Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Arsenic-containing hydrocarbons are toxic in the in vivo model Drosophila melanogaster†
S.
Meyer
ab,
J.
Schulz
c,
A.
Jeibmann
c,
M. S.
Taleshi
de,
F.
Ebert
b,
K. A.
Francesconi
d and
T.
Schwerdtle
*b
aGraduate School of Chemistry, University of Muenster, Wilhelm-Klemm-Straße 10, 48149 Muenster, Germany
bInstitute of Nutritional Science, University of Potsdam, Arthur-Scheunert-Allee 114-116, 14558 Nuthetal, Germany. E-mail: tanja.schwerdtle@uni-potsdam.de
cInstitute of Neuropathology, University Hospital Muenster, Pottkamp 2, 48149 Muenster, Germany
dInstitute of Chemistry – Analytical Chemistry, University of Graz, Universitaetsplatz 1, 8010 Graz, Austria
eDepartment of Marine Chemistry, Faculty of Marine Science, University of Mazandaran, Babolsar, Iran
First published on 29th September 2014
Abstract
Arsenic-containing hydrocarbons (AsHC) constitute one group of arsenolipids that have been identified in seafood. In this first in vivo toxicity study for AsHCs, we show that AsHCs exert toxic effects in Drosophila melanogaster in a concentration range similar to that of arsenite. In contrast to arsenite, however, AsHCs cause developmental toxicity in the late developmental stages of Drosophila melanogaster. This work illustrates the need for a full characterisation of the toxicity of AsHCs in experimental animals to finally assess the risk to human health related to the presence of arsenolipids in seafood.
Inorganic arsenic (iAs) is classified as a human carcinogen. Additionally, chronic ingestion of iAs in humans has been associated with skin lesions, neurotoxicity, cardiovascular diseases, abnormal glucose metabolism, diabetes as well as a disturbance of foetal and infant development.1 In 2009 the EFSA Panel on Contaminants in the Food Chain carried out a risk characterisation for arsenic in food and concluded that risks to human health related to the presence of inorganic arsenic cannot be excluded.2 In the case of arsenosugars and arsenolipids, which occur mainly in seafood, no risk characterisation exists because of a lack of toxicological data. However, when arsenolipids are ingested by humans, they are bioavailable and can be efficiently metabolised.3,4
This class of fat-soluble arsenicals can be separated into arsenosugar-phospholipids (AsPL),5 arsenic-containing fatty acids (AsFA),6 the recently identified cationic trimethylarsonio fatty alcohols (TMAsFOH)7 and the arsenic-containing hydrocarbons (AsHC).8
AsHCs consist of a polar dimethylarsinoyl group and a long hydrocarbon chain, which is important for the lipophilic character of these compounds. AsHCs have been identified in edible fish oils,7–9 several fish meat samples, including herring,10 tuna11 and cod12,13 and also in some edible brown algae.5
Recently, three AsHCs (Fig. 1) have been shown to exert toxic effects in cultured human urothelial and liver cells with IC50 values similar to that of arsenite (iAsIII). However, the toxic modes of action seem to differ between iAsIII and the AsHCs. Because of their lipophilic properties, the AsHCs strongly accumulated in the cells, where, in contrast to arsenite, they strongly disturbed the cellular energy level.14
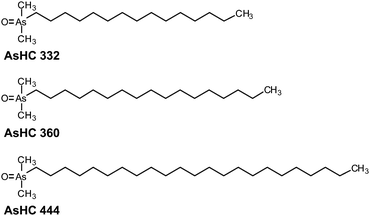 | ||
| Fig. 1 Chemical structures and abbreviations of three arsenic-containing hydrocarbons investigated in this study. | ||
