Diastereoselective self-assembly of clofarabine lipids
Abstract
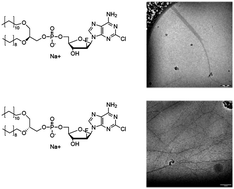
Clofarabine is a nucleosidic chemotherapeutic approved for the treatment of acute lymphoblastic leukemia, currently undergoing clinical trials for the treatment of solid tumors. Like for many others, lipidic derivatization of this drug improves pharmacokinetics and relieves the heavy side effects. Since the self-assembly pattern can modulate the bioactivity profile, a precise knowledge of the aggregation behavior of these nucleolipids and of the microstructure of the aggregates is central to design formulations that efficiently provide sufficient bioavailability. In this contribution we investigate the self-assembly behavior of two Clofarabine dioxy-ether derivatives, the diastereomers (1-2S and 1-2R). These clofarabine lipid derivatives showed an unexpected diastereoselective self-assembly effect, which we have monitored by observing the time evolution of self-assemblies and their microstructural response to thermal treatment. Dynamic Light Scattering and Circular Dichroism provide an ensemble of results which correlate with the occurrence of a hierarchical association of wormlike aggregates, imaged using Electron Microcopy. A strikingly different behavior for the two diastereomers was observed. This behavior can be crucial for the different bioavailability observed in vivo.
- This article is part of the themed collection: Bioinspired systems in supramolecular chemistry and nanotechnology

 Please wait while we load your content...
Please wait while we load your content...