Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iron uptake and ferrokinetics in healthy male subjects of an iron-based oral phosphate binder (SBR759) labeled with the stable isotope 58Fe
Hans-Peter
Gschwind
*a,
Dietmar G.
Schmid
a,
Friedhelm
von Blanckenburg†
b,
Marcus
Oelze†
b,
Kirsten
van Zuilen‡
b,
Alan J.
Slade
c,
Sylvie
Stitah
d,
Daniel
Kaufmann
e and
Piet
Swart
a
aDrug Metabolism & Pharmacokinetics (DMPK)/Integrated Drug Disposition (IDD), Novartis Pharma AG, 4002 Basel, Switzerland. E-mail: hans-peter.gschwind@bluewin.ch
bInstitute for Mineralogy, Leibniz University of Hannover, Germany
cNovartis Institute for Biomedical Research (NIBR)/Translational Medicine, Novartis Corporation, East Hanover, USA
dClinical Science and Innovation, Novartis Pharma AG, Basel, Switzerland
eChemical and Analytical Development, Novartis Pharma AG, Basel, Switzerland
First published on 14th July 2014
Abstract
SBR759 is a novel polynuclear iron(III) oxide–hydroxide starch·sucrose·carbonate complex being developed for oral use in chronic kidney disease (CKD) patients with hyperphosphatemia on hemodialysis. SBR759 binds inorganic phosphate released by food uptake and digestion in the gastro-intestinal tract increasing the fecal excretion of phosphate with concomitant reduction of serum phosphate concentrations. Considering the high content of ∼20% w/w covalently bound iron in SBR759 and expected chronic administration to patients, absorption of small amounts of iron released from the drug substance could result in potential iron overload and toxicity. In a mechanistic iron uptake study, 12 healthy male subjects (receiving comparable low phosphorus-containing meal typical for CKD patients: ≤1000 mg phosphate per day) were treated with 12 g (divided in 3 × 4 g) of stable 58Fe isotope-labeled SBR759. The ferrokinetics of [58Fe]SBR759-related total iron was followed in blood (over 3 weeks) and in plasma (over 26 hours) by analyzing with high precision the isotope ratios of the natural iron isotopes 58Fe, 57Fe, 56Fe and 54Fe by multi-collector inductively coupled mass spectrometry (MC-ICP-MS). Three weeks following dosing, the subjects cumulatively absorbed on average 7.8 ± 3.2 mg (3.8–13.9 mg) iron corresponding to 0.30 ± 0.12% (0.15–0.54%) SBR759-related iron which amounts to approx. 5-fold the basal daily iron absorption of 1–2 mg in humans. SBR759 was well-tolerated and there was no serious adverse event and no clinically significant changes in the iron indices hemoglobin, hematocrit, ferritin concentration and transferrin saturation.
1 Introduction
Serum phosphate homeostasis is primarily regulated by the body's ability to excrete excess dietary phosphate via the kidneys. Patients with chronic renal insufficiency, who have varying degrees of renal function, accumulate excess phosphate resulting in a condition known as hyperphosphatemia. First generation, calcium- or aluminum-containing phosphate binders are effective in reducing the absorption of phosphate from the diet, but require large doses and may result in accumulation of calcium or aluminum. Intense research efforts have led to the development of a second generation of potent, calcium and aluminum-free, phosphate binders such as sevelamer hydrochloride (HCl) (RenaGel®), a cross-linked poly-allylamine hydrochloride (Bleyer, 1999)1 or fosrenol™, a lanthanum carbonate compound (D'Haese, 2003).2 While sevelamer-HCl has proven efficacy it is associated with possible induction of metabolic acidosis and gastrointestinal (GI) side effects (Brezina, 2004).3 In addition, sevelamer-HCl does not bind phosphate efficiently in an acidic environment. Lanthanum carbonate also demonstrates significant phosphate-binding properties and good efficacy but has also been associated with hypercalcemia (Hutchison, 2009)4 (Hutchison, 2005)5 (D'Haese, 2003).2 Studies in both rats (Slatopolsky, 2005)6 and humans (Spasovski, 2006)7 have suggested that long-term lanthanum administration carries the risk of blood and tissue accumulation.SBR759 is a novel polynuclear iron(III) oxide–hydroxide·starch–sucrose·carbonate complex designed for oral use to specifically bind with high affinity and excrete inorganic phosphate. SBR759 is an odorless, water-insoluble, slightly sweet tasting powder formulation. The compound was developed with SeBo GmbH, Germany for the treatment of hyperphosphatemia commonly observed in patients with CKD. SBR759 has been shown to be an effective phosphate binder in vivo and shows rapid, selective, and high in vitro binding of phosphate at both highly acidic and neutral pH with phosphate-binding capacity similar to currently available agents (Hergesell, 1999)8a (Hergesell, 1999).8b SBR759 lowered serum phosphate concentrations rapidly and to a clinically meaningful extent across a wide dose range with good tolerability (Block, 2010).9 In phase II dose titration studies assessing Asian patients requiring hemodialysis, SBR759 demonstrated superior efficacy in phosphate control compared with sevelamer-HCl, an approved phosphate binder (Chen, 2011)10 (Fukagawa, 2014).11
SBR759 is a chemical complex of polymeric nature. It is a novel member of the iron-oxide–hydroxide compound class, which is different from previously described iron-oxide–hydroxides such as goethite, akaganéite or lepidocrocite. Even though it resembles ferrihydrite in its characteristics, it is different to ferrihydrite and known iron-oxides–hydroxides as follows: (i) sucrose, carbonate and very likely starch are included in the coordination sphere of the iron atoms and bound to the iron atoms by a coordinative bond; (ii) Mössbauer spectroscopy showed that SBR759 is different to standard iron(III)-oxides. Thus, it can be described formally as the polynuclear iron(III) oxide–hydroxide·starch·sucrose·carbonate complex and is therefore not a simple mixture of these starting materials. Phosphate binding by SBR759 is accomplished by a ligand exchange mechanism displacing sucrose carbonate or hydroxyl groups, the latter similar to the iron(III) oxide–hydroxide complex described in (Sigg, 1980)12 forming predominantly binuclear bidentate complexes (Parfitt, 1975).13 The iron in SBR759 is covalently bound to the starch–saccarose complex and is very stable against digestion in the human gastro-intestinal tract (GIT). However, due to the high iron content (∼20% w/w) of SBR759 and gram quantity doses required for treatment, chronic administration may be accompanied by absorption of iron, released from the iron(III) complex. With chronic treatment often required in patients with chronic kidney disease, absorption of this exogenous iron may result in the potential for iron overload and toxicity. Iron toxicity results when free iron not bound to transferrin (NTBI) appears in the blood, forming labile plasma iron (LPI) and catalyzing the generation of reactive oxygen species (ROS) (Anderson, 2007)14 that can damage tissues. Likewise, generation of ROS in the gut for prolonged periods of time could lead to epithelial cell damage and may facilitate pathogen entry to the organism (Chávez, 2007).15In vitro tests have shown that the iron oxides present in SBR759 are not redox-active, a prerequisite for the above mentioned side effects (Novartis, unpublished data).