In the present study we further characterised the toxicological profiles of the three AsHCs (Fig. 1) by investigating their effects on an entire and intact organism. For the first model organism, we chose the common fruit fly Drosophila melanogaster because it is a well-characterised in vivo model exhibiting a high degree of homology to mammalian genes.15 Further advantages of Drosophila melanogaster over other animals are that they are easy and fast to breed, and thus a whole life cycle from the parental flies over the larval and pupal stages to flies of the F1 generation can be studied in a few weeks. For these reasons, Drosophila melanogaster is frequently applied as an in vivo model to screen compounds for their toxicity to get a first insight into their toxic modes of action.16
As toxicity markers, we assessed the survival of the parental flies, the resulting number of pupae as well as the eclosion, which describes the number of hatching adult flies (F1 generation) from the pupal stage. These lethality endpoints are eminently suitable for an efficient toxicological screening.16 Effects of the three AsHCs were studied in direct comparison to the toxic reference arsenical iAsIII. All crosses of wild type Drosophila melanogaster (OregonR kindly provided by C. Klaembt, Muenster, Germany) were performed at 25 °C and raised on Drosophila melanogaster medium (Formula 4-21, Carolina Biological Supply Company, Burlington, USA). Media were incubated with the stock solutions of the investigated arsenicals, yielding final concentrations of 50 to 200 μM. iAsIII (>99.0%, Fluka Biochemika, Buchs, Germany) stock solutions were always freshly prepared in bi-distilled water before each experiment. AsHC 332, AsHC 360 and AsHC 444 were synthesised and purified (purity >99%) as described elsewhere.17 Stock solutions (10 mM) of the AsHCs were prepared in 100% EtOH and stored at 4 °C. For incubation of media, the stock solutions were diluted shortly before the experiment with EtOH. The final EtOH concentration in the Drosophila melanogaster media (incubated with the AsHCs) was 2% in all experiments. An identical experiment incubated with 2% of EtOH, but without an arsenical, was used as reference. This EtOH concentration did not induce any significant effects compared to untreated control experiments (data not shown).
To start the experiment two male and three female flies were placed in each vial. On day three of the experiment the parental generation was removed. The third instar larval and pupal stage as well as the adult F1 generation flies were collected at the end of the experiment. For collecting, the flies were anaesthetized with CO2 and stored at −20 °C until analysis (see below). For toxicity testing, pupae were marked on day 14 and the number of hatched flies was counted 7 days later to determine the rate of eclosion (Fig. 2).
 | ||
| Fig. 2 Experimental set up for toxicity testing and representative pictures of the developmental stages of Drosophila melanogaster. | ||
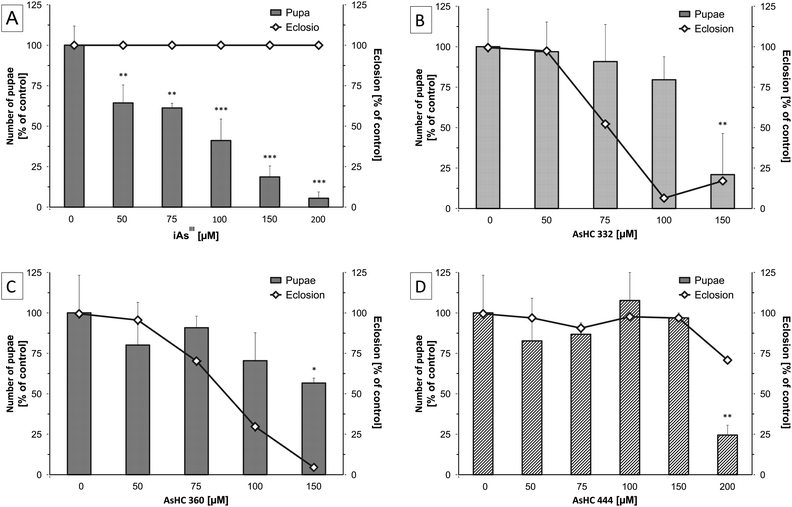
