorbance and fluorescence intensity of these SAs highly depend on the pH values of the solution, and thus they can be used as pH probes. The pH responses were measured in the mixed solvents of MeCN/aqueous Britton–Robinson (B–R) buffer solution (a mixture of 0.04 mol L−1 H3BO3, H3PO4 and CH3COOH in water). The concentration of SAs is 1.0 × 10−5 mol dm−3 and the f values are listed in Table 1. The absorption (Fig. S6, ESI†) and emission (Fig. 6) properties of 3-Cl are strongly dependent on pH values. The red emission intensity at 565 nm (I565) that belongs to the protonated 3-Cl remains constant at pH < 7.0. And then it reduces along with the increase of pH values from 7.0 to 11.0 and finally remains constant again at pH > 11.0. At the same time, blue-green I515 belonging to the deprotonated 3-Cl has a totally adverse pH response, which is zero, increased, and constant at pH <7.0, 7.0–11.0, >11.0, respectively (Fig. 6b). Moreover, the ratio of I515 to I565 exhibits adverse pH responses with I565 (Fig. 6c). Therefore, 3-Cl provides not only a ratiometric method of detection but also a perceived colour change (red to blue-green) for rapid visual sensing (Fig. 6a).
Among these SAs, 3-Cl, SA, 3-F, and 4-OMe exhibit ratiometric fluorescence responses to pH values (Fig. 7). Since the λem difference (Δλem = 50 nm) between protonated (λem = 565 nm) and deprotonated 3-Cl (λem = 515 nm) is bigger than that of SA (Δλem = 23 nm, Fig. S7, ESI†), 3-F (Δλem = 38 nm, Fig. S8, ESI†), and 4-OMe (Δλem = 16 nm, Fig. S9, ESI†), 3-Cl is more suitable for ratiometric detection.
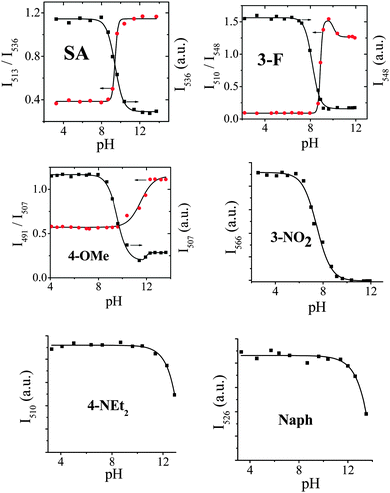 | ||
| Fig. 7 Emission intensity versus pH value in B–R buffer solution for SAs (1.0 × 10−5 mol dm−3 and f values are listed in Table 1; black and red points for intensity and ratiometric measurements, respectively). | ||
3-OMe has too weak fluorescence to be used as a fluorescent probe. 3-NO2, 4-NEt2 (Fig. 8), and Naph (Fig. S10, ESI†) serve as turn-off fluorescent pH probes but not ratiometric fluorescent probes (Fig. 7), because they are nonemissive after deprotonation. For example, the I566 of 3-NO2 remains constant, decreasing, and quenching totally at pH <5.1, 5.1–10, >10, respectively, revealing that the pKa of 3-NO2 is 7.5 (Fig. 8). It is worthwhile that the fluorescence of 4-NEt2 and Naph remains constant at pH 3–10 and 3–11, respectively, and then decreases upon alkalinity increase, which indicates that they can be used as fluorescent pH probes even at very high pH values. In the literature, such fluorescent probes for the detection of high pH values are still scarce. The pH values of the different subcellular compartments are 4.7–8. The pKa values of the SAs are 7.5–13.3. And thus some of them can be potentially used in intracellular pH sensing. The pH probes with a high pKa of 9–12 might be used in some special applications, such as wastewater treatment.
 | ||
| Fig. 8 Emission spectra of 3-NO2 and 4-NEt2 at different pH values in B–R buffer solution (1.0 × 10−5 mol dm−3 and f = 90%). | ||
It is well-known that the pKa of phenol is about 10.22SA (pKa = 9.3) in the presence of an EA salicylaldimine moiety is more acidic than phenol, because the inductive effect of the EA moiety reduces the electron density of the benzene ring, resulting in the negative charge of phenolic hydroxyl being delocalized into the benzene ring. Consequently, the resultant phenolate anion is more stable and readily releases a proton. On the other hand, ED substituents have an opposite effect to decrease the acidity.