Nonclinical testing with 59Fe-radiolabeled SBR759 in dogs demonstrated that less than 0.2% of the SBR759-related iron dose was absorbed (Novartis, unpublished data). In order to gain insight into the ferrokinetics and to quantify the extent of SBR759-related iron absorption following oral administration of SBR759, a phase I clinical study with stable 58Fe isotope-labeled SBR759 in healthy male subjects was performed. The ferrokinetics of [58Fe]SBR759-related total iron was followed in whole blood (over 3 weeks) and in plasma (over 26 hours) by analyzing the isotope ratios of the natural iron isotopes by MC-ICP-MS. Apart from whole blood, plasma was chosen because it comprises a more dynamic compartment concerning the turnover of transferrin-bound iron as compared to red blood cells which are characterized by a 120 day erythrocyte life span in circulation (Walczyk, 2005).16 Aside from tracing the ferrokinetics and the extent of the oral absorption of [58Fe]SBR759-related iron in blood and plasma determined upon changing 58Fe/56Fe-isotope ratios, a possible difference in the 56Fe/54Fe isotope signature between erythrocytes (whole blood) and plasma was measured by MC-ICP-MS in samples collected between 0 and 26 hours post first SBR759 dose. These results are presented in a companion paper that also contains the measured iron concentrations and iron isotope ratio data used for the present study in an Online Supplement S4 (von Blanckenburg, submitted).17 This study also provided insight into the applicability of the MC-ICP-MS technique in measuring isotope-labeled iron in human blood and plasma.
2 Materials and methods
2.1 Selecting iron isotopes to investigate iron uptake in human
Biomarkers such as transferrin concentration and ferritin saturation can be used as clinical indicators as well as the state of iron stores including iron overload. Direct quantification of ferrokinetics was typically obtained through the use of isotope labeled iron compounds. Applying radioactive iron isotopes such as 59Fe is a very sensitive and a specific approach, but it presents several disadvantages: (1) the radioisotopes 59Fe and 55Fe decay via X- and/or β-ray emission with physical half-lives of 45 days and 2.7 years, respectively; (2) absorbed iron is not actively excreted from and is salvaged in the human body during hematopoiesis (red blood cell production) which raises ethical concerns exposing human subjects to radioactivity over a long time; (3) formation of unknown impurities due to radiolysis of the drug and therefore radiochemical stability might not be determinable; (4) this is in particular the case for SBR759 which is generally insoluble and has a very complex molecular structure; (5) radiation during the Good Manufacturing Practice (GMP) production of the drug, shipping, administration and analysis requires special precautions, facilities and handling procedures to minimize exposure to radioactivity and cross-contamination; (6) the short physical half-life of 45 days of 59Fe does not simply allow prolonged synthesis processes under GMP production or keeping retained samples for analysis afterwards; (7) factors contributing to measurement variability have to be minimized as much as possible, and therefore, it is essential that the drug product is labeled homogeneously with the iron isotope by applying GMP production which is extremely difficult to achieve using radioactive iron.The disadvantages mentioned above can be circumvented by applying the isotope dilution concept using stable iron isotopes, e.g.58Fe or 57Fe and determination of isotope ratios by MC-ICP-MS. Many clinical iron uptake studies were conducted in the past using stable iron isotopes and applying the isotope dilution concept, mainly in nutrition research in adults (Whittaker, 1989)18 (Barrett, 1992)19 and children (Janghorbani, 1986)20 (Fomon, 1988)21 (Fomon, 1989)22 (Woodhead, 1988)23 (Walczyk, 1997)24 (Vasquez Garibay, 2001).25
2.2 Study design
The clinical part of this open-label study was performed at Covance Clinical Research Unit AG (former Swiss Pharma Contract Ltd), Allschwil, Switzerland. This study was approved by the Cantonal Ethics Committee and was conducted in accordance with the declaration of Helsinki (1964 and subsequent revisions) and International Conference on Harmonization-Good Clinical Practice guidelines. The analytical investigations of the ferrokinetics and iron uptake of [58Fe]SBR759-related iron were not required to be conducted under GLP but were carried out according to the available current scientific standards.| Subject number | Total [58Fe]SBR759 dose (g) | Body weight (kg) | Body height (cm) | Total Fe concentration in whole blooda,b | Calculated blood volumec (mL) | Total amount of Fe in whole blood (mg) | |
|---|---|---|---|---|---|---|---|
| (ppm) | (μg mL−1) | ||||||
