Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Quantification of pharmaceutical peptides using selenium as an elemental detection label
Laura Hyrup
Møller
a,
Charlotte
Gabel-Jensen
a,
Henrik
Franzyk
b,
Jesper Søborg
Bahnsen
a,
Stefan
Stürup
a and
Bente
Gammelgaard
*a
aUniversity of Copenhagen, Department of Pharmacy, Denmark. E-mail: bente.gammelgaard@sund.ku.dk
bUniversity of Copenhagen, Department of Drug Design and Pharmacology, Denmark
First published on 7th July 2014
Abstract
The aim of the present work was to demonstrate how selenium labelling of a synthetic cell-penetrating peptide may be employed in evaluation of stability and quantitative estimation of cellular uptake by inductively coupled plasma mass spectrometry (ICP-MS). Two analogues of the cell-penetrating peptide, penetratin, were synthesized, one with selenomethionine (SeMet) added at the N-terminal of the peptide (N-PenMSe) and the other with the internal methionine (Met) replaced with SeMet (i-PenMSe). The purity of the synthesized peptides was 92% for N-PenMSe and 89% for i-PenMSe as determined by liquid chromatography (LC)-ICP-MS. The selenium-labelled peptides were investigated by cell uptake studies in HeLa WT cells. The stability of the peptides was monitored in water, cell medium and during cell uptake studies. Total uptake of selenium was quantified by flow injection (FI)-ICP-MS. Speciation analysis of cell samples by LC-ICP-MS showed mainly uptake of the intact peptides, while the amount of intact peptides in cell lysates was semi-quantitatively determined. The selenium-containing penetratin analogues were to some extent degraded in pure cell medium, while an extensive degradation was observed during cell uptake studies. The major degradation products were determined by LC-electrospray ionization mass spectrometry (ESI-MS). The labelling method in combination with FI-ICP-MS, LC-ICP-MS and LC-ESI-MS techniques provided detailed information on the fate of penetratin in cellular uptake studies. Most pharmaceutical peptides, including penetratin, are synthetic analogues of endogenous peptides, and incorporation of selenium may improve the critical assessment of the native drug or drug delivery candidate early in the drug development process.
Introduction
Peptides are involved in a large variety of physiological processes and cell functions and several peptide drugs have already been successfully applied for treatment of, for instance, certain cancer forms and type 2 diabetes. The interest in exploiting peptides as drug substances has increased rapidly during the last few decades. Although the annual sale of peptide drugs is only a few percent of the global drug market, it is increasing twice as fast as the market for classical small molecules.1 In the period of 2000–2012, marketing approvals for 19 peptides were obtained in USA, six of these were approved in 2012. A large number of peptides are in the pipeline and several have reached the Phase II and Phase III clinical trials. The therapeutic areas of the Phase I and Phase II candidates are mainly metabolic diseases and oncology, while the major indications for the Phase III candidates are oncology and infectious diseases.2Peptides often offer high efficacy, selectivity and specificity as drug candidates, and as their degradation products are amino acids and smaller peptides they are associated with low risk of systemic toxicity. However, peptides exhibit low oral bioavailability owing to their high propensity to enzymatic degradation in the gastro-intestinal tract combined with a low intestinal barrier passage.3 Development of drug delivery systems is therefore an integrated research area for improving the bioavailability of potential drug candidates.4 Drug delivery by nanoparticles and development of cell penetrating peptides (CPPs) are examples of such efforts.5
In order to monitor the stability, pharmacokinetics and metabolism of pharmaceutical peptides, development of sensitive quantitative analytical methods for peptides is of great importance. A challenge in monitoring the fate of peptides in complex biological systems is to obtain adequate selectivity and sensitivity.
Pharmaceutical peptides comprising 5–50 amino acids are predominantly produced by solid-phase peptide synthesis (SPPS). This opens up the possibility of introducing a label for detection and thereby enhance analytical selectivity.3,6
Quantification of peptides has traditionally been performed by liquid chromatography (LC) with detection by UV absorption7–9 or fluorescence emission spectroscopy.10,11 However, UV detection has limited sensitivity and specificity, whereas fluorescence detection demands either inherent fluorescent properties of the biomolecule or labelling with a fluorophore. With development of high-resolution mass spectrometry (MS), this technique is increasingly applied in quantitative peptide analysis.12 Recently, inductively coupled plasma mass spectrometry (ICP-MS) was proposed as a complementary method to molecular MS for quantification of peptides. The advantages of ICP-MS are low detection limits, a wide dynamic range and in principle matrix-independent sensitivity of different species of the heteroelements. This opens up the opportunity for quantification by using a single inorganic element standard in contrast to species-specific standards needed for molecular MS.13,14 However, detection of C, O and N, the main constituents of biomolecules, is not possible by ICP-MS due to use of an open plasma causing interferences from the air. Fortunately, a wide range of proteins naturally contain ICP-MS detectable heteroelements, e.g. S in methionine (Met) and cysteine (Cys), Se in selenoproteins, P in phosphoproteins or I in thyroid hormones. The occurrence of selenoproteins, phosphoproteins and thyroid hormones is moderate in number, and sulfur analysis is restricted due to low ionization efficiency and spectral interferences in ICP-MS resulting in lower sensitivity.13 In order to enhance sensitivity, several different ICP-MS sensitive labels have been suggested, and these possibilities have been reviewed by Prange and Pröfrock,13 Kretschy et al.15 and Wang et al.16 Two different approaches are generally pursued when labelling biomolecules: pre-labelling and post-labelling. In proteomics, post-labelling is often applied via labelled antibodies targeting the biomolecule17 or through direct conjugation with derivatizing agents.18 In the pre-labelling approach the biomolecule is labelled prior to its introduction into a biological system and hence it remains detectable throughout the experiment. This approach is mainly used when analyzing smaller biomolecules as in the present work.