Except 3-OMe, the pKa values (7.5–13.3) of these SAs (Table 1) were calculated by the fluorescence method. A tendency of pKa is observed in the order of 3-NO2 (pKa = 7.5) < 3-F (pKa = 8.2) < 3-Cl (pKa = 8.5) < SA (pKa = 9.3) < 4-OMe (pKa = 9.5) < 4-NEt2 (pKa = 13.0) < Naph (pKa = 13.3), which is consistent with the fact that EA and ED substituents cause the acidity to increase and decrease, respectively, as previously mentioned. Understanding this inherent relationship between chemical structures and pKa values would be helpful to design pH probes.
Our previous work demonstrated a salicylaldehyde-based Salen Schiff base that has a similar chemical structure to SA showing multiple different pH-sensitive segments including –OH and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.9d For SA, as expected, adding a base would lead to the deprotonation of –OH, which can be confirmed by the 1H nuclear magnetic resonance (1H NMR) analysis (Fig. 9). The 1H NMR signals of SA (δ = 11.15 ppm) in (CD3)2SO belonging to the –OH disappear after adding base. In contrast, there is no obvious change in the 1H NMR signals if an acid was added, which is consistent with the fact that the emission spectra of SA show little change upon adding acid. These results reveal that, in our case, SA has only one type of pH-sensitive segment (–OH) to achieve ratiometric detection.
C.9d For SA, as expected, adding a base would lead to the deprotonation of –OH, which can be confirmed by the 1H nuclear magnetic resonance (1H NMR) analysis (Fig. 9). The 1H NMR signals of SA (δ = 11.15 ppm) in (CD3)2SO belonging to the –OH disappear after adding base. In contrast, there is no obvious change in the 1H NMR signals if an acid was added, which is consistent with the fact that the emission spectra of SA show little change upon adding acid. These results reveal that, in our case, SA has only one type of pH-sensitive segment (–OH) to achieve ratiometric detection.
 | ||
| Fig. 9 1H NMR spectra of SA (bottom) and deprotonated SA (top, adding 2 equiv. of NaOH in D2O) in (CD3)2SO. | ||
The previous work10a reported that SA molecules aggregate in water to form nanoparticles (<5 nm), which reveals that SA molecules are not dissolved but dispersed in water. One of the problems of these nanoparticles without any surface protective agents is that the dispersed nanoparticles are not stable and would precipitate through further aggregation. Therefore, the dynamic stability of 3-Cl nanoparticles in MeCN–water was examined (Fig. 10). After adding water to the MeCN solution of 3-Cl, the emission of 3-Cl reaches saturation (>95%) in 15 minutes, indicating that 3-Cl molecules aggregate to form nanoparticles very quick. Moreover, the nanoparticles are stable at least for 150 minutes, which is enough for sensing applications.