| a As determined by ICP-OES in blood collected at 0 h (pre-dose, baseline). b Using a blood density of 1.050 g mL−1 at 37 °C (Geigy Scientific Tables).34 c Blood volume calculated according to eqn (12). | |||||||
| 5101 | 12.47 | 71.7 | 174 | 460 | 483 | 4400 | 2127 |
| 5102 | 12.48 | 97.8 | 177 | 490 | 515 | 5320 | 2735 |
| 5103 | 12.47 | 86.8 | 172 | 450 | 473 | 4820 | 2282 |
| 5104 | 12.49 | 84.9 | 179 | 470 | 494 | 4960 | 2450 |
| 5105 | 12.45 | 57.2 | 165 | 500 | 525 | 3690 | 1937 |
| 5106 | 12.48 | 78.0 | 186 | 460 | 483 | 4950 | 2389 |
| 5107 | 12.42 | 79.5 | 182 | 460 | 483 | 4880 | 2357 |
| 5108 | 12.47 | 59.2 | 166 | 460 | 483 | 3780 | 1827 |
| 5109 | 12.47 | 67.2 | 179 | 450 | 473 | 4410 | 2084 |
| 5110 | 12.46 | 102.1 | 186 | 490 | 515 | 5710 | 2939 |
| 5111 | 12.47 | 97.4 | 183 | 510 | 536 | 5470 | 2934 |
| 5112 | 12.46 | 81.1 | 180 | 510 | 536 | 4870 | 2612 |
| Mean | 12.47 | 80.2 | 177 | 475.8 | 499.9 | 4770 | 2389 |
| SD | 0.017 | 14.7 | 7.0 | 22.7 | 24.0 | 620 | 365 |
| CV (%) | 0.13 | 18.3 | 3.9 | 4.8 | 4.8 | 13.0 | 15.3 |
| Range | 12.42–12.49 | 57.2–102.1 | 165–186 | 450–510 | 473–536 | 3690–5710 | 1827–2939 |
Healthy, nonsmoking men between 18 and 45 years of age were enrolled. Subjects with (i) hematocrit <41%, (ii) hemoglobin <13.8 or >17.2 g dL−1, (iii) serum ferritin <20 or >320 ng mL−1, (iv) transferrin saturation <20%, (v) reticulocyte count >1.5% or platelets <100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μL−1, (vi) history of anemia, hemochromatosis or other dyscrasia(s), e.g. thalassemia, myelodysplastic syndrome, etc., treated or not had to be excluded from the study. A total of 12, iron replete i.e., nonanemic, subjects were recruited. This population was selected as their propensity for iron absorption was expected to be similar to that of a CKD patient on dialysis based on similarities in absorption of therapeutic iron salts (Skikne, 2000).28
000 μL−1, (vi) history of anemia, hemochromatosis or other dyscrasia(s), e.g. thalassemia, myelodysplastic syndrome, etc., treated or not had to be excluded from the study. A total of 12, iron replete i.e., nonanemic, subjects were recruited. This population was selected as their propensity for iron absorption was expected to be similar to that of a CKD patient on dialysis based on similarities in absorption of therapeutic iron salts (Skikne, 2000).28
Stable 58Fe isotope labeling of SBR759. Research grade 58Fe-enriched (99.49%) iron was purchased from Isoflex, San Francisco, CA, USA. 58Fe was completely dissolved in 37% (w/w) aqueous hydrochloric acid at ambient temperature. The resulting aqueous FeCl2-solution was filtered and subsequently oxidized by addition of excess of gaseous chlorine. The resulting aqueous 58FeCl3 solution was evaporated to dryness yielding 58FeCl3·6H2O as colorless hygroscopic powder (Tessenderlo AG, Bad Zurzach, Switzerland). 58FeCl3·6H2O was diluted with commercially available FeCl3·6H2O (Merck, Darmstadt, Germany) to a target 58Fe content of 2.5%. 58Fe stable isotope-labeled SBR759 was prepared starting from 58Fe-stable isotope-labeled FeCl3·6H2O using the same synthesis protocol as for the GMP production of the SBR759 drug substance for human use (Novartis Pharma, AG; Basel, Switzerland).
The drug product (SBR759 58Fe Moda Saccharose, iron aqua carbonate hydroxyl-oxo starch sucrose complex (CAS registration No: 1041180-02-2)) contained 20.4% w/w of iron consisting of 5.706% 54Fe, 89.552% 56Fe, 2.077% 57Fe and 2.665% 58Fe isotopes as determined by MC-ICP-MS.
The molar iron isotope ratios 58Fe/56Fe, 58Fe/54Fe, 57Fe/54Fe and 56Fe/54Fe measured by MC-ICP-MS in the [58Fe]SBR759 drug product are listed in Table 2 as relative to the “IRMM-014” iron reference standard (IRMM-014 Fe Standard).29 The raw data are reported in Online Supplement S4 (von Blanckenburg, submitted).17
| Iron isotope ratios | ||||
|---|---|---|---|---|
| 58Fe/56Fe | 58Fe/54Fe | 57Fe/54Fe | 56Fe/54Fe | |
| Iron isotope ratio | 0.029761 | 0.46705 | 0.363943 | 15.69313 |
| Relative error (2σ; n = 3) | 0.000034 | 0.00054 | 0.000012 | 0.00030 |
| CV (%) | 0.11 | 0.12 | 0.32 | 0.0019 |
In order to minimize hemolysis during Day 1 and Day 2 when several blood withdrawals took place, blood samples were collected via a plastic cannula placed in a forearm vein that remained through sampling 26 hours post last dose. For blood sampling at Days 5, 11, 14 and 21 a metallic needle (venipuncture) was used for individual samplings. Blood was collected (0–16 h: 35 mL; 22–490 h: 5 mL) via gentle aspiration into special 7.5 mL S-Monovettes® for metal analysis containing only a small and specified amount of metal impurities (<50 ng iron per tube; Sarstedt, Germany). These tubes contained about 4–7 μL of a lithium heparin solution corresponding to about 0.1% of the final blood sample volume. After blood collection the tubes were inverted gently several times. Immediately after collection, five aliquots of whole blood were exactly weighed into pre-labeled polypropylene cryotubes and stored at ≤−20 °C.