In the tested incubation range, none of the arsenicals affected the survival of the parenteral flies (data not shown). However, all three AsHCs and iAsIII showed significant effects on the development of Drosophila melanogaster. In the case of iAsIII a concentration-dependent reduction in the number of pupae was observed, with significant effects occurring already at 50 μM iAsIII (Fig. 3A). Nevertheless, iAsIII had no influence on the late development cycle of Drosophila melanogaster from the larval over the pupal stage to hatched flies of the F1 generation. Pupae of incubated parental flies were still able to pupate and hatch, albeit less successfully compared to untreated flies (Fig. 3A). No dead larvae were visible in the vials. These findings match results from a previous study18 with Drosophila melanogaster exposed to iAsIII for 7 days, where a LC50 of 540 μM was found and 250 μM iAsIII was not toxic to parental flies, but induced a yield reduction of about 60%. These results indicate that iAsIII interrupts the life circle or development of Drosophila melanogaster at an early stage. Thereby iAsIII might affect not only the laying of eggs but also disturbs the development from the embryonal to the early larval stage, an effect that has been postulated before.19
The effects for the AsHCs were different from those observed for iAsIII. All three AsHCs decreased the number of pupae only in the highest incubation concentration, but in contrast to arsenite they disturbed the hatching of flies from pupae (Fig. 3B–D). These data indicate that these three arsenolipids exert an impact on the late developmental stages of Drosophila melanogaster. AsHC 332 and AsHC 360 nearly completely inhibited the hatching of flies (F1 generation) from the pupal stage. Interestingly, the most lipophilic arsenolipid investigated, AsHC 444, exerted the lowest developmental toxicity in Drosophila melanogaster. This fits nicely to the in vitro studies, where AsHC 444 was less toxic than both AsHC 332 and AsHC 360. In liver cells, the lower toxicity of AsHC 444 correlated well with its lower cellular bioavailability.14
To study the bioavailability of arsenite and the arsenic-containing hydrocarbons in Drosophila melanogaster as well, the total arsenic contents in samples of the different developmental stages of Drosophila melanogaster were determined by inductively coupled plasma mass spectrometry (ICP-MS/MS, Agilent ICP-QQQ 8800, Waldbronn, Germany). Incubated flies of the parental, larval and pupal stage as well as flies of the F1 generation were washed three times with PBS and digested with a mixture of 65% HNO3 and 30% H2O2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for at least 12 h at 95 °C to dryness. The residue was dissolved and diluted with 0.15 M HNO3 for ICP-MS/MS measurement. Arsenic was quantified in the MS/MS mode using oxygen as reaction gas as described before (for ICP-MS parameters see ESI†).14
1) for at least 12 h at 95 °C to dryness. The residue was dissolved and diluted with 0.15 M HNO3 for ICP-MS/MS measurement. Arsenic was quantified in the MS/MS mode using oxygen as reaction gas as described before (for ICP-MS parameters see ESI†).14
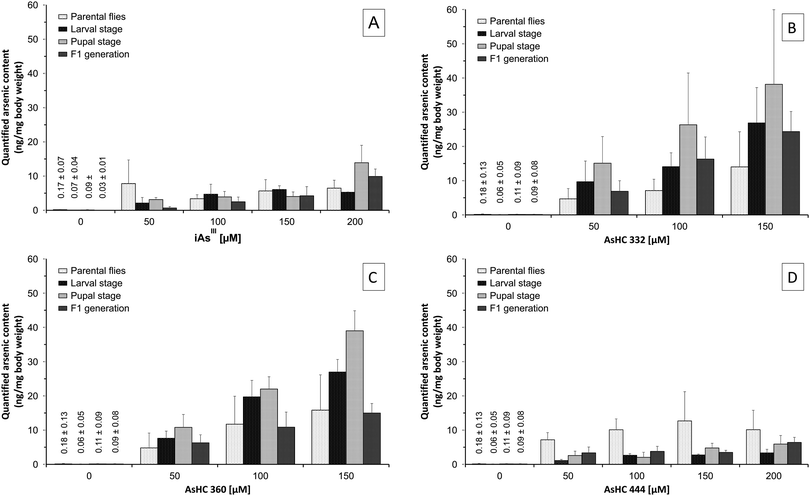
For all arsenicals, the arsenic content of the Drosophila melanogaster samples increased with increasing exposure concentration, although there were differences between the arsenicals. Thus, after incubation with iAsIII the arsenic content in all stages of development of Drosophila melanogaster was between 2–14 ng mg−1 body weight (Fig. 4A), whereas after incubation with AsHC 332 or 360, the total arsenic content was higher, especially in the larvae and pupae, ranging between 8–40 ng mg−1 body weight (Fig. 4B and C). AsHC 444 produced different results: it exerted the lowest bioavailability of the investigated arsenolipids (Fig. 4D), and in contrast to the other arsenicals studied, arsenic contents in