pH test paper
One of the best advantages of AIE-active dyes is the nature of strong fluorescence in solid medium, and thus we tried to use 3-Cl as a pH test paper. The homemade test papers were fabricated by dipping filter papers into the MeCN solution of 3-Cl. When the solvent evaporates, the adsorbed 3-Cl molecules in the test paper aggregate to emit strong red fluorescence like that in MeCN–water solutions (Fig. 11). This aggregation is also confirmed by the fact that the red fluorescence does not distribute uniformly in the test paper under UV irradiation. When the test papers are dipped into aqueous B–R buffer solution with different pH values, they exhibit different color changes under room light and 360 nm UV light, respectively. Under room light, the color of the test papers changes from white to yellow at high pH values, which accords with the absorption enhancement at high pH values (Fig. S6, ESI†). Under UV irradiation, the test papers undergo a much more obvious color change. The test papers display red, yellow, and green fluorescence at pH values of 6–9, 10–11, and 12–14, respectively. It should be noted that the test papers emit green fluorescence at high pH values, under the same conditions, however, the MeCN–water solutions display blue-green fluorescence (Fig. 6a). At high pH values, 3-Cl would be deprotonated to form phenolate consequently, which has better solubility and a less AIE effect than protonated 3-Cl in water. This less AIE effect coincides with the fact that the green fluorescence from test papers containing deprotonated 3-Cl is more uniform (Fig. 11). Since deprotonated 3-Cl is a traditional organic dye rather than an AIE-active dye, its fluorescence in solid medium is red-shifted compared with that in the dilute solution. | ||
| Fig. 11 Colour changes (top: under room light; bottom: under 360 nm UV light) of homemade 3-Cl test papers at different pH values. | ||
Conclusion
We have systematically synthesized and studied the photophysical properties and pH sensing applications of a series of salicylaldehyde azines. The chemical structures of these salicylaldehyde azines have a significant influence on their molecular arrangements and consequently aggregation-induced emission properties. Strong cross stacking of molecular arrangement with a small d and weak face-to-face π–π interactions is a key factor to enhance aggregation-induced emission. 3-Cl, 3-F, and 4-OMe exhibit aggregation-induced emission at low pH values, while they become water soluble and show blue-shifted fluorescence at high pH values, and thus they can be used as ratiometric fluorescent pH probes with a broad pH range in water and solid medium (test paper). Moreover, their pKa values ranging from 7.5 to 13.3 can be varied and well tuned by their chemical structures. Therefore, we believe that these simple salicylaldehyde azines provide a new paradigm in the design of aggregation-induced emission-active dyes for some useful sensing applications.Experimental section
Materials and instrumentation
All reagents were purchased from commercial suppliers and used without further purification. UV/visible absorption spectra were recorded using a UV 765 spectrophotometer with quartz cuvettes of 1 cm pathlength. Fluorescence spectra were obtained using an F-7000 Fluorescence spectrophotometer (Hitachi) at room temperature. The slit width was 2.5 or 5.0 nm for both excitation and emission. The photomultiplier voltage was 400 V. All SAs were prepared according to the previous reports.19 The X-ray structures of SA,23a 3-OMe,23b 4-OMe,23c and Naph23d were previously reported.Measurement of fluorescence quantum yield (Φ)
Φ was measured by the optical dilute method with a standard of quinine sulfate (Φr = 0.55, quinine in 0.05 mol dm−3 sulfuric acid) calculated by: Φs = Φr(Br/Bs)(ns/nr)2(Ds/Dr), where the subscripts s and r refer to the sample and reference standard solution respectively; n is the refractive index of the solvents; D is the integrated intensity. The excitation intensity B is calculated by B = 1 − 10−AL, where A is the absorbance at the excitation wavelength and L is the optical path length (L = 1 cm in all cases). The refractive indices of the solvents at room temperature are taken from a standard source. Errors for Φ values (±10%) are estimated.Measurement of pH sensing
The pH responses were measured in the mixed solvents of MeCN–aqueous B–R buffer solution (a mixture of 0.04 mol L−1 H3BO3, H3PO4 and CH3COOH in water). The concentration of SAs is 1.0 × 10−5 mol dm−3 and the f values are listed in Table 1. All types of absorption and fluorescence measurements were monitored 1 hour after the addition of the probe to the buffer solution at room temperature.Synthesis
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21172160 and 21372169).Notes and references
- A. S. Vasylevska, A. A. Karasyov, S. M. Borisov and C. Krause, Anal. Bioanal. Chem., 2007, 387, 2131 CrossRef CAS PubMed.
- (a) J. Srivastava, D. L. Barber and M. P. Jacobson, Physiology, 2007, 22, 30 CrossRef CAS PubMed; (b) R. Wang, C. W. Yu, F. B. Yu and L. X. Chen, TrAC, Trends Anal. Chem., 2010, 29, 1004 CrossRef CAS; (c) J. Y. Han and K. Burgess, Chem. Rev., 2010, 110, 2709 CrossRef CAS PubMed.