Plasma was obtained from heparinized blood (0, 6, 12, 16, 22 and 26 h post first dose) by centrifugation at 2000 × g, at 4 °C for 10 min. Plasma was recovered into pre-weighed tubes (S-Monovettes® for metal analysis, Sarstedt) and stored at ≤−20 °C. Plasma samples which displayed signs of hemolysis were excluded from data analysis.
| Mean ± SD (range) | Iron toxicity-specific laboratory parameters | |||
|---|---|---|---|---|
| Hemoglobina (mmol L−1) | Hematocrit (%) | Ferritin (μg L−1) | TSAT (%) | |
| a To obtain g dL−1 divide nmol L−1 values by 0.6206. b Outlier value: not repeated nor excluded from average and standard deviation. | ||||
| Screening (Day-21 to Day-2) | 9.7 ± 0.52 (9.0–10.6) | 45.1 ± 2.0 (42.5–49.8) | 94.6 ± 52.9 (30–217) | 34.6 ± 11.2 (20.8–54.6) |
| Baseline (Day-1) | 9.4 ± 0.48 (8.7–10.2) | 44.0 ± 2.3 (40.6–48.7) | 86.4 ± 56.4 (28–219) | 35.8 ± 19.0 (22.0–93.6b) |
| Day 11 | 8.9 ± 0.65 (8.2–9.8) | 41.9 ± 2.4 (38.4–46.2) | 70.5 ± 43.6 (28–167) | 24.3 ± 8.9 (10.1–36.9) |
| Day 21 | 8.8 ± 0.62 (8.0–10.1) | 41.8 ± 2.8 (37.0–47.9) | 68.3 ± 54.3 (30–191) | 24.0 ± 12.5 (10.5–45.2) |
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |
|---|---|---|---|---|---|---|---|---|
| Normal ranges | 8.7 | 10.9 | 40.1 | 51 | 30 | 400 | 16 | 45 |
2.3 Iron isotope determination in blood and plasma
Iron standard for controlling MC-ICP-MS measurements. Commercially available “IRMM-014” iron reference material (from EU Institute for Reference Materials and Measurements, Geel, Belgium) was used as the “bracketing standard” in measurements (Schoenberg, 2005),30 and to calculate normalized isotope ratio differences according to (eqn (1)). Iron in the “IRMM-014” standard is reported to be at a molar weight of 55.84515 ± 0.00048 g mol−1 consisting of the following isotope mass fractions: 54Fe = 5.845%, 56Fe = 91.754%, 57Fe = 2.1192% and 58Fe = 0.2818%. For MC-ICP-MS measurements, the “IRMM-014” metallic iron standard was dissolved in 0.3 mol L−1 HNO3 (Merck KGaA, Darmstadt, Germany). Before use, HNO3 was distilled until content in iron was <0.2 ppb (controlled by ICP-OES).
Interference correction of 54Cr on 54Fe and 58Ni on 58Fe. To avoid artifacts introduced by a high Cr or Ni corrections, Fe isotope analyses with 54Cr/54Fe > 0.1‰ were rejected, while δ58/56Fe values of samples with 58Ni/58Fe > 10‰ were rejected from the data sets (von Blanckenburg, submitted).17
2.4 Data evaluation and equations used
Isotope ratio measurements by ICP-MS never yield absolute ratios. They are affected by a variable instrumental mass bias, the degree of which depends on run conditions. To make the measurements of unknown sample compositions comparable, a reference material of known composition is measured (meas) before and after each sample. In this so-called “bracketing technique” sample ratios are normalized to the nominal (nom) value of the reference material. We measured the “IRMM-014” iron reference standard before and after each sample. The normalized 58Fe/56Fe ratio of the sample 58Fe/56Fesample then is: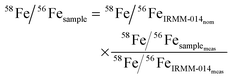 | (1) |
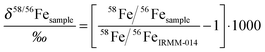 | (2) |
The molar amount of absorbed [58Fe]SBR759-related iron niso is calculated by eqn (3), derived from (Walczyk, 1997).24
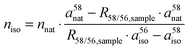 | (3) |
The atomic weight of iron of natural composition (awtFe,nat) is calculated for each subject by eqn (4):
| awtFe,nat = m54·a54nat + m56·a56nat + m57·a57nat + m58·a58nat | (4) |
m 5x, the atomic weight of 5xFe (DeLaeter, 2003);31a5xnat, the natural abundance of 5xFe (x = 4, 6, 7 and 8) at baseline; The latter is calculated by eqn (5) and (6):
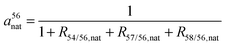 | (5) |
| a5xnat = a56nat·R5x/56,nat | (6) |
R 5x/56,nat, the 5xFe/56Fe ratio relative to the “IRMM-014” iron standard according to eqn (2), measured at baseline (x = 4, 7 and 8).
The abundances of the iron isotopes in SBR759 (a5xiso) are calculated by eqn (7) and (8):
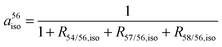 | (7) |
| a5xiso = a56iso·R5x/56,iso | (8) |
R 5x/56,iso, the 5xFe/56Fe ratio relative to the “IRMM-014” iron standard according to eqn (2) of SBR759 (x = 4, 7 and 8).
The molar amount of absorbed [58Fe]SBR759-related iron (niso) can be converted to the amount of adsorbed iron in mg (miso) by eqn (9):
| miso = niso·awtFe,iso·1000 | (9) |
The atomic weight of iron in SBR759 (awtFe,iso) is calculated by eqn (10):
| awtFe,iso = m54·a54iso + m56·a56iso + m57·a57iso + m58·a58iso | (10) |
Due to the long life span of erythrocytes (∼120 days), the total amount of absorbed [58Fe]SBR759-related iron can be determined 14 days (or if more conservative 21 days) after the administration. Since not all of the absorbed [58Fe]SBR759-related iron is incorporated into erythrocytes, an incorporation factor (finc) is included (Walczyk, 1997).24 According to (Bernat, 1983),32 70–90% of absorbed [58Fe]SBR759-related iron is incorporated into erythrocytes. Therefore, finc will be set to the mean value of 80%. The final, absolute amount of absorbed [58Fe]SBR759-related iron mabs is calculated by eqn (11):
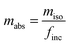 | (11) |
We note that during intestinal absorption light isotopes are preferred by mass-dependent isotope fractionation. For European male omnivores this fractionation amounts to a shift in the 58Fe/56Fe ratio by a factor of 0.9985 (von Blanckenburg, 2013).33 As this shift is negligible when compared to the shifts in 58Fe/56Fe introduced by [58Fe]SBR759, we ignore this absorption effect.
The blood volume (BV; in mL) is calculated by eqn (12) (Geigy Scientific Tables, 1984)34 which is based on the body length (L; in cm) and the body mass (B; in kg) (Table 1):
| BV = L × 28.5 + B × 31.6 − 2820 | (12) |
The corresponding plasma volume is calculated by multiplying the calculated blood volume BV with the blood/plasma volume ratio of 0.5769 calculated from Table II in (Davies, 1993).35
3 Results and discussion
3.1 Safety of subjects and drug tolerability assessment
Doses of 12 g 58Fe-labeled SBR759, divided in three doses of 4 g, each, and administered with a low phosphorus meal, were generally well tolerated by healthy male subjects. There was no serious adverse event reported in the study. The most common adverse events were flatulence, discolored feces, diarrhea, and headache which were mild or moderate and transient. There were no clinically significant changes in laboratory parameters, vital signs, ECGs, or physical examinations over the course of the study. There was no sign of overt or acute iron toxicity as there were no clinically significant changes in laboratory parameters hemoglobin, hematocrit, ferritin and transferrin saturation (TSAT) (Table 3).The mean TSAT values at Days-1, 11 and 21 were 35.8, 24.3 and 24.0% which were in the normal range (16–45%) and distinctly below the toxic limit of 85% leading to labile redox active plasma iron (LPI). It should be noted that an obvious TSAT outlier of 93.6% at baseline (prior to treatment) was reported for subject 5110. This value was not repeated nor excluded from the listing. There was no impact on the mean versus median values, therefore mean values are reported.
There was a trend to numerically lower mean and median hemoglobin, hematocrit, ferritin and TSAT values. These trends were likely due to the volume of blood drawn from subjects in the immediate post dose time period (∼246 mL) which amounts to ∼108 mg of iron lost during the first 26 hours of the study and ∼132 mg iron during the entire study (300 mL × Hct × 1 mg mL−1). These losses occurred in the time period after dosing. Hence they are not expected to drive iron absorption as it is expected that the 58Fe-isotope-labeled-SBR759 would have passed through the proximal small bowel before the blood/iron losses would have stimulated the need to iron absorption. Therefore this is not expected to have an impact on the conclusion of iron absorption from SBR759.
These safety results in healthy volunteers are in agreement with those of three clinical trials in chronic kidney disease patients on hemodialysis (Block, 2010)9 (Chen, 2011)10 (Fukagawa, 2014)11 where SBR759 was well tolerated within the anticipated clinical dose range of 3.75 to 15 g SBR759 per day. No treatment-related serious adverse events were observed nor were there clinically relevant changes in iron indices.
3.2 Ferrokinetics in blood and plasma
The ferrokinetics of SBR759-related iron in whole body blood of the individual subjects was investigated in the time period from 1 to 21 days (0–490 h) post [58Fe]SBR759 dose (Fig. 1).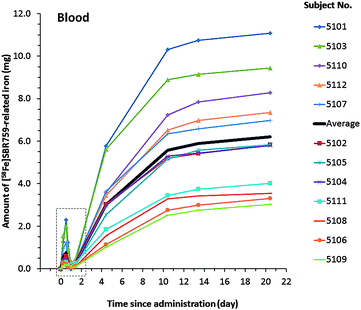 | ||
| Fig. 1 Amount of total [58Fe]SBR759-related iron in whole body blood collected between Day 1 and Day 21 post 12 g (3 × 4 g) of [58Fe]SBR759 dose to 12 healthy male subjects. The amount of total blood iron was determined by MC-ICP-MS. Total blood iron calculation based on 58Fe/56Fe isotope ratios. The time window between 0 and 26 hours (dashed rectangle) is shown enlarged in Fig. 2A. Almost identical total blood iron values were calculated by using 58Fe/54Fe isotope ratios (not shown). | ||
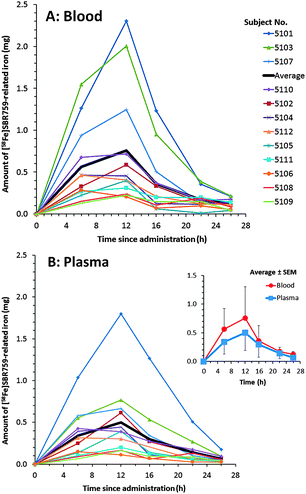
In order to monitor the rapid systemic uptake of [58Fe]SBR759-related iron into the bloodstream, also whole body blood and plasma in the early time window between 0 and 26 hours were investigated (Fig. 2A and B).
The calculation of total blood iron and plasma iron was based on 58Fe/56Fe isotope ratios. In contrast to blood, plasma 58Fe/56Fe and 58Fe/54Fe ratio values were very high due to the 320- to 590-fold lower amount of iron in plasma (adult males: 0.75–1.75 mg L−1) (Geigy Scientific Tables, 1984).34 Within 0–26 h, most subjects reached a first maximum at 12 hours amounting to 0.22 to 2.3 mg in blood (Fig. 2A) and 0.15 to 1.8 mg in plasma (Fig. 2B). Thereafter, total blood and plasma iron values dropped by about 26 hours to low levels of 0.01–0.22 mg in blood and 0.02 to 0.18 mg in plasma. Comparable to our study, healthy volunteers treated with oral phosphate binders combined with supplemental iron salts reached plasma iron plateaus by about 6 hours following dosing (Pruchnicki, 2002).36 In our study the ferrokinetic curves were formed out of three single curves related to each of the three consecutive [58Fe]SBR759 doses given at 0, 4 and 10 hours. The early maximum at approx. 12 hours can be attributed to a transient appearance of transferrin-bound SBR759-related iron in blood and plasma. After 26 hours, SBR759-related total blood iron steadily increased, each subject reaching a different plateau between 14 and 21 days (Fig. 1). Total SBR759-related blood iron ranged between 3.03 and 11.1 mg revealing high inter-subject variability (CV: 41–104%).
Apart from the upstream intestinal enterocytes, blood plasma is the primary central compartment for iron absorption since virtually all cells in the organism take up iron from transferrin (the major iron transporter protein: apo-transferrin containing bound iron) (Walczyk, 2005).16 Iron transport-capacity of transferrin is limited and transferrin-bound iron turnover is relatively short-lived due to the quick transfer of freshly absorbed iron to other tissue compartments, i.e., mainly to the bone marrow for erythrocyte synthesis but also to liver and muscle tissues.