The ideal peptide labelling probe should enhance the sensitivity and neither affect the functionality of the peptide nor the analytical properties. Incorporation of the label should be well controlled, easy to handle and stoichiometrically unequivocal. All these requirements are not readily achieved. Only a few studies on elemental labelling for ICP-MS analysis of therapeutic peptides have been reported, mainly involving chelating agents.15,19 Chelating agents typically consist of a lanthanide/metal chelating moiety linked to a reactive group targeting a specific functional group in the peptide, e.g. primary amines or thiols in cysteines. Liu et al. addressed the importance of monitoring the labelling procedure ensuring 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 labelling stoichiometry. They reported that a mixture of labelled bradykinin with peptide
1 labelling stoichiometry. They reported that a mixture of labelled bradykinin with peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) label ratios of 1
label ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained by use of a reactive moiety containing two reactive groups, diethylenetriamine-N,N,N′,N′′,N′′-pentaacetic dianhydride (DTPAA), selective for conjugation to primary amines.20 In addition, labelling with DTPAA may result in diastereomeric mixtures of the labelled peptides challenging the subsequent analysis.21 Labelling reactions have been optimized to avoid formation of diastereomers,22 however, consideration for the possible presence of multiple labelling sites in the peptide is important. Furthermore, subsequent separation of free label and labelled peptide is necessary during pre-labelling.23 A few studies have demonstrated labelling with indium by use of DOTA (1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid), introducing the label during peptide synthesis, ensuring 1
1 was obtained by use of a reactive moiety containing two reactive groups, diethylenetriamine-N,N,N′,N′′,N′′-pentaacetic dianhydride (DTPAA), selective for conjugation to primary amines.20 In addition, labelling with DTPAA may result in diastereomeric mixtures of the labelled peptides challenging the subsequent analysis.21 Labelling reactions have been optimized to avoid formation of diastereomers,22 however, consideration for the possible presence of multiple labelling sites in the peptide is important. Furthermore, subsequent separation of free label and labelled peptide is necessary during pre-labelling.23 A few studies have demonstrated labelling with indium by use of DOTA (1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid), introducing the label during peptide synthesis, ensuring 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 labelling stoichiometry.24,25 The advantage of lanthanide or metal chelating agents is considerably enhanced sensitivity of the biomolecule during ICP-MS analysis, however, the relatively large chelating moiety will affect the peptide structure and thereby the physico-chemical properties and potentially cell uptake, which is undesirable in studies employing pre-labelled peptides.
1 labelling stoichiometry.24,25 The advantage of lanthanide or metal chelating agents is considerably enhanced sensitivity of the biomolecule during ICP-MS analysis, however, the relatively large chelating moiety will affect the peptide structure and thereby the physico-chemical properties and potentially cell uptake, which is undesirable in studies employing pre-labelled peptides.
In the present study, we introduce selenium as a label in peptides by substitution of Met with the naturally occurring selenium analogue, selenomethionine (SeMet) during peptide synthesis. Selenium labelling constitutes an attractive non-interfering method of probing biological systems, while ensuring unambiguous labelling stoichiometry. Sulfur and selenium belong to the group of chalcogens in the periodic table and thus display similar chemical characteristics.26 Due to these comparable properties, it may be assumed that peptides incorporating SeMet instead of Met will display similar biological effects. This is corroborated by the fact that SeMet may randomly be exchanged with Met in humans as the body cannot distinguish between Met and SeMet.27 Synthetic selenopeptides have been examined for a wide range of applications, for instance, synthetic exchange of disulfide bridges in peptides with diselenide or selenosulfo bridges has been reported for a wide range of bioactive peptides in order to improve stability.28 Lang et al. have proposed introduction of SeMet as an improved target for thiophilic labelling with iodoacetamide reagents as compared to Met, since selenium is more nucleophilic as compared to sulfur.29 To the best of our knowledge, selenium has not previously been introduced as a synthetic label for detection in quantitative analysis.
The aim of the present work was to explore whether selenium labelling of a synthetic cell-penetrating peptide may be employed in evaluation of stability and quantitative cell uptake by ICP-MS. Labelling with SeMet was demonstrated for the cell-penetrating peptide (CPP) penetratin, consisting of 16 amino acids including a Met residue, RQIKIWFQNRRMKWKK. The peptide was originally derived from the Drosophila antennapedia transcription protein, and this 16-residue sequence was shown to be one of the minimal peptide sequences maintaining cell-penetrating properties.30 In addition, penetratin is able to deliver covalently conjugated drug cargoes through cell membranes, thus offering potential opportunities in the development of new drug delivery systems.5,31 Two selenium-containing analogues were investigated, one with SeMet added at the N-terminal of the peptide (N-PenMSe) and the other with the internal Met replaced with SeMet (i-PenMSe).