- Y. Feng, J. H. Cheng, L. Zhou, X. G. Zhou and H. F. Xiang, Analyst, 2012, 137, 4885 RSC.
- (a) C. G. Niu, X. Q. Gui, G. M. Zeng and X. Z. Yuan, Analyst, 2005, 130, 1551 RSC; (b) H. S. Peng, J. A. Stolwijk, L. N. Sun, J. Wegener and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2010, 49, 4246 CrossRef CAS PubMed; (c) S. Wu, Z. Li, J. Han and S. Han, Chem. Commun., 2011, 47, 11276 RSC; (d) D. Aigner, B. Ungerbock, T. Mayr, R. Saf, I. Klimant and S. M. Borisov, J. Mater. Chem. C, 2013, 1, 5685 RSC.
- (a) S. Chen, Y. Hong, Y. Liu, J. Liu, C. W. T. Leung, M. Li, R T. K. Kwok, E. Zhao, J. W. Y. Lam, Y. Yu and B. Z. Tang, Chem. Sci., 2012, 3, 1804 RSC; (b) M. H. Lee, J. H. Han, J. H. Lee, N. Park, R. Kumar, C. Kang and J. S. Kim, Angew. Chem., Int. Ed., 2013, 52, 6206 CrossRef CAS PubMed; (c) G. Men, G. Zhang, C. Liang, H. Liu, B. Yang, Y. Pan, Z. Wang and S. Jiang, Analyst, 2013, 138, 2847 RSC; (d) X. D. Liu, Y. Xu, R. Sun, Y. J. Xu, J. M. Lu and J. F. Ge, Analyst, 2013, 138, 6542 RSC; (e) S. Chen, Y. Hong, Y. Liu, J. Liu, C. W. T. Leung, M. Li, R. T. K. Kwok, E. Zhao, J. W. Y. Lam, Y. Yu and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 4926 CrossRef CAS PubMed; (f) P. Y. Gu, J. Gao, Q. Zhang, G. Liu, F. Zhou, Q. F. Xu and J. M. Lu, J. Mater. Chem. C, 2014, 2, 1539 RSC.
- (a) C. K. Koo, B. Lam, S. K. Leung, M. H. W. Lam and W. Y. Wong, J. Am. Chem. Soc., 2006, 128, 16434 CrossRef CAS PubMed; (b) R. Pal and D. Parker, Chem. Commun., 2007, 474 RSC; (c) J. Wang, Y. Sun, W. Zhang, Y. Liu, X. Yu and N. Zhao, Talanta, 2014, 129, 241 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, B. Z. Tang, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740 RSC.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC; (b) M. Wang, G. Zhang, D. Zhang, D. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858 RSC; (c) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- (a) L. Zhou, P. Y. Cai, Y. Feng, J. H. Cheng, H. F. Xiang, J. Liu, D. Wu and X. G. Zhou, Anal. Chim. Acta, 2012, 735, 96 CrossRef CAS PubMed; (b) L. Zhou, Y. Feng, J. H. Cheng, N. Sun, X. G. Zhou and H. F. Xiang, RSC Adv., 2012, 2, 10529 RSC; (c) J. H. Cheng, K. Y. Wei, X. F. Ma, X. G. Zhou and H. F. Xiang, J. Phys. Chem. C, 2013, 117, 16552 CrossRef CAS; (d) J. H. Cheng, Y. H. Zhang, X. F. Ma, X. G. Zhou and H. F. Xiang, Chem. Commun., 2013, 49, 11791 RSC; (e) J. H. Cheng, X. F. Ma, Y. H. Zhang, J. Y. Liu, X. G. Zhou and H. F. Xiang, Inorg. Chem., 2014, 53, 3210 CrossRef CAS PubMed.