larvae, pupae and flies of the F1 generation were always lower compared to arsenic contents in parenteral flies after feeding with AsHC 444. This lower bioavailability of AsHC 444 in larvae, pupae and flies of the F1 generation might be one reason for the less pronounced developmental toxicity of this arsenical. Interestingly, applied arsenic content of media (20–80 mg kg−1) as well as the determined total arsenic content in all developmental stages of Drosophila melanogaster (2–40 mg kg−1) were in the area of arsenic contents in commercial fish oils and certain organs of marine fish (1–19 mg kg−1).20
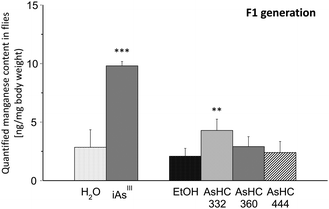
In the next step, we quantified the content of the essential trace elements manganese, iron, copper and zinc in all developmental stages of Drosophila melanogaster after incubation of media each with 100 μM of the respective arsenicals (for ICP-MS parameters see ESI†). Neither arsenite nor the AsHCs affected the manganese, iron, copper or zinc content of parenteral flies, larvae or pupae (data not shown). Likewise, the contents of iron, copper and zinc were not disturbed by the arsenicals in flies of the F1 generation (data not shown). Nevertheless, arsenite and AsHC 332 significantly increased the manganese content in flies of the F1 generation (Fig. 5). The mechanisms behind as well as the consequences of the disturbed manganese homeostasis in the flies of the F1 generation are unclear and should be further investigated.
Conclusions
In this first in vivo toxicity study with Drosophila melanogaster, arsenic-containing hydrocarbons showed toxicity in a concentration range similar to that for arsenite. These results illustrate the need for a full characterisation of the toxicity of arsenic-containing hydrocarbons in experimental mammals in order to assess the risk to human health related to the presence of arsenolipids in seafood.Acknowledgements
This work was supported by the DFG grant number SCHW903/4-1, the Austrian Science Fund (FWF), project number I550-N17, and the Graduate School of Chemistry (University of Muenster, Germany).Notes and references
- IARC, A review of human carcinogens. Part C: arsenic, metals, fibres, and dusts, IARC Monographs 2012, pp. 196–211.
- EFSA, EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on Arsenic in Food, EFSA Journal, 2009, 7, 1351–1355 Search PubMed.
- E. Schmeisser, A. Rumpler, M. Kollroser, G. Rechberger, W. Goessler and K. A. Francesconi, Arsenic fatty acids are human urinary metabolites of arsenolipids present in cod liver, Angew. Chem., Int. Ed., 2006, 45, 150–154, DOI:10.1002/anie.200502706.
- E. Schmeisser, W. Goessler and K. A. Francesconi, Human metabolism of arsenolipids present in cod liver, Anal. Bioanal. Chem., 2006, 385, 367–376, DOI:10.1007/s00216-006-0401-x.
- S. Garcia-Salgado, G. Raber, R. Raml, C. Magnes and K. A. Francesconi, Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae, Environ. Chem., 2012, 9, 63–66, DOI:10.1071/EN11164.
- A. Rumpler, J. S. Edmonds, M. Katsu, K. B. Jensen, W. Goessler, G. Raber, H. Gunnlaugsdottir and K. A. Francesconi, Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity?, Angew. Chem., Int. Ed., 2008, 47, 2665–2667, DOI:10.1002/anie.200705405.
- K. O. Amayo, A. Raab, E. M. Krupp, H. Gunnlaugsdottir and J. Feldmann, Novel identification of arsenolipids using chemical derivatizations in conjunction with RP-HPLC-ICPMS/ESMS, Anal. Chem., 2013, 85, 9321–9327, DOI:10.1021/ac4020935.