Experimental
Instrumentation
Reagents
Rink amide resin, coupling reagents and amino acid building blocks for solid-phase peptide synthesis as well as supplementary solvents and chemicals were acquired from Iris Biotech (Marktredwitz, Germany). L-Selenomethionine (SeMet) was obtained from TCI Europe. For mobile phases, glacial acetic acid, trifluoroacetic acid (TFA) and methanol were obtained from BDH Prolabo (France).Procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN 95
MeCN 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with 0.1% TFA) in eluent A (H2O
5 with 0.1% TFA) in eluent A (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN 5
MeCN 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 with 0.1% TFA) within 20 min was applied. The flow rate was 20 mL min−1.
95 with 0.1% TFA) within 20 min was applied. The flow rate was 20 mL min−1.
Cell culture
The human cervical cancer cell line HeLa WT was obtained from American Type Culture Collection (ATCC) and maintained in Eagle's minimal essential medium (EMEM) supplemented with penicillin (100 U mL−1), streptomycin (100 μg mL−1), L-glutamine (2 mM), non-essential amino acids (0.1 mM), sodium pyruvate (1 mM) and 10% fetal bovine albumin (FBS) (Fischer Scientific, Slangerup, Denmark). The cells were grown in an atmosphere of 5% CO2 at 37 °C to 80% confluence and then sub-cultured at a ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 twice a week. Cells, 7.6 × 104, were seeded onto 24-well plates (Fischer Scientific, Slangerup, Denmark).
5 twice a week. Cells, 7.6 × 104, were seeded onto 24-well plates (Fischer Scientific, Slangerup, Denmark).
Cell uptake studies
Experiments were performed 22–24 h after seeding of the HeLa WT cells; by then, a sub-confluent monolayer (80–90% confluence) was achieved. Peptide solutions (10 μM) in Hanks Balanced Salt Solution (HBSS; Gibco, Paisley, UK) supplemented with 10 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES, SigmaUltra) were prepared immediately prior to use in cell uptake studies from aqueous 200 μM peptide stock solutions prepared within 2 weeks from the uptake experiment and stored at 4 °C. The uptake study was initiated by washing the cells once with 37 °C phosphate buffered saline (PBS, Sigma), and subsequent addition of 400 μL of peptide solution. The cells were then incubated with peptide solution for 2 h on an orbital shaker (90 rpm) at 37 °C. After exactly 2 h, medium was collected and immediately acidified with acetic acid to a final concentration of 2%. The cell uptake was terminated by washing the cells four times with ice-cold PBS, and then the cells were lysed by addition of 100 μL of 0.1% Triton-X 100 in 2% acetic acid and kept on ice for 10 min. The lysis mixture was transferred into low-binding Eppendorf tubes (Alpha Laboratories), centrifuged at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g and the supernatant was collected for analysis. All samples were stored at −18 °C until analysis.
000 g and the supernatant was collected for analysis. All samples were stored at −18 °C until analysis.
Determination of total selenium by flow injection analysis
Total selenium was determined by flow injection analysis (FI) using an aqueous carrier fluid of 0.02% trifluoroacetic acid (TFA), 0.1% acetic acid and 5% methanol. The flow rate was 200 μL min−1 and the injection volume was 10 μL. Quantification was performed by single standard calibration based on peak areas of a 50 μg L−1 solution of certified selenium element standard (Se concentration of 1010 mg L−1, PlasmaCAL, SCP Science, Canada).LC-ICP-MS (Aridus)
For LC-ICP-MS analysis, an Agilent 1100 series HPLC system equipped with a degasser, a quaternary pump, an autosampler and a column oven was used. The column was an Aeris PEPTIDE XB-C18, 3.6 μm, 100 × 2.1 mm ID column protected by a C18-Peptide SecurityGuard ULTRA cartridge (Phenomenex, SupWare, Denmark). A linear gradient of 20–80% methanol, (with 0.1% acetic acid and 0.05% TFA added) within 10 min was applied with a flow rate of 200 μL min−1, a column temperature of 60 °C, and an injection volume of 5 μL. Semiquantitative peptide uptake was determined by use of a single standard solution of 2.4 μM i-PenMSe standardized for total selenium content with a certified selenium element standard (PlasmaCAL, SCP Science, Canada), concentration of 1010 mg L−1 of Se.LC-ESI-MS
UHPLC-ESI-MS analysis was performed on a Dionex Ultimate 3000 UHPLC (Thermo Scientific) equipped with a degasser, a quaternary pump, an autosampler, a thermostated column compartment and a diode array detector (DAD) and hyphenated to a Q Exactive Orbitrap mass spectrometer. The column and mobile phase described for LC-ICP-MS were applied for this system as well. However, the linear gradient was shortened to 20–62% methanol (with 0.1% acetic acid and 0.05% TFA added) within 7 min to reduce time of analysis. MS data were collected between 2.5 and 10 min.Results and discussion
Peptide synthesis
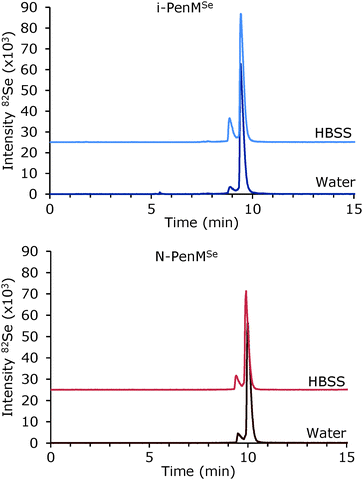
The obvious approach for labelling a peptide with selenium would be to substitute a sulfur-containing amino acid with its selenium analogue, i.e. selenocysteine (SeCys) or selenomethionine (SeMet). Cysteine is usually avoided in peptide synthesis because of high redox potentials and thereby low stability. Selenoamino acids are generally more readily oxidized during synthesis and purification compared to the sulfur analogues; with the reactivity of SeCys exceeding that of SeMet.26 Thus, SeMet was chosen for selenium labelling in the present study. Exchange of Met in penetratin with other neutral amino acids, leucine (Leu) or alanine (Ala), has been reported in the literature without a significant change in cell uptake in HeLa WT cells and HaCAT cells respectively.32,33 Hence, exchange of Met appears to be a convenient labelling site in penetratin.Synthesis was carried out by employing microwave (MW)-assisted automated solid-phase peptide synthesis (SPPS). In order to test whether it was possible to introduce SeMet into the peptide sequence with a reasonable yield and purity, a penetratin analogue with SeMet added at the N-terminal (N-PenMSe) was initially synthesized (sequence shown in Table 1). As a standard C → N SPPS strategy was applied, the peptide was conveniently modified by introduction of SeMet at the N-terminal subsequently to MW-assisted automatic synthesis. Thereby, minimum exposure of SeMet to reagents was ensured, and thus the risk of SeMet oxidation was minimized. Also, it was intended to avoid MW irradiation in this initial labelling experiment as it might not be compatible with SeMet. Furthermore, N-terminal labelling of penetratin has been widely used for introduction of fluorescent dyes and covalent attachment of drug cargoes.34–36 Synthesis and purification of N-PenMSe was accomplished, and purity of the resulting selenopeptide was determined by LC-ICP-MS to be 92% as illustrated in Fig. 1. It appears from the figure that an impurity, eluting immediately before the desired peptide, was present. By LC-ESI-MS, a signal was observed at m/z 606.831, corresponding to the [M + 4H]4+ ion of a peptide with mass 2423.293 confirming N-PenMSe (2423.290 Da) as the primary product (ΔM ≤ 1 ppm). A signal from the impurity was seen at m/z 606.326 corresponding to the [M + 4H]4+ ion representing a mass of 2421.273 Da (ΔM ≤ 1 ppm) which is 2.020 Da less than that of N-PenMSe. A difference of 2.020 Da might arise from loss of two hydrogen atoms (2.016 Da) due to cyclization or formation of a double bond.
| Abbreviation | Sequence | M monoisotopic | M average |
|---|---|---|---|
| i-PenMSe | RQIKIWFQNRR(MSe)KWKK-NH2 | 2292.250 | 2292.638 |
| N-PenMSe | MSe-RQIKIWFQNRRMKWKK-NH2 | 2423.290 | 2423.834 |
Introduction of SeMet within the peptide sequence by exchange of Met was then pursued. By fully automated MW-assisted SPPS, SeMet was incorporated at the original position of Met into penetratin i-PenMSe, the sequence is shown in (Table 1). Purity of the synthesized peptide was determined by LC-ICP-MS to be 89%. LC-ESI-MS revealed a signal at m/z 1147.132 corresponding to the [M + 2H]2+ ion of the peptide, confirming the identity of i-PenMSe (2292.250 Da) with a mass of 2292.248 Da (ΔM ≤ 1 ppm) as the primary product. The [M + 2H]2+ ion originating from the impurity was observed at m/z 1155.131 corresponding to a mass of 2308.246 Da (ΔM ≤ 1 ppm). The mass increase of 15.998 Da indicated the presence of an additional oxygen atom (15.995 Da) that may arise from oxidation of the peptide, most likely in the SeMet residue. Oxidation of SeMet may have occurred during synthesis due to the exposure to oxygen at elevated temperatures in the synthesizer or during the purification process.
As only the oxidized impurity was identified for i-PenMSe, mild synthesis conditions appear to be important in order to avoid oxidation of SeMet. Furthermore, a slightly higher peptide purity (92%) was obtained for N-PenMSe as compared to i-PenMSe (89%). The purity of i-PenMSe might be improved in future by manual synthesis of the peptide or by using lower temperatures during coupling and deprotection steps. However, manual peptide synthesis is considerably more time-consuming, favoring automated synthesis. Improving the preparative HPLC method might also result in increased purity of the final product.