3.3 Extent of systemic iron absorption
The absorption of [58Fe]SBR759-related iron was investigated in blood samples collected at baseline and compared to those measured 14 and 21 days after administration (Table 4) (Fig. 1).| Subject | Blood collected after 14 days | Blood collected after 21 days | ||
|---|---|---|---|---|
| Calculated amount of absorbed iron | Calculated amount of absorbed iron | |||
| Absolute value (mg) | Relative to total iron dose (%) | Absolute value (mg) | Relative to total iron dose (%) | |
| 5101 | 13.4 | 0.53 | 13.9 | 0.54 |
| 5102 | 6.8 | 0.27 | 7.3 | 0.29 |
| 5103 | 11.5 | 0.45 | 11.8 | 0.46 |
| 5104 | 6.8 | 0.27 | 7.3 | 0.28 |
| 5105 | 7.0 | 0.27 | 7.3 | 0.28 |
| 5106 | 3.8 | 0.15 | 4.1 | 0.16 |
| 5107 | 8.2 | 0.33 | 8.7 | 0.34 |
| 5108 | 4.3 | 0.17 | 4.4 | 0.17 |
| 5109 | 3.5 | 0.14 | 3.8 | 0.15 |
| 5110 | 9.8 | 0.39 | 10.4 | 0.41 |
| 5111 | 4.7 | 0.18 | 5.0 | 0.20 |
| 5112 | 8.7 | 0.34 | 9.2 | 0.36 |
| Arithmetic mean | 7.4 | 0.29 | 7.8 | 0.30 |
| Median | 6.9 | 0.27 | 7.3 | 0.29 |
| Minimum | 3.5 | 0.14 | 3.8 | 0.15 |
| Maximum | 13.4 | 0.53 | 13.9 | 0.54 |
| First quartile (25%) | 4.6 | 0.18 | 4.9 | 0.19 |
| Third quartile (75%) | 9.0 | 0.35 | 9.5 | 0.37 |
| Standard deviation | 3.1 | 0.12 | 3.2 | 0.12 |
| CV (%) | 42 | 41 | 41 | 40 |
Total blood iron was measured by MC-ICP-MS and applying the stable isotope dilution principle. Assuming that approx. 80% of absorbed SBR759-related iron is incorporated into erythrocytes (Bernat, 1983),32 the amount of absorbed iron ranged from 3.8 mg (subject 5109) to 13.9 mg (subject 5101) at Day 21 following [58Fe]SBR759 administration. The arithmetic mean was 7.8 mg which corresponds to about 0.3% of the iron dose in SBR759. Compared to the daily iron uptake of 1 to 2 mg in healthy male adults, a mean uptake of 7.8 mg appears to be relatively high. An extrapolation of these data to the target CKD patient population is tainted with uncertainties because in contrast to healthy subjects, CKD patients do not absorb orally administered iron salts well (Hörl, 2007).37
In a recent iron absorption study (Geisser, 2010)38 with 10 g 59Fe-radiolabeled PA21 (an iron-based polynuclear iron(III) oxy-hydroxide compound), which was administered to CKD patients and healthy volunteers (HVs), the HVs have taken up 8.3 mg iron, and CKD patients 10-fold less (∼0.8 mg). This malabsorption has been linked to the inflammatory state associated with CKD and up-regulation of hepcidin expression (Hörl, 2007)37 (Andrews, 2007).39 Although there is a hypothetical risk for local toxicity of SBR759 with chronic administration, the assessment of such a risk is out of scope for this abbreviated trial and is a more appropriate question for chronic toxicology studies in animals and additional clinical testing in the target patient population which would be required prior to health authority approval (Block, 2010)9 (Chen, 2011)10 (Fukagawa, 2014).11 In addition, considering the low bioavailability of iron from the SBR759 drug substance compared to that expected from therapeutic oral iron supplements (e.g. ferrous sulfate) which are administered by thousands of patients per day, the risk of ROS-related GIT damage appears to be minimal.
4 Conclusions
• The results of this phase I mechanistic iron uptake study provide insight into the potential for iron absorption from a daily dose of 12 g (divided in 3 × 4 g) of [58Fe]SBR759 to healthy male subjects.• The study demonstrated absorption of 0.15 to 0.54% of the total daily administration of [58Fe]SBR759-related iron which amounted on average to 7.8 mg; approx. 5-fold more than the basal daily iron absorption (1 to 2 mg).
• [58Fe]SBR759-related iron transiently appeared in blood and plasma within the first 26 hours post first dose, followed by second later phase in blood ranging over 3 weeks which is characterized by redistribution of [58Fe]SBR759-related iron from blood into other relevant iron storage tissue compartments such as bone marrow, liver and muscle.
• In an interdisciplinary collaboration of the pharmaceutical industry with the academic environment in the field of Geosciences, the MC-ICP-MS technique was successfully applied for reliably measuring iron absorption and ferrokinetics in human blood and plasma with high specificity, sensitivity, accuracy and precision.
Abbreviations
| CKD | Chronic kidney disease |
| GMP | Good manufacturing practice |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| MC-ICP-MS | Multi-collector inductively coupled plasma mass spectrometry. |
Authors contribution
Authors in alphabetical order.Participated in research design: Gschwind, Schmid, Slade, Stitah, Swart, von Blanckenburg. Conducted experiments: Kaufmann, van Zuilen, Oelze, Schmid, Stitah. Performed data analysis: Gschwind, van Zuilen, Oelze, Schmid, von Blanckenburg. Wrote or contributed to the writing of the manuscript: Gschwind, Kaufmann, Schmid, Slade, Stitah, Swart, von Blanckenburg.