- M. S. Taleshi, K. B. Jensen, G. Raber, J. S. Edmonds, H. Gunnlaugsdottir and K. A. Francesconi, Arsenic-containing hydrocarbons: natural compounds in oil from the fish capelin, Mallotus villosus, Chem. Commun., 2008, 4706–4707, 10.1039/b808049f.
- K. O. Amayo, A. Petursdottir, C. Newcombe, H. Gunnlaugsdottir, A. Raab, E. M. Krupp and J. Feldmann, Identification and quantification of arsenolipids using reversed-phase HPLC coupled simultaneously to high-resolution ICP-MS and high-resolution electrospray MS without species-specific standards, Anal. Chem., 2011, 83, 3589–3595, DOI:10.1021/ac2005873.
- S. Lischka, U. Arroyo-Abad, J. Mattusch, A. Kuhn and C. Piechotta, The high diversity of arsenolipids in herring fillet (Clupea harengus), Talanta, 2013, 110, 144–152, DOI:10.1016/j.talanta.2013.02.051.
- M. S. Taleshi, J. S. Edmonds, W. Goessler, M. J. Ruiz-Chancho, G. Raber, K. B. Jensen and K. A. Francesconi, Arsenic-containing lipids are natural constituents of sashimi tuna, Environ. Sci. Technol., 2010, 44, 1478–1483, DOI:10.1021/es9030358.
- U. Arroyo-Abad, J. Mattusch, S. Mothes, M. Moder, R. Wennrich, M. P. Elizalde-Gonzalez and F. M. Matysik, Detection of arsenic-containing hydrocarbons in canned cod liver tissue, Talanta, 2010, 82, 38–43, DOI:10.1016/j.talanta.2010.03.054.
- U. Arroyo-Abad, S. Lischka, C. Piechotta, J. Mattusch and T. Reemtsma, Determination and identification of hydrophilic and hydrophobic arsenic species in methanol extract of fresh cod liver by RP-HPLC with simultaneous ICP-MS and ESI-Q-TOF-MS detection, Food Chem., 2013, 141, 3093–3102, DOI:10.1016/j.foodchem.2013.05.152.
- S. Meyer, M. Matissek, S. M. Muller, M. S. Taleshi, F. Ebert, K. A. Francesconi and T. Schwerdtle, In vitro toxicological characterisation of three arsenic-containing hydrocarbons, Metallomics, 2014, 6, 1023–1033, 10.1039/c4mt00061g.
- T. F. Mackay and R. R. Anholt, Of flies and man: Drosophila as a model for human complex traits, Annu. Rev. Genomics Hum. Genet., 2006, 7, 339–367, DOI:10.1146/annurev.genom.7.080505.115758.
- M. D. Rand, Drosophotoxicology: the growing potential for Drosophila in neurotoxicology, Neurotoxicol. Teratol., 2010, 32, 74–83, DOI:10.1016/j.ntt.2009.06.004.
- M. S. Taleshi, R. K. Seidler-Egdal, K. B. Jensen, T. Schwerdtle and K. A. Francesconi, Synthesis and Characterization of Arsenolipids: Naturally Occurring Arsenic Compounds in Fish and Algae, Organometallics, 2014, 33, 1397–1403, DOI:10.1021/om4011092.
- S. H. Goldstein and H. Babich, Differential effects of arsenite and arsenate to Drosophila melanogaster in a combined adult/developmental toxicity assay, Bull. Environ. Contam. Toxicol., 1989, 42, 276–282, DOI:10.1007/BF01699411.
- J. G. Muniz Ortiz, R. Opoka, D. Kane and I. L. Cartwright, Investigating arsenic susceptibility from a genetic perspective in Drosophila reveals a key role for glutathione synthetase, Toxicol. Sci., 2009, 107, 416–426, DOI:10.1093/toxsci/kfn192.
- V. Sele, J. J. Sloth, A. K. Lundebye, E. H. Larsen, M. H. G. Berntssen and H. Amlund, Arsenolipids in marine oils and fats: a review of occurrence, chemistry and future research needs, Food Chem., 2012, 133, 618–630, DOI:10.1016/j.foodchem.2012.02.004.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4mt00249k |
| This journal is © The Royal Society of Chemistry 2014 |