The stability of i-PenMSe in water was monitored by LC-ICP-MS during a two-month storage period. The ratio between the intact peptide and the impurity remained constant throughout the period (data not shown) confirming that the SeMet-labelled peptide was stable in aqueous solution. By dilution of the selenopeptides in HBSS medium, a decrease of the intact peptide was immediately observed in LC-ICP-MS chromatograms (Fig. 1). The mechanism behind this increased degradation is not clarified, but may be due to the higher ionic strength of the HBSS medium. The content of intact i-PenMSe decreased from 89% to 81%, while the content of intact N-PenMSe decreased from 92% to 86%. Only degradation to the formerly identified impurities was observed, thus no oxidation of N-PenMSe was seen, whereas the presence of an oxidation product of i-PenMSe increased. This indicated that oxidation may be dependent on the position of SeMet in the peptide sequence.
The stability of fluorescently labelled penetratin has been investigated in a number of studies, as the translocation of the peptide and its function as a drug carrier may be dependent on the stability. Tréhin and colleagues have shown considerable degradation of carboxyfluorescein-labelled penetratin (CF-penetratin) in HBSS after 21 days of incubation, since only 76% of the intact peptide remained.10 In PBS supplemented with 1 g L−1D-glucose, CF-penetratin was shown to be stable for only 1 h.37 Thus, instability is also observed for the native Met-based penetratin.
Cell uptake of selenopeptides
The total uptake of selenium from the peptides was determined by flow injection ICP-MS (FI-ICP-MS), (Table 2). SeMet was included as a positive control of the cell uptake experiment setup, as extensive cell uptake of SeMet has formerly been reported in e.g. the Caco-2 cell line.39 Considerable amounts of i-PenMSe and N-PenMSe were taken up by the cells, 17.4 ± 2.2 pmol per 104 cells of i-PenMSe and 25.4 ± 2.9 pmol per 104 cells of N-PenMSe, respectively. However, the selenium uptake from the peptides was not as pronounced as from SeMet, 49.6 ± 3.3 pmol per 104 cells. Blank lysates contained no selenium.
| Start Se | Total Se | Intact peptide | |
|---|---|---|---|
| pmol per 104 cells | pmol per 104 cells | pmol per 104 cells | |
| i-PenMSe | 505 | 17.4 ± 2.2 | 16.1 |
| N-PenMSe | 484 | 25.4 ± 2.9 | 18.4 |
| SeMet | 508 | 49.6 ± 3.3 | — |
The cellular uptake of penetratin has been studied by several groups in a variety of different human cell lines. The majority of these studies are based on fluorophore-labelled penetratin. Determination of CF-penetratin uptake in four different human carcinoma cell lines by total fluorescence spectrophotometry showed very different degrees of peptide internalization depending on the cell line.40,41 Lindgren and colleagues reported 14% uptake of total fluorescence of 2-aminobenzoic acid (Abz)-labelled penetratin in Caco-2 cells after 30 min incubation with 10 μM peptide.34 Sarko et al. investigated the cellular uptake of 111In-DOTA-labelled penetratin in six different human carcinoma cell lines, where the uptake was determined using a γ-counter. Upon incubation for 30 min with cells, the amount of internalized radioactive label varied remarkably between different cell lines.25 As HeLa WT cells were not among the investigated cell lines, these data are not directly comparable to those obtained in the present work. Bahnsen et al. reported a semi-quantitative uptake of CF-labelled penetratin analogues in HeLa WT cells via determination by flowcytometry.32 However, the results were presented as relative to CF-penetratin uptake and they are thus not comparable to those of the present work.
 | ||
| Fig. 2 LC-ICP-MS chromatograms of cell lysates from HeLa WT cells incubated with 10 μM selenopeptide for 2 h. | ||
FI-ICP-MS and LC-ICP-MS of HeLa WT cell lysates demonstrated that cellular uptake of i-PenMSe and N-PenMSe mainly represented internalization of intact peptides. A direct comparison of these results to the existing literature was not possible, as different cell models and labels were applied.
 | ||
| Fig. 3 Chromatograms of samples of Se-peptides in HBSS medium detected by ESI-MS and ICP-MS. MS chromatograms are extracted ion chromatograms (EIC) (i-PenMSem/z: [M + 2H]2+ 495.798; [M + 3H]3+ 515.637; [M + 2H]2+ 694.899; [M + 3H]3+ 722.721; [M + 3H]3+ 765.092; [M + 3H]3+ 770.425. N-PenMSem/z: [M + 3H]3+ 523.264; [M + 3H]3+ 573.997; [M + 3H]3+ 575.998; [M + 2H]2+ 585.290; [M + 3H]3+ 697.409; [M + 3H]3+ 766.400; [M + 3H]3+ 808.100; [M + 3H]3+ 808.774). Annotation of the peaks corresponds to peptide sequences presented in Table 3. | ||
In order to investigate the mode of degradation further, the samples from the HBSS medium from uptake studies were analyzed by LC-ESI-MS. Degradation products of i-PenMSe and N-PenMSe were identified via their m/z values, and sequences corresponding to these masses are suggested in Table 3. The main compound observed for both i-PenMSe and N-PenMSe was the intact peptide (Fig. 3, peak a). Similar cleavage sites were observed for i-PenMSe and N-PenMSe as illustrated in Fig. 4. Previous unpublished studies on penetratin stability by UPLC-QTOF identified cleavage sites in native penetratin when exposed to human and rat gastrointestinal fluid that correspond to those observed for selenium-labelled penetratin analogues in the present study.43 Cleavage at the C-terminal site of arginine (R) and lysine (K) was confirmed to be caused by trypsin whereas cleavage at the C-terminal site of phenylalanine (F) was caused by chymotrypsin.43 Thus, the degradation products c, d and e shown in Fig. 3 may be due to the presence of trypsin while product f may be a result of chymotrypsin. The composition of enzymes produced by HeLa cells is not reported, but some proteolytic activity is expected, which may include trypsin and chymotrypsin.