Acknowledgements
Michael Seiberling, MD, Clinical Investigator (Covance Clinical Research Unit AG, Switzerland). Andreas Helfrich, PhD, (Solvias AG, Analytics, Basel, Switzerland) for ICP-OES measurements. Zdenek Zencak, PhD, (Technical Research and Development, Novartis, Basel, Switzerland) for analyzing the [58Fe]SBR759 drug product. Prof. Kaspar Hegetschweiler, PhD, (Universität des Saarlandes, Germany) for scientific consultancy, Prof. Jochen Litterst, PhD, (Technische Universität Braunschweig, Germany) for Mössbauer spectroscopic investigations and Ernst Küsters, PhD, (Novartis Basel, Switzerland) for investigations of the redox activity of SBR759. Hendrik Andres, PhD, and Thomas Moenius, PhD, for scientific advices and managing the purchase order of 58Fe metal (Isotope Laboratory, Novartis Basel, Switzerland). We acknowledge Ingo Horn, PhD, (Leibniz Universität Hannover, Germany) for supporting mass spectrometric measurements, Prof. Ronny Schoenberg, PhD, (University Tübingen, Germany) for advice in the design of this study. We thank Prof. Thomas Walczyk, PhD, (National University of Singapore) and an anonymous manuscript reviewer for their constructive comments.References
- A. J. Bleyer, S. K. Burke, M. Dillon, B. Garrett, K. S. Kant, D. Lynch, S. N. Rahman, P. Schoenfeld, I. Teitelbaum, S. Zeig and E. Slatopolsky, A comparison of a calcium free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in haemodialysis patients, Am. J. Kidney Dis., 1999, 33, 694–701 CrossRef CAS.
- P. C. D'Haese, G. B. Spasovski, A. Sikole, A. Hutchison, T. J. Freemont, S. Sulkova, C. Swanepoel, S. Pejanovic, L. Djukanovic, A. Balducci, G. Coen, W. Sulowicz, A. Ferreira, A. Torres, S. Curic, M. Popovic, N. Dimkovic and M. E. De Broe, A multicenter study on the effects of lanthanum carbonate (Fosrenol™) and calcium carbonate on renal bone disease in dialysis patients, Kidney Int., 2003, 63, S73–S78 CrossRef.
- B. Brezina, W. Y. Qunibi and C. R. Nolan, Acid loading during treatment with sevelamer hydrochloride: mechanisms and clinical implications, Kidney Int., 2004, 66(suppl 90), S39–S45 CrossRef PubMed.
- A. J. Hutchison, Oral phosphate binders, Kidney Int., 2009, 75(9), 906–914 CrossRef CAS PubMed.
- A. J. Hutchison, B. Maes, J. Vanwalleghem, G. Asmus, E. Mohamed, R. Schmieder, W. Backs, R. Jamar and A. Vosskühler, Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6 month, randomized, comparative trial versus calcium carbonate, Nephron, 2005, 100, c8–c19 CAS.
- E. Slatopolsky, H. Liapis and J. Finch, Progressive accumulation of lanthanum in the liver of normal and uremic rats, Kidney Int., 2005, 68, 2809–2813 CrossRef CAS PubMed.
- G. B. Spasovski, A. Sikole, S. Gelev, J. Masin-Spasovska, T. Freemont, I. Webster, M. Gill, C. Jones, M. E. De Broe and P. C. D'Haese, Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up, Nephrol., Dial., Transplant., 2006, 621, 2217–2224 CrossRef PubMed.
- (a) O. Hergesell and E. Ritz, Stabilized polynuclear iron hydroxide is an efficient oral phosphate binder in uraemic patients, Nephrol., Dial., Transplant., 1999, 14, 863–867 CrossRef CAS PubMed; (b) O. Hergesell and E. Ritz, Phosphate binders on iron basis: a new perspective?, Kidney Int., 1999, 73(suppl), S42–S45 CrossRef CAS.
- G. A. Block, S. L. Brillhart, M. S. Persky, A. Amer and A. J. Slade, Efficacy and safety of SBR759, a new iron-based phosphate binder, Kidney Int., 2010, 77(10), 897–903 CrossRef CAS PubMed.
- J. B. Chen, S. S. Chiang, H. C. Chen, S. Obayashi, M. Nagasawa, J. M. Hexham, A. Balfour, G. Junge, T. Akiba and M. Fukagawa, Efficacy and safety of SBR759, a novel calcium-free, iron(III)-based phosphate binder, in Asian patients undergoing hemodialysis: A 12 week, randomized, open-label, dose-titration study versus sevelamer hydrochloride, Nephrology, 2011, 16(8), 743–750 CrossRef CAS PubMed.
- M. Fukagawa, H. Kasuga, D. Joseph, H. Sawata, G. Junge, A. Moore and T. Akiba, Efficacy and safety of SBR759, a novel calcium-free, iron(III)-based phosphate binder, versus placebo in chronic kidney disease stage V Japanese patients on maintenance renal replacement therapy, Clin. Exp. Nephrol., 2014, 18(1), 135–143, DOI:10.1007/s10157-013-0815-7.
- L. Sigg and W. Stumm, The interaction of anions and weak acids with the hydrous goethite (α-FeOOH) surface, Colloids Surf., 1980, 2(2), 101–117 CrossRef.
- R. L. Parfitt, R. J. Atkinson and R. S. C. Smart, The mechanism of phosphate fixation by iron oxides, Soil Sci. Soc. Am. Proc., 1975, 39, 837–841 CrossRef CAS.
- G. J. Anderson, Mechanisms of iron loading and toxicity, Am. J. Hematol., 2007, 82, 1128–1131 CrossRef CAS PubMed.
- V. Chávez, A. Mohri-Shiomi, A. Maadani, L. A. Vega and D. A. Garsin, Oxidative Stress Enzymes Are Required for DAF-16-Mediated Immunity Due to Generation of Reactive Oxygen Species by Caenorhabditis elegans, Genetics, 2007, 176(3), 1567–1577 CrossRef PubMed.
- T. Walczyk and F. von Blanckenburg, Deciphering the iron isotope message of the human body, Int. J. Mass Spectrom., 2005, 242(2–3), 117–134 CrossRef CAS PubMed.
- F. von Blanckenburg, M. Oelze, D. G. Schmid, K. van Zuilen, H.-P. Gschwind, A. J. Slade, S. Stitah, D. Kaufmann and P. Swart, An iron stable isotope comparison between human erythrocytes and plasma, Metallomics 10.1039/C4MT00124A.