- (a) W. Tang, Y. Xiang and A. Tong, J. Org. Chem., 2009, 74, 2163 CrossRef CAS PubMed; (b) R. Wei, P. Song and A. Tong, J. Phys. Chem. C, 2013, 117, 3467 CrossRef CAS; (c) X. Chen and A. Tong, J. Lumin., 2014, 145, 737 CrossRef CAS; (d) L. Peng, Z. Zhou, R. Wei, K. Li, P. Song and A. Tong, Dyes Pigm., 2014, 108, 24 CrossRef CAS.
- M. Ziolek, M. Gil, J. A. Organer and A. Douhal, Phys. Chem. Chem. Phys., 2010, 12, 2107 RSC.
- (a) D. X. Xie, Z. J. Ran, Z. Jin, X. B. Zhang and D. L. An, Dyes Pigm., 2013, 96, 495 CrossRef CAS; (b) X. Cao, X. Zeng, L. Mu, Y. Chen, R. Wang, Y. Zhang, J. Zhang and G. Wei, Sens. Actuators, B, 2013, 177, 493 CrossRef CAS.
- S. Dalapati, S. Jana, Md. A. Alam and Ni. Guchhait, Sens. Actuators, B, 2011, 160, 1106 CrossRef CAS.
- Q. Li, Y. Guo, Ji. Xu and S. Shao, Sens. Actuators, B, 2011, 158, 427 CrossRef CAS.
- (a) W. T. Gong, Q. L. Zhang, L. Shang, B. Gao and G. L. Ning, Sens. Actuators, B, 2013, 177, 322 CrossRef CAS; (b) P. Zhang, B. B. Sh, X. M. You, Y. M. Zhang, Q. Lin, H. Yao and T. B. Wei, Tetrahedron, 2014, 70, 1889 CrossRef CAS.
- L. Peng, R. Wei, K. Li, Z. Zhou, P. Song and A. Tong, Analyst, 2013, 138, 2068 RSC.
- H. Liu, P. Song, R. Wei, K. Li and A. Tong, Talanta, 2014, 118, 348 CrossRef CAS PubMed.
- (a) Z. Li, Y. Q. Dong, J. W. Y. Lam, J. Sun, A. Qin, M. Hauler, Y. P. Dong, H. H. Y. Sung, I. D. Williams, H. S. Kwok and B. Z. Tang, Adv. Funct. Mater., 2009, 19, 905 CrossRef CAS; (b) Z. Yang, W. Qin, J. W. Y. Lam, S. Chen, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Chem. Sci., 2013, 4, 3725 RSC.
- H. Gorner, S. Khanra, T. Weyhermuller and P. Chaudhuri, J. Phys. Chem. A, 2006, 110, 2587 CrossRef PubMed.
- (a) X. Chen, R. Wei, Y. Xiang, Z. Zhou, K. Li, P. Song and A. Tong, J. Phys. Chem. C, 2011, 115, 14353 CrossRef CAS; (b) S. Jana, S. Dalapati and N. Guchhait, J. Phys. Chem. A, 2012, 116, 10948 CrossRef CAS PubMed.
- H. F. Xiang, J. H. Cheng, X. F. Ma, X. G. Zhou and J. J. Chruma, Chem. Soc. Rev., 2013, 42, 6128 RSC.
- K. Gross and P. G. Seybold, Int. J. Quantum Chem., 2001, 85, 569 CrossRef CAS.
- (a) X. X. Xu, X. Z. You, Z. F. Sun, X. Wang and H. X. Liu, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 1169 CrossRef; (b) S. G. Teoh, S. B. Teo, G. Y. Yeap and H. K. Fun, Main Group Met. Chem., 1994, 17, 595 CrossRef CAS; (c) H. Takakashi, K. Kubo, H. Takechi, T. Matsumoto and K. Ideta, Yukagaku, 2006, 55, 483 Search PubMed; (d) D. Guo, J. H. Li, J. J. Xie, C. Y. Duan and Q. J. Meng, Chin. J. Inorg. Chem., 2002, 18, 1215 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Photophysical properties and applications of fluorescent pH probes of SAs. See DOI: 10.1039/c4nj01908c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |