Open Access Article
Open Access ArticleSelected metal ions protect Bacillus subtilis biofilms from erosion†
S.
Grumbein
ab,
M.
Opitz
c and
O.
Lieleg
*ab
aZentralinstitut für Medizintechnik, Technische Universität München, 85748 Garching, Germany. E-mail: oliver.lieleg@tum.de
bFakultät für Maschinenwesen, Technische Universität München, 85748 Garching, Germany
cFakultät für Physik, Ludwig-Maximilians-Universität München, 80333 Munich, Germany
First published on 16th April 2014
Abstract
Many problems caused by bacterial biofilms can be traced back to their high resilience towards chemical perturbations and their extraordinary sturdiness towards mechanical forces. However, the molecular mechanisms that link the mechanical properties of a biofilm with the ability of bacteria to survive in different chemical environments remain enigmatic. Here, we study the erosion stability of Bacillus subtilis (B. subtilis) biofilms in the presence of different chemical environments. We find that these biofilms can utilize the absorption of certain metal ions such as Cu2+, Zn2+, Fe2+, Fe3+ and Al3+ into the biofilm matrix to avoid erosion by shear forces. Interestingly, many of these metal ions are toxic for planktonic B. subtilis bacteria. However, their toxic activity is suppressed when the ions are absorbed into the biofilm matrix. Our experiments clearly demonstrate that the biofilm matrix has to fulfill a dual function, i.e. regulating both the mechanical properties of the biofilm and providing a selective barrier towards toxic chemicals.
Introduction
Bacteria can grow in adverse and challenging environments, e.g. in the presence of toxic substances such as disinfectants or metal ions.1–3 Yet, to do so, they need to rely on the protective abilities of self-produced biopolymers into which the bacteria embed themselves. Such a community of embedded bacteria is referred to as a bacterial biofilm.4–7 The protective abilities of those biofilm biopolymers can cause severe problems in situations where biofilms are undesirable, e.g. in health care where they can colonize catheters, implants and contact lenses and resist antibiotic treatments.8,9Other problems due to the formation of biofilms are observed in industrial settings. There, biofilms can interrupt the cooling water supply due to plugging and corrosion of pipes,10 and biofilms on surfaces in food production enhance the risk for product contamination with pathogenic microflora.11 As a consequence, the deactivation and removal of biofilms from surfaces have become a main goal in biofilm research, and the mechanical properties of those biofilms have gained considerable attention recently.12–20
It has been shown that the presence of shear forces and different ionic conditions during growth can have an impact on biofilm mechanics.21–25 Also the viscoelastic properties of mature biofilms can be altered by shear forces and chemicals.26–29 When those shear forces are generated by flowing liquids, detachment of individual bacteria or erosion of biofilm fragments has been described.30–34 The erosion stability of biofilms depends on the biofilm species35,36 and on the chemical environment the biofilm is exposed to.29,37–39 However, it is not clear whether chemical conditions that are beneficial for the mechanical properties of a biofilm might be harmful for the embedded bacteria.
Here, we analyze the erosion stability of biofilms grown by the bacterium Bacillus subtilis B1 (B. subtilis B1). B. subtilis is a soil-dwelling gram-positive bacterium, but it has recently also been found in the human gut.40B. subtilis strains are used for biotechnology applications and have emerged as model organisms for biofilm formation.41,42 The strain used in this study was isolated from an oil field43 and can form biofilms on solid surfaces. We show that those B. subtilis B1 biofilms can protect themselves from erosion by shear forces by absorbing metal ions into the biofilm matrix. This mechanical protection is often accompanied by an increase in the shear stiffness of the biofilm that is induced by the absorbed metal ions. On top of providing this mechanical fortification, the ion absorption established by the biofilm biopolymer matrix also helps to shield the embedded bacteria from ions that are toxic for planktonic B. subtilis B1 bacteria. This illustrates that the biopolymer matrix of bacterial biofilms has to fulfill a dual function, i.e. regulating both the mechanical properties and the permeability of the biofilm.
