Theoretical investigation of atmospheric chemistry of volatile anaesthetic sevoflurane: reactions with the OH radicals and atmospheric fate of the alkoxy radical (CF3)2CHOCHFO: thermal decomposition vs. oxidation†
Abstract
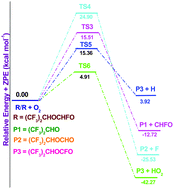
A theoretical study on the mechanism and kinetics of the gas phase reactions of a volatile anaesthetic compound (CF3)2CHOCH2F (Sevoflurane) with the OH radicals has been carried out using the hybrid HF–density functional M06-2X/6-31+G(d,p) method. Three conformations are predicted for the Sevoflurane molecule. Among the three conformers, the most stable one is considered for a detailed study. Reaction profiles are modeled including the formation of pre-reactive and post-reactive complexes at entrance and exit channels. Single point energy calculations have been performed by using the 6-311++G(d,p) basis set. The hydrogen abstraction from the –CH2F group is found to be the dominant reaction channel for hydrogen abstraction by OH radicals. Theoretically the calculated rate constant is found to be in good agreement with the experimentally measured ones. Using group-balanced isodesmic reactions, the standard enthalpies of formation for (CF3)2CHOCH2F, (CF3)2COCH2F and (CF3)2CHOCHF radicals are also reported for the first time. The atmospheric fate of the alkoxy radical, (CF3)2CHOCHFO, is also investigated for the first time using the same level of theory. Out of four prominent plausible decomposition channels including oxidation, our results clearly point out that reaction with O2 is the dominant path for the decomposition of (CF3)2CHOCHFO in the atmosphere involving the lowest energy barrier which is in accord with recent experimental findings.

 Please wait while we load your content...
Please wait while we load your content...