| i-PenMSe (1) | M monoisotopic | N-PenMSe (2) | M monoisotopic | |
|---|---|---|---|---|
| a | RQIKIWFQNRRMSeKWKK-NH2 | 2292.250 | MSeRQIKIWFQNRRKWKK-NH2 | 2423.290 |
| b | Oxidized i-PenMSe | 2308.245 | — | — |
| c | RQIKIWFQNRR-OH | 1543.885 | MSeRQIKIWFQNRR-OH | 1722.870 |
| d | RQIKIWFQNR-OH | 1387.784 | MSeRQIKIWFQNR-OH | 1566.769 |
| e | RQIKIWFQNRRMSeKWK-OH | 2165.139 | MSeRQIKIWFQNRRKWK-OH | 2296.17 |
| f | RQIKIWF-OH | 989.581 | MSeRQIKIWF-OH | 1168.566 |
| g | — | — | N-PenMSe – 2 Da | 2421.275 |
| h | — | — | QIKIWFQNRRKWKK-NH2 | 2088.204 |
 | ||
| Fig. 4 Cleavage sites of i-PenMSe and N-PenMSe after 2 h of incubation at 37 °C with HeLa WT-cells. Annotation corresponds to peaks in chromatograms in Fig. 3. | ||
The degradation products identified from i-PenMSe and N-PenMSe are listed in Table 3 and differed in the presence of selenium. In extracted ion chromatograms (EIC) of the LC-ESI-MS analysis, dissimilarities were at first observed by just a minor difference in the retention time of the peaks in the chromatograms (Fig. 3). Comparison to the LC-ICP-MS chromatograms revealed that the difference was due to the selenium labelling site. For instance, peak f was present in the LC-ICP-MS chromatogram of N-PenMSe in medium, but not in that of i-PenMSe in medium, whereas the peak appeared in both LC-ESI-MS EICs. Zooming in on the isotope patterns of the LC-ESI-MS peaks revealed a characteristic pattern for compounds containing selenium which was easily distinguished from a carbon pattern, exemplified for peak f in Fig. 5. The short peptide RQIKIWF from i-PenMSe exhibited the common peptide carbon pattern (12C, 98.90%; 13C, 1.10%). The selenium-containing degradation product from N-PenMSe, MSeRQIKIWF, displayed a combined pattern of carbon and selenium (76Se, 9.0%; 77Se, 7.6%; 78Se, 23.6%; 80Se, 49.7%; 82Se, 9.2%). Hence, the characteristic isotope pattern of selenium demonstrates that selenium is also a useful molecular MS label.
 | ||
| Fig. 5 Simulated isotope patterns of single charged peptides of [RQIKIWF + H]+ and [MSeRQIKIWF + H]+ (peaks f1 and f2 in Fig. 3). Left: degradation product from i-PenMSe, isotope pattern without selenium. Right: degradation product of N-PenMSe containing selenium. | ||
Thus, differences between selenium-containing degradation products obtained from i-PenMSe and N-PenMSe, respectively, were revealed by LC-ICP-MS (chromatograms in Fig. 3) as only selenium-containing compounds were present. In general, amino acids and short polar peptides eluted in the beginning of the LC-ICP-MS chromatograms, while larger fragments and oxidation products appeared around the intact peptide. In N-PenMSe, SeMet was added at the N-terminal, and was thus retained in most of the cleaved peptides resulting in larger selenium-containing degradation products. By contrast, SeMet was incorporated towards the highly positively charged C-terminal in i-PenMSe, and therefore SeMet was predominantly cleaved off the parent peptide as very polar smaller Se-containing peptides eluting in the beginning of the chromatogram. Thus, the position of the label in the peptide sequence must be considered carefully in development and optimization of the LC methods. However, these results demonstrated that selenium labelling of penetratin indeed may be used as a readily detectable probe in evaluation of peptide degradation in various matrices by LC-ICP-MS and LC-ESI-MS. The possibility of specific detection of selenium offered by the ICP-MS technique implies that degradation in even more complex sample matrices may be analyzed.
Analytical figures of merit
Total selenium uptake in HeLa cells was successfully quantified by FI-ICP-MS while uptake of the intact peptide was semi-quantitatively determined by LC-ICP-MS, exploiting the high selectivity and robustness of the ICP ionization. The limit of detection (LOD) of FI-ICP-MS was estimated to be 6.0 ng Se mL−1 (0.76 nmol Se injected) as 3σ of the peak area of 2.4 μM i-PenMSe. FI-ICP-MS precision of three repeated injections of 2.4 μM i-PenMSe was 1.0% relative standard deviation (RSD). The LOD for the LC-ICP-MS method was 6.5 ng Se mL−1 (0.41 nmol Se on column) estimated as 3σ of the peak area of 2.4 μM i-PenMSe. The column recovery was 101.5 ± 1.1% (n = 3) and 101.9 ± 0.3% (n = 2) for i-PenMSe and N-PenMSe respectively. The precision of three repeated injections of 2.4 μM i-PenMSe was 1.3% RSD.The LOD for the FI-ICP-MS method in this study was higher than what has previously been reported for low molecular selenium compounds.39 Peptides are known to exhibit unspecific adsorption onto different materials, e.g. glass, metal and plastic.7 Adsorption onto vials, injection needle or tubing might explain the increased LOD. The flow injection method was thoroughly optimized to avoid unspecific adsorption by acidification and the increase of the peptide concentration in samples. However, adsorption might still contribute to increased deviation between injections, resulting in a higher LOD for peptides as compared to smaller selenium compounds. Furthermore, the low-abundant selenium isotope 82Se (9.2%) was measured in the present work, due to the polyatomic interference of 40Ar2 of the most abundant isotope 80Se (49.7%) using the ELAN 6000 quadrupole instrument. Sufficient sensitivity was achieved by ELAN 6000 for the measurements of the present study. However, detection limits may be reduced by application of improved ICP-MS instrumentation, e.g. by applying a dynamic reaction cell (DRC) instrument.44
Conclusion
In this study, selenium incorporated as SeMet was demonstrated to be a useful label for quantification of pharmaceutically relevant peptides exemplified by the cell-penetrating peptide, penetratin. Selenium was readily introduced during solid-phase peptide synthesis, however, the stability of SeMet at higher temperatures must be considered. The introduction of the non-toxic SeMet label is considered to have a minimal effect on the biological activity of the peptide. Selenium labelling thus offers minimal disturbance of the native structure of the peptide of interest as compared to labelling with fluorophores or metal-chelating agents. The labelling technique and a combination of FI-ICP-MS, LC-ICP-MS and LC-ESI-MS techniques provided detailed information on the fate of penetratin in cell uptake studies. In addition to qualitative information on degradation products obtainable from LC-ESI-MS, LC-ICP-MS allowed for quantification of the peptide. Most therapeutic peptides are synthetic analogues of endogenous peptides, and incorporation of selenium allows for improved critical assessment of the native drug or drug delivery candidate early in the drug development process.References
- D. J. Craik, D. P. Fairlie, S. Liras and D. Price, Chem. Biol. Drug Des., 2013, 81, 136–147 CAS.
- A. A. Kaspar and J. M. Reichert, Drug Discovery Today, 2013, 18, 807–817 CrossRef CAS PubMed.
- P. Vlieghe, V. Lisowski, J. Martinez and M. Khrestchatisky, Drug Discovery Today, 2010, 15, 40–56 CrossRef CAS PubMed.
- M. Grant and A. Leone-Bay, Ther. Delivery, 2012, 3, 981–996 CrossRef CAS.
- e. Khafagy and M. Morishita, Adv. Drug Delivery Rev., 2012, 64, 531–539 CrossRef CAS PubMed.
- T. Bruckdorfer, O. Marder and F. Albericio, Curr. Pharm. Biotechnol., 2004, 5, 29–43 CAS.
- H. Grohganz, M. Rischer and M. Brandl, Eur. J. Pharm. Sci., 2004, 21, 191–196 CrossRef CAS PubMed.
- D. Farin, G. A. Piva, I. Gozlan and R. Kitzes-Cohen, J. Pharm. Biomed. Anal., 1998, 18, 367–372 CrossRef CAS.
- D. W. Backes, H. I. Aboleneen and J. A. Simpson, J. Pharm. Biomed. Anal., 1998, 16, 1281–1287 CrossRef CAS.
- R. Tréhin, H. M. Nielsen, H. G. Jahnke, U. Krauss, A. G. Beck-Sickinger and H. P. Merkle, Biochem. J., 2004, 382, 945–956 CrossRef PubMed.
- T. Holm, S. Netzereab, M. Hansen, U. Langel and M. Hallbrink, FEBS Lett., 2005, 579, 5217–5222 CrossRef CAS PubMed.
- I. van den Broek, R. W. Sparidans, J. H. Schellens and J. H. Beijnen, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 872, 1–22 CrossRef CAS PubMed.
- A. Prange and D. Pröfrock, J. Anal. At. Spectrom., 2008, 23, 432–459 RSC.
- E. Svantesson, J. Pettersson and K. E. Markides, J. Anal. At. Spectrom., 2002, 17, 491–496 RSC.
- D. Kretschy, G. Koellensperger and S. Hann, Anal. Chim. Acta, 2012, 750, 98–110 CrossRef CAS PubMed.