Experimental
Biofilm formation
B. subtilis strain B143 was used for all experiments. Overnight cultures were grown from a frozen glycerol stock in LB (Luria/Miller) medium at 37 °C and 90 rpm shaking (Certomat BS-1, Sartorius AG, Göttingen, Germany). Biofilms were grown by plating 100 μL of an overnight culture onto 1.5% (w/v) agar plates containing LB (Luria/Miller) and incubating those agar plates at 37 °C for 24 hours.Erosion assay
Customized sample holders were crafted from PTFE plates. Each sample holder has a cylindrical hole of 20 mm diameter and 3 mm depth. Sample holders were sterilized prior to filling with 1 mL of 1.5% (w/v) agar containing LB (Luria/Miller) generating a circular agar patch. Afterwards, 20 μL of an overnight culture was mixed with 80 μL of fresh LB and distributed over the agar surface of the sample holders. Sample holders were then incubated at 37 °C for 24 hours for biofilm growth. An example of such a biofilm-covered agar patch is depicted in Fig. 1a. To conduct the erosion tests, 25 mL of a testing liquid was prepared and filled into centrifuge tubes. The sample holders were placed into the centrifuge tubes (Fig. 1b) which in turn were mounted onto a lab shaker (Unimax 1010, Heidolph Instruments, Schwabach, Germany). The lab shaker was then set into rotation at 300 rpm for defined time intervals which generated a shear stress of ∼1.6 mPa (see ESI†). After exposure to this shaking-induced shear force, the agar layer was carefully removed from the sample holder ensuring that the remaining biofilm layer was not disturbed. Images of the biofilm-covered agar patches were acquired using a perimeter stand at a defined height with a digital camera (Canon PowerShot SX240 HS). Images were then converted into black and white using a binarization procedure implemented in the software ImageJ, and the percentage of biofilm erosion was calculated by determining the ratio of the area covered with biofilm and the total area of the agar patch.Rheological characterization
Rheological measurements were performed using a commercial rheometer (MCR 302, Anton Paar GmbH, Graz, Austria) with a 25 mm plate–plate geometry and 300 μm plate separation in strain-controlled mode. Mature biofilms were removed from agar plates by manual scraping and pooled. Small amounts of biofilm (∼500 mg) were transferred to micro test tubes and weighed. 5% (v/w) of a chemical stock solution was then added to the tubes and the chemical was distributed through the biofilm by gentle stirring with a pipette tip. Treated biofilm samples were kept at room temperature for 1 hour prior to their rheological characterization. Frequency spectra were obtained at 21 °C using small torques (∼1 μNm) to guarantee linear material response.Minimal inhibitory concentration
Different ionic solutions were prepared at increasing concentrations in LB medium. We then inoculated each of those solutions with 100 μL of an overnight culture of B. subtilis B1 and measured the optical densities (Victor3 Multilabel Counter, PerkinElmer, Waltham, Massachusetts, USA) of the bacterial suspensions after 24 hours of incubation at 37 °C.Regrowth assay – planktonic bacteria
700 μL of an overnight culture of B. subtilis B1 was mixed with 700 μL of an ionic solution in micro test tubes. Tubes were placed onto a vertical lab rotator at 2 rpm for 1 hour to ensure good mixing. 10 μL of the chemically challenged culture were then transferred into 990 μL of fresh LB to remove the chemical challenge and allow for bacterial growth. Optical densities were determined (GeneQuant Pro, Amersham Biosciences, Amersham, UK) directly after mixing with fresh LB and after 15 hours of incubation at 37 °C and 90 rpm.Regrowth assay – biofilm
For this assay, comparable amounts of untreated biofilm (∼500 mg each) were weighed into sterile micro test tubes to ensure comparable starting conditions. Those biofilm pieces were then treated with different ionic solutions in full analogy to the chemical treatment performed for our macrorheological experiments. After an incubation time of 1 hour, the micro test tubes containing the chemically treated biofilms were rinsed on the outside with ethanol to avoid contamination and placed into 50 mL centrifuge tubes which then were filled with 20 mL of liquid LB medium. Those centrifuge tubes were then mounted onto a lab shaker and incubated at 37 °C for 15 hours to wash out bacteria from the biofilm matrix and allow for their growth. Optical densities (GeneQuant Pro) of the bacterial solutions were then obtained every hour for a total duration of 15 hours.Results
In this study, we aim at quantifying how the erosion stability of bacterial biofilms towards shear forces depends on the chemical environment. For this purpose, we have developed a new biofilm erosion assay (Fig. 1) which employs similar principles to the macroscopic erosion test introduced by Simões et al.39 but translates them into a miniaturized version to render it more high-throughput compatible (see Experimental section for details). Fig. 1c shows a biofilm patch with a very low degree of erosion and Fig. 1d represents a high degree of erosion, respectively.Bacillus subtilis biofilms are sensitive to erosion by shear forces
When the erosion test described before is performed in double distilled water (ddH2O), it takes about 5 minutes before any measurable biofilm erosion is induced. Following this initial erosion, we observe a linear increase in the biofilm removal with time. After ∼40 minutes, more than 90% of the biofilm is washed off the surface, and only small chunks of biofilm material remain (Fig. 2a). A similar linear increase in the eroded biofilm mass is observed when the removed biofilm material is quantified by lyophilization, i.e. when the liquid in which the erosion assay is performed is freeze-dried and the eroded biomass is determined by weighing the lyophilized material (Fig. S1, ESI†). This demonstrates that our optical analysis correctly measures the degree of biofilm removal as a function of time. Furthermore, our results show that the B. subtilis biofilm cannot withstand the prolonged application of shear forces in an aqueous environment. Of course, swelling of the biofilm in water could also contribute to the observed erosion process. Therefore, we repeat the same experiment with the lab shaker being switched off. In the absence of shear forces, the degree of biofilm erosion is much lower and saturates at ∼10% after 15 minutes (Fig. 2a). This finding verifies the suitability of our erosion assay to quantify the stability of B. subtilis B1 biofilms towards shear forces. Moreover, it demonstrates that B. subtilis B1 biofilms are sensitive towards shear forces as they may occur in liquid environments such as river beds or waste water pipes.However, in their natural environment, biofilms are able to withstand shear forces, e.g. when they grow on the surface of pipes or catheters in the presence of flowing fluids. Accordingly, this indicates the existence of a mechanism that allows biofilms to protect themselves from erosion. Of course, in pipes or catheters the biofilms are exposed to a more complex chemical environment than ultrapure water. Thus, we next ask whether and how variations in the chemical composition of the aqueous environment affect the erosion stability of B. subtilis B1 biofilms.
Absorption of multivalent ions protects Bacillus subtilis biofilms from erosion and increases the biofilm shear stiffness
When growing in pipes that are typically made of copper or iron, biofilms are exposed to Cu2+ and Fe3+ ions. Thus, we next test if the presence of Cu2+ and Fe3+ ions influences the erosion stability of B. subtilis B1 biofilms. We repeat our shear erosion test with biofilm samples exposed to solutions of 50 mM CuSO4 or 50 mM FeCl3 and then compare the kinetics of biofilm erosion to the results obtained with ultrapure water (Fig. 2a). We find that, in the presence of Cu2+ ions, the measured biofilm erosion is comparable to when the biofilm is placed in ultrapure water without applying shear forces: over a time span of 42 minutes, less than 20% of the biofilm is removed. The virtually same stabilization effect is obtained when FeCl3 solutions are used. Apparently, both ions can stabilize the biofilm and protect it from surface erosion. In addition to this stabilization effect, we also observe a change in the material quality of the biofilm. For both ions, the color of the biofilm is altered after incubation (Fig. 2b–d): whereas the biofilm acquires an orange/brown color after exposure to FeCl3, it turns blue after exposure to CuSO4. This clearly indicates an uptake of the respective ions into the biofilm matrix. Moreover, when trying to remove the biofilm from the agar surface by manual scraping, we note a significant stiffening of the biofilm matrix. This suggests that the absorption of Cu2+ and Fe3+ ions might alter the viscoelastic properties of the biofilm.In order to quantify the putative alterations in biofilm stiffness observed from manual scraping, we next aim at measuring the viscoelastic properties of biofilms which have been exposed to selected chemicals. As a material parameter describing the mechanical properties of the biofilm in response to shear forces, we choose the dynamic shear modulus G*(f), a quantity that depends on the frequency of the applied shear deformation. This dynamic shear modulus is a complex quantity. Its real part, the so called storage modulus G′(f), describes the elastic properties of the biofilm whereas the imaginary part, the loss modulus G′′(f), represents its viscous properties. Experimentally, those two parts of the complex shear modulus are determined from the in-phase and out-of-phase part of the biofilm deformation in response to an oscillatory shear force (Fig. 3a). We measure those two parameters over a range of biologically relevant frequencies, i.e. between 10 mHz and 10 Hz corresponding to time scales of 0.1 s to 100 s. We observe that the storage modulus dominates over the loss modulus over the whole frequency regime tested (Fig. 3b) underscoring the pronounced elastic properties of a bacterial biofilm in contrast to the purely viscous properties of a bacterial suspension. Furthermore, we find that B. subtilis biofilms obtained from different growth batches show relatively little variation in their viscoelastic properties as we measure G′ values in the range of 50 to 125 Pa.
In order to determine the effect a given chemical might have on the viscoelastic properties of the biofilm, we prepare stock solutions and then add 5% (v/w) of those solutions to harvested biofilms. Those chemicals are then distributed throughout the biofilm matrix by gentle stirring with a pipette tip to homogenize the sample (see Experimental section for details). As a negative control, ddH2O is added to the biofilm. By this slight dilution of the biofilm material, the storage and loss modulus are only minimally changed (Fig. 3b), and the overall shape of the frequency spectrum is unaffected. When adding CuSO4 to a harvested B. subtilis biofilm, the qualitative stiffening effect observed by manual scraping is confirmed: after treatment with 5% (v/w) of 1 M CuSO4 (corresponding to a final CuSO4 concentration of 50 mM as used in the erosion assay) the storage and the loss modulus are increased approximately 100 fold compared to the addition of ddH2O. In comparison, when 5% (v/w) of 1 M FeCl3 is added to the biofilm, the alteration in the viscoelastic moduli is even more pronounced and the resulting viscoelastic moduli are ∼1000 times larger than for the treatment with ddH2O (Fig. 3b). When we calculate the loss factor of the samples, which is the ratio of the loss modulus to the storage modulus, we find no significant changes (Fig. S2, ESI†).
As a next step, we test if this increase in the viscoelastic moduli occurs immediately when the biofilm is brought into contact with the metal ions, or if a certain time span is required until the maximum effect is reached. Therefore, we cover fully grown biofilms with a 50 mM solution of FeCl3 or CuSO4 for short time intervals. We then discard the solution, harvest the exposed biofilms by scraping them from the agar surface and measure the viscoelastic properties. We observe a fast increase of both viscoelastic moduli within the first 10–15 minutes (see Fig. S4, ESI†), but this increase in the biofilm viscoelasticity saturates after approximately 15 min. However, in all those cases the dependence of the viscoelastic moduli on the shear frequency is weak. Thus, we can subsume the viscoelastic behavior of the biofilms into a single material parameter. For simplicity, G′ (1 Hz) is chosen as such a material parameter to compare the elastic properties of different biofilms for the remainder of this article.
Both CuSO4 and FeCl3 form acidic solutions in water. Thus, it is a priori not clear whether the observed alterations of the viscoelastic biofilm properties are due to the ions in the aqueous solution or whether they are caused by pH differences.
To clarify this, we repeat our rheological experiment with biofilms that have been treated with 5% (v/w) of aqueous solutions buffered to pH 3, 7 or 10.
We find that the viscoelastic properties of the biofilm remain unaffected by those pH alterations (Fig. 4a, upper panel and Table S2, ESI†). This demonstrates that the changes in biofilm stiffness observed above are indeed caused by the ions in the CuSO4 and FeCl3 solution.