- P. G. Whittaker, T. Lind, J. G. Williams and A. L. Gray, Inductively coupled plasma mass spectrometric determination of the absorption of iron in normal women, Analyst, 1989, 114(6), 675–678 RSC.
- J. F. R. Barrett, P. G. Whittaker, J. G. Williams and T. Lind, Absorption of non-heme iron in normal women measured by the incorporation of two stable isotopes into erythrocytes, Clin. Sci., 1992, 83(2), 213–219 CAS.
- M. Janghorbani, B. T. G. Ting and S. J Fomon, Erythrocyte incorporation of ingested stable isotope of iron (58Fe), Am. J. Hematol., 1986, 21(3), 277–288 CrossRef CAS.
- S. J. Fomon, M. Janghorbani, B. T. Ting, E. E. Ziegler, R. R. Rogers, S. E. Nelson, L. S. Ostedgaard and B. B. Edwards, Erythrocyte incorporation of ingested 58 iron by infants, Pediatr. Res., 1988, 24(1), 20–24 CrossRef CAS PubMed.
- S. J. Fomon, E. E. Ziegler, R. R. Rogers, S. E. Nelson, B. B. Edwards, D. G. Guy, J. C. Erve and M. Janghorbani, Iron absorption from infant foods, Pediatr. Res., 1989, 26(3), 250–254 CrossRef CAS PubMed.
- J. C. Woodhead, J. M. Drulis, R. R. Rogers, E. E. Ziegler, P. J. Stumbo, M. Janghorbani, B. T. Ting and S. J. Fomon, Use of the stable isotope, 58Fe, for determining availability of nonheme iron in meals, Pediatr. Res., 1988, 23(5), 495–499 CrossRef CAS PubMed.
- T. Walczyk, L. Davidsson, N. Zavaleta and R. F. Hurrell, Stable isotope labels as a tool to determine the iron absorption by Peruvian school children from a breakfast meal, Fresenius' J. Anal. Chem., 1997, 359(4–5), 445–449 CrossRef CAS.
- E. Vasquez Garibay, I. Santos Torres, S. E. Nelson, E. E. Ziegler, R. R. Rogers, M. Janghorbani and S. J. Fomon, Iron absorption during recovery from malnutrition, J. Am. Coll. Nutr., 2001, 20(4), 286–292 CrossRef CAS.
- A. N. Halliday, D. C. Lee, J. N. Christensen, M. Rehkämper, W. Yi, X. Luo, C. M. Hall, C. J. Ballentine, T. Pettke and C. Stirling, Applications of multiple collector-ICPMS to cosmochemistry, geochemistry and paleoceanography, Geochim. Cosmochim. Acta, 1998, 62, 919–940 CrossRef CAS.
- N. Jakubowski, T. Prohaska, F. Vanhaecke, P. H. Roos and T. Lindemann, Inductively coupled plasma- and glow discharge plasma-sector field mass spectrometry, J. Anal. At. Spectrom., 2011, 26, 727–757 RSC.
- B. S. Skikne, N. Ahluwalia, B. Fergusson, A. Chonko and J. D. Cook, Effects of erythropoietin therapy on iron absorption in chronic renal failure, J. Lab. Clin. Med., 2000, 135(6), 452–458 CrossRef CAS PubMed.
- Institute for Reference Materials and Measurements, European Commission Joint Research Centre, Geel, Belgium, http://https://irmm.jrc.ec.europa.eu/refmat_pdf/IRMM-014_cert.pdf.
- R. Schoenberg and F. von Blanckenburg, An assessment of the accuracy of stable Fe isotope ratio measurements on samples with organic and inorganic matrices by high-resolution multicollector ICP-MS, Int. J. Mass Spectrom., 2005, 242(2–3), 257–272 CrossRef CAS PubMed.
- J. R. DeLaeter, J. K. Boehlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor, Atomic weights of the elements: Review 2000 (IUPAC technical report), Pure Appl. Chem., 2003, 75(6), 683–800 CAS.
- I. Bernat, Incorporation of radioiron into the erythroblasts and reticulocytes. Iron utilization in the course of red cell production. Effective erythropoiesis, in Iron Metabolism, ed. A. Kiado, Plenum Press, New York, 1983, ch. 14, pp. 167–181 Search PubMed.
- F. von Blanckenburg, J. Noordmann and M. Guelke-Stelling, The iron stable isotope fingerprint of the human diet, J. Agric. Food Chem., 2013, 61, 11893–11899 CrossRef CAS PubMed.
- Geigy, Scientific Tables, Geigy Scientific Tables, ed. C. Lentner, Physical Chemistry, Composition of blood, Hematology, Somatometric Data, Ciba-Geigy Limited, Medical Education Division, Ciba-Geigy Corporation, Basle, Switzerland, West Caldwell, New Jersey, 8th edn, 1984, vol. 3, pp. 65–67 and pp. 84–85 Search PubMed.
- B. Davies and T. Morris, Physiological parameters in laboratory animals and humans, Pharm. Res., 1993, 10(7), 1093–1095 CrossRef CAS.
- M. C. Pruchnicki, J. D. Coyle, S. Hoshaw-Woodard and W. H. Bay, Effect of phosphate binders on supplemental iron absorption in healthy subjects, J. Clin. Pharmacol., 2002, 42(10), 1171–1176 CrossRef CAS PubMed.
- W. H. Hörl, Iron therapy for renal anemia: how much needed, how much harmful, Pediatr. Nephrol., 2007, 22, 480–489 CrossRef PubMed.
- P. Geisser and E. Philipp, PA21: A novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease, Clin. Nephrol., 2010, 74(1), 4–11 CrossRef CAS.
- N. C. Andrews, in Principles of Molecular Medicine, ed. M. S. Runge and C. Patterson, Iron metabolism, Humana Press, Inc., Totowa, NJ, 2nd edn, 2007, ch. 87 Search PubMed.
Footnotes |
| † Present address: GFZ German Research Center for Geosciences, Helmholtz Centre Potsdam, Germany. |
| ‡ Present address: Institute of Geological Sciences, University of Berne, Switzerland. |
| This journal is © The Royal Society of Chemistry 2014 |