- M. Wang, W. Y. Feng, Y. L. Zhao and Z. F. Chai, Mass Spectrom. Rev., 2010, 29, 326–348 CrossRef CAS PubMed.
- A. Sanz-Medel, M. Montes-Bayon, M. del Rosario Fernandez de la Campa, J. R. Encinar and J. Bettmer, Anal. Bioanal. Chem., 2008, 390, 3–16 CrossRef CAS PubMed.
- S. Bomke, M. Sperling and U. Karst, Anal. Bioanal. Chem., 2010, 397, 3483–3494 CrossRef CAS PubMed.
- Y. He, Y. Zhang, C. Wei, C. Li, Y. Gao and R. Liu, Appl. Spectrosc. Rev., 2013, 49, 492–512 CrossRef.
- R. Liu, X. Hou, Y. Lv, M. McCooeye, L. Yang and Z. Mester, Anal. Chem., 2013, 85, 4087–4093 CrossRef CAS PubMed.
- M. W. Yang, Z. W. Wang, L. Fang, J. P. Zheng, L. J. Xu and F. F. Fu, J. Anal. At. Spectrom., 2012, 27, 946–951 RSC.
- G. Schwarz, S. Beck, M. G. Weller and M. W. Linscheid, Anal. Bioanal. Chem., 2011, 401, 1203–1209 CrossRef CAS PubMed.
- D. Esteban-Fernandez, C. Scheler and M. W. Linscheid, Anal. Bioanal. Chem., 2011, 401, 657–666 CrossRef CAS PubMed.
- G. Koellensperger, M. Groeger, D. Zinkl, P. Petzelbauer and S. Hann, J. Anal. At. Spectrom., 2009, 24, 97–102 RSC.
- D. Sarko, B. Beijer, B. R. Garcia, E. M. Nothelfer, K. Leotta, M. Eisenhut, A. Altmann, U. Haberkorn and W. Mier, Mol. Pharmaceutics, 2010, 7, 2224–2231 CrossRef CAS PubMed.
- L. A. Wessjohann, A. Schneider, M. Abbas and W. Brandt, Biol. Chem., 2007, 388, 997–1006 CrossRef CAS PubMed.
- P. Whanger, J. Am. Coll. Toxicol., 1986, 5, 101–110 CrossRef CAS.
- M. Muttenthaler and P. F. Alewood, J. Pept. Sci., 2008, 14, 1223–1239 CrossRef CAS PubMed.
- S. Lang, D. E. Spratt, J. G. Guillemette and M. Palmer, Anal. Biochem., 2005, 342, 271–279 CrossRef CAS PubMed.
- D. Derossi, A. H. Joliot, G. Chassaing and A. Prochiantz, J. Biol. Chem., 1994, 269, 10444–10450 CAS.
- C. Foged and H. M. Nielsen, Expert Opin. Drug Delivery, 2008, 5, 105–117 CrossRef CAS PubMed.
- J. S. Bahnsen, H. Franzyk, A. Sandberg-Schaal and H. M. Nielsen, Biochim. Biophys. Acta, 2013, 1828, 223–232 CrossRef CAS PubMed.
- P. M. Fischer, N. Z. Zhelev, S. Wang, J. E. Melville, R. Fahraeus and D. P. Lane, J. Pept. Res., 2000, 55, 163–172 CrossRef CAS.
- M. E. Lindgren, M. M. Hallbrink, A. M. Elmquist and U. Langel, Biochem. J., 2004, 377, 69–76 CrossRef CAS PubMed.
- P. E. Thoren, D. Persson, P. Isakson, M. Goksor, A. Onfelt and B. Norden, Biochem. Biophys. Res. Commun., 2003, 307, 100–107 CrossRef CAS.
- R. Tréhin, U. Krauss, A. G. Beck-Sickinger, H. P. Merkle and H. M. Nielsen, Pharm. Res., 2004, 21, 1248–1256 CrossRef.
- C. Palm, M. Jayamanne, M. Kjellander and M. Hallbrink, Biochim. Biophys. Acta, 2007, 1768, 1769–1776 CrossRef CAS PubMed.
- M. Zorko and U. Langel, Adv. Drug Delivery Rev., 2005, 57, 529–545 CrossRef CAS PubMed.
- B. Gammelgaard, L. H. Rasmussen, C. Gabel-Jensen and B. Steffansen, Biol. Trace Elem. Res., 2012, 145, 248–256 CrossRef CAS PubMed.
- A. Elmquist, M. Lindgren, T. Bartfai and U. Langel, Exp. Cell Res., 2001, 269, 237–244 CrossRef CAS PubMed.
- P. Lundberg and Ü. Langel, Int. J. Pept. Res. Ther., 2006, 12, 105–114 CrossRef CAS.
- C. Palm, S. Netzerea and M. Hallbrink, Peptides, 2006, 27, 1710–1716 CrossRef CAS PubMed.
- M. V. Christensen, Master thesis, University of Copenhagen, 2012.
- S. D. Tanner, V. I. Baranov and U. Vollkopf, J. Anal. At. Spectrom., 2000, 15, 1261–1269 RSC.
| This journal is © The Royal Society of Chemistry 2014 |