Can such a stiffening of the biofilm matrix only be induced by Cu2+ and Fe3+ ions or have other ions a similar effect? To answer this question, we next perform a rheological screening testing the viscoelastic properties of biofilms treated with other metal ions ranging from monovalent ions such as Na+ and K+ to multivalent ions such as Zn2+ or Al3+. As the naturally grown biofilm might already contain a significant amount of ions, we also test chelating agents such as citrate, EDTA and EGTA. For all those conditions we try to maximize putative effects by using stock solutions close to the solubility limit of the respective chemical when adding 5% (v/w) of a chemical to the biofilm matrix for rheological evaluation. However, for most chemicals we do not detect a relevant change in the biofilm elasticity (Fig. 4a, upper panel and Table S2, ESI†): neither chelating agents nor monovalent ions significantly alter the elastic properties of the biofilm. However, Zn2+ and Al3+ induce a strong fortification of the biofilm matrix similar to what we observe for biofilms treated with Cu2+ and Fe3+. In contrast, for the reduced form of iron ions (i.e. Fe2+), the biofilm elasticity is only weakly increased, and other divalent ions such as Mg2+ and Ca2+ hardly alter the biofilm elasticity at all.
The observation that both Cu2+ and Fe3+ ions can have a dual effect on the biofilm, i.e. enhancing the biofilm stiffness and preventing biofilm erosion, suggests that a change in the bulk material properties of the biofilm might be responsible for the erosion protection of biofilms formed by the strain Bacillus subtilis B1. To test this hypothesis, we repeat our erosion assay and quantify the biofilm erosion stability for the whole range of chemical conditions screened with macrorheology. To ensure comparable situations, the concentrations of the aqueous solutions for the erosion assay were chosen such that they match the final concentrations of the chemical agents in the biofilm as used in the rheological screening assay. We find that, in addition to Cu2+ and Fe3+ ions, also the multivalent ions Ca2+, Zn2+, Fe2+ and Al3+ significantly reduce biofilm erosion (Fig. 4a, lower panel). In contrast, for all other ions and test conditions the biofilm erosion is comparable to that obtained in pure water.
For most conditions tested so far, we have observed that the absorption of metal ions into the biofilm matrix can both induce a stiffening of the biofilm matrix and protect the biofilm layer from erosion. This seems to suggest that these two phenomena are related. However, in the case of Ca2+ ions we have observed a protection from erosion without a measurable increase in the biofilm shear stiffness. This indicates that other biofilm properties besides their shear stiffness might play a role in their stability towards shear forces induced by fluid flow.
We further test this hypothesis by repeating our evaluation of the biofilm shear stiffness and its erosion stability using lower concentrations of the metal ions tested so far. Our rationale is that the increase in the biofilm shear stiffness might depend on the ion concentration, and that below a critical biofilm stiffness the erosion protection will fail. However, if the biofilm would still be protected from erosion at those lower ion concentrations without showing increased shear stiffness, this would underscore our notion that another, independent biofilm property needs to be considered to understand its erosion resistance. For this set of experiments, we focus on multivalent ions since only those showed significant effects in our previous experiments. We compare the influence of those metal ions at identical concentrations of 50 mM, which also allows us to quantitatively compare the efficiency of those ions. We find that – in contrast to CuSO4, FeCl3 and Al2(SO4)3 – the addition of 50 mM ZnCl2 or FeCl2 does not induce a significant increase in the biofilm shear stiffness anymore. Yet, the degree of biofilm erosion remains very low (Fig. 4b and Table S2, ESI†). In conclusion, we have now observed several conditions where erosion protection is achieved without a significant alteration in the biofilm shear stiffness. This shows that, whereas the absorption of ions into a biofilm matrix can simultaneously influence the shear stiffness of a biofilm as well as its erosion stability, those biofilm properties are not necessarily linked.
Selected metal ions lose their toxicity when they are absorbed in the biofilm matrix
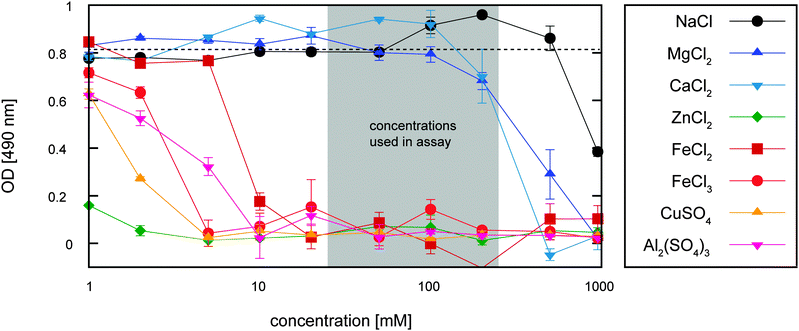
So far, we have described how the absorption of selected multivalent ions into the biofilm matrix can both enhance the shear stiffness of the biofilm and protect the biofilm from erosion. However, those absorbed ions might have an adverse effect on the embedded biofilm bacteria. In general, the metabolism of many bacteria requires certain metal ions, e.g. Fe3+, at low concentrations.44 Yet, at high concentrations, Fe3+ ions may become toxic – at least for bacteria in their planktonic state, i.e. when they are freely floating in a liquid environment. One could imagine that the erosion protection of the biofilm comes at a cost: does a certain percentage of the biofilm bacteria have to sacrifice their life for the greater good? To answer this question, we next test if Fe3+ ions (or the other metal ions tested here) are toxic for B. subtilis B1 bacteria. In principle, there are two possibilities how a given chemical might harm bacteria. First, the chemical might inhibit bacterial growth, and second the chemical might induce bacterial death. To address the first possibility, we determine the minimal inhibitory concentration (MIC) of the different metal ions by employing a growth assay using planktonic bacteria of B. subtilis B1 (see Experimental section for details). As depicted in Fig. 5, bacterial growth is not impaired by NaCl, CaCl2 and MgCl2 up to concentrations of ∼200 mM. In contrast, we observe a strong reduction of bacterial growth for FeCl2, FeCl3, CuSO4, ZnCl2 and Al2(SO4)3 even at concentrations of 10 mM or less. This indicates that the concentrations of certain metal ions used in our previous assays, i.e. in the erosion test and macrorheological screening is already high enough to inhibit growth of planktonic bacteria; in other words, they are bacteriostatic.Besides their bacteriostatic activity, some of the metal ions studied here might even be lethal for the B. subtilis B1 bacteria. To test this, we set up a regrowth assay in which we first perform a chemical challenge by incubating planktonic bacteria in different ionic solutions and then measure the ability of the bacteria to grow again when the chemical stress is released by transferring the bacteria into standard LB medium (see Experimental section for details). Then, the optical densities of the bacterial solutions are obtained after 15 hours of incubation in LB medium at 37 °C. Our results show that for NaCl, CaCl2 and MgCl2 the bacteria are able to grow again when the chemical challenge is released. The same result is obtained for a transient chemical challenge with FeCl2: even though FeCl2 can inhibit the growth of B. subtilis B1, once the chemical pressure is released, the optical density of the bacteria solution increases identically to that of planktonic bacteria which have not been chemically challenged (Table 1 and Table S1, ESI†). In contrast, when the chemical challenge is performed with FeCl3, CuSO4, ZnCl2 or Al2(SO4)3, we observe a significantly weaker bacterial growth when the bacteria are transferred into LB medium. This indicates that this group of metal ions is not only bacteriostatic but can even induce death of planktonic B. subtilis B1 bacteria – at least at high enough concentrations.
| ddH2O | 250 mM NaCl | 250 mM CaCl2 | 250 mM MgCl2 | 150 mM FeCl2 | 150 mM FeCl3 | 50 mM CuSO4 | 50 mM Al2(SO4)3 | 50 mM ZnCl2 | |
|---|---|---|---|---|---|---|---|---|---|
| + indicates growth after chemical challenge, − indicates no growth after chemical challenge. | |||||||||
| Planktonic B. subtilis B1 | + | + | + | + | + | − | − | − | − |
| Biofilm embedded B. subtilis B1 | + | + | + | + | + | + | + | + | − |
However, in a biofilm bacteria are embedded in a biopolymer matrix which might shield the microorganisms from toxic agents. Such a shielding effect has already been observed for several bacterial species where the tolerance of a mature biofilm towards antibiotics is much higher than for planktonic bacteria. We hypothesize that a similar protective mechanism towards toxic metal ions might also occur for B. subtilis B1 bacteria that reside inside a biofilm matrix.
To test this hypothesis, we repeat our regrowth assay using defined amounts of a chemically pre-treated biofilm instead of chemically challenged planktonic bacteria. Similar to our previous assay, we then measure the ability of bacteria to grow when the chemical stress is released (see Experimental section for details). Indeed, when the regrowth kinetics of different chemically challenged biofilm samples are compared to that of an untreated biofilm, we find a very similar increase in the optical density for almost all chemical challenges tested (Table 1 and Fig. S3, ESI†). Only when a B. subtilis B1 biofilm is pre-treated with 250 mM ZnCl2, the increase in the optical density over time is weak and saturates at a final value that is ∼5 times lower than the one obtained for all other conditions. Apparently, there are chemicals that can permanently suppress bacterial growth in a biofilm after a transient chemical challenge suggesting that a large fraction of the biofilm bacteria have been killed. Most metal ions, however, seem to be trapped in the biofilm matrix in such a way that they are not able to harm the bacteria and can therefore increase the resistance of the biofilm towards shear forces without decimating the bacterial population.
The erosion protection of absorbed metal ions can be partially countered by the chelating agent EDTA
So far we have shown that the biofilm matrix can absorb metal ions, prevent putative toxic ions from harming the embedded biofilm bacteria and even protect the biofilm from mechanical erosion. However, in a biological or medical setting, the chemical composition of the surrounding liquid might significantly vary over time. Thus, we next ask whether the erosion protection observed so far can persist once the biofilm is exposed to a different chemical environment where the stabilizing metal ions are not present anymore. For this experiment we pre-treat the biofilm with selected solutions of metal ions prior to performing the erosion assay. This pre-treatment is performed by incubating the biofilm-covered agar patches in a solution containing 50 mM of a metal ion for ∼40 minutes. After this incubation time, the samples are transferred into ddH2O and the erosion assay is performed as in our previous experiments. As depicted in Fig. 4c, the stabilizing effect induced by the metal ions indeed persists when the biofilm sample is transferred into ddH2O. This indicates that the absorbed metal ions are strongly bound by the biofilm matrix and that they cannot easily be washed out again by pure water. However, a strong chelating agent might still be able to remove the ions from the biofilm matrix. If this indeed is possible, then the initial properties of the biofilm should be restored and the biofilm should become erosion-sensitive again. To test this hypothesis, we transfer the pre-treated biofilm samples into a 25 mM EDTA solution instead of distilled water and test the erosion stability of those pre-treated biofilms in the presence of this chelating agent. EDTA is known to form highly stable chelate complexes with metal ions and thus might be able to remove the metal ions from the biofilm matrix. We find that for biofilm samples that have been pre-treated with Al3+, Ca2+ or Zn2+ ions, the stabilizing effect remains even when the erosion assay is performed in the EDTA solution (Fig. 4c). In contrast, when Fe2+, Fe3+ or Cu2+ ions are used for biofilm pre-treatment, the biofilm color is reverted to that of an untreated biofilm after incubation with the EDTA solution. In addition to this change of the biofilm color, the biofilms also become erosion sensitive again and more than 50% of the biofilm can be removed in our erosion assay.Discussion
In this study, we have developed a new assay to measure the stability of bacterial biofilms towards erosion by shear forces. With this assay, we are able to quantify the time-dependent biofilm erosion process in the presence of different metal ions. As a model system for bacterial biofilms, we have studied biofilms formed by the non-pathogenic strain B. subtilis B1. A great advantage of the B. subtilis B1 strain used here is the large amount of biomass the strain produces within a short time. With a sufficient supply of nutrition, about 1500 mg of biofilm material can be harvested from a standard petri dish after 24 hours of incubation which is very convenient for a macrorheological screening assay as performed here. Of course, our assay could also be applied to biofilms that are generated by other bacteria – provided that they grow reasonably well on agar. Such other biofilm types may include those formed by pathogenic bacteria including Pseudomonas aeruginosa or Vibrio cholerae.The assay presented here has several advantages compared to other methods that have been introduced to study biofilm erosion: microfluidic erosion tests in microcapillaries45 may allow for following the dynamic detachment process of bacterial microcolonies from the biofilm surface, but cannot that easily be used to quantitatively compare the influence of different chemical environments on the erosion process. Such a quantitative comparison is possible with the macroscopic erosion assay introduced by Simões et al.39 which measures the decrease in biomass of biofilms that are grown on metal cylinders with a surface of 34.6 cm2. Those cylinders are rotated in a chemical bath to induce shear forces on the biofilm surface. However, this macro-assay is very time consuming as it requires several days to grow a biofilm layer of sufficient thickness on the metal cylinders. Thus, it is not a convenient platform for screening a broad range of chemical conditions. In our miniaturized erosion assay, we optically determine the decrease of biofilm coverage on test geometries with a total biofilm area of 3.1 cm2. The processing of the digital pictures obtained after shear force application is fast and robust and should also be suitable for automization. Therefore, our erosion assay constitutes a cost-efficient and easy-to-handle platform for high-throughput screening of chemicals that are considered for biofilm removal strategies.
Using this erosion assay in combination with macrorheological measurements, we have shown that the absorption of metal ions into the biofilm matrix can both induce a stiffening of the biofilm matrix and protect the biofilm layer from erosion. We have observed significant erosion protection for ion concentrations of 50 mM and higher. Although the ion concentrations used here are larger than typical release values of metal ions in water distribution pipes,46 an accumulation of those ions in the biofilm matrix beyond the ion concentrations found in the aqueous medium might still occur. Indeed, we find a first indication for such an oversaturation behaviour from our experiments with 50 mM CuSO4 (see Fig. 4 and Fig. S4, ESI†): when the biofilm is covered with the 50 mM CuSO4 solution for more than 20 minutes, a 10 fold higher biofilm shear stiffness is achieved compared to when 50 mM of CuSO4 is directly forced into the biofilm by mechanical stirring. This suggests that the concentration of absorbed Cu2+ ions in the biofilm is larger than the concentration of Cu2+ ions in the surrounding liquid. Of course, this scenario would require the metal ions to have a high affinity for the biofilm matrix components – which agrees with our finding that the chelating agent EDTA cannot easily wash out all absorbed metal ions (see Fig. 4c).
One possible explanation for the increased matrix stiffness could be a simple ionic cross-linking of the biofilm matrix by the metal cations.47 Indeed, the polyanionic biopolymer poly-γ-glutamate is a main component of the biofilm matrix formed by B. subtilis B1,48 so ionic cross-linking should be possible per se. Also Pseudomonas aeruginosa biofilms can be mechanically fortified by selected metal ions,27,49 and the polyanionic biopolymer alginate has been put forward as a main component of mucoid P. aeruginosa biofilms. However, for those biofilms ionic cross-linking was not sufficient to account for the observed increase in biofilm stiffness upon exposure to metal ions since only trivalent and not divalent ions had a significant effect. For the B. subtilis biofilms studied here, we find an even higher degree of specificity as only selected metal ions induce a mechanical fortification and erosion protection whereas other metal ions of the same valency do not.
Furthermore, a detailed comparison of different metal ions revealed that an increase in the biofilm shear stiffness is not necessarily linked to a reduction of biofilm erosion. It seems probable that the adhesion strength of the biofilm to the surface it has been grown on is a second important parameter that needs to be addressed to understand how the stability of a bacterial biofilm towards surface erosion is regulated on a molecular scale. This question and how the adhesion strength of bacterial biofilms can be tuned by different ionic conditions needs to be addressed in future investigations.
Interestingly, neither the viscoelastic properties of a biofilm27,50 nor its erosion stability19 are directly linked to the viability of the embedded bacteria. Nevertheless, in response to the exposure to toxic ion concentrations, sporulation could be induced for a fraction of the embedded bacteria population, and such a sporulation event might alter the mechanical properties of the biofilm. However, as most of our experiments are performed within a time-period smaller than the time needed for spore formation in B. subtilis,51 this is not a likely scenario. In fact, biofilm-embedded bacteria are more resistant towards heavy metals than planktonic cells,52 and biofilm cells bind metals in different quantities than planktonic cells.53 By absorbing metal ions into the biofilm matrix in such a way that the embedded bacteria are not harmed, a B. subtilis biofilm can make use of toxic ions to optimize its mechanical properties.
This ability of B. subtilis B1 biofilms to deactivate toxic ions by absorption is a hallmark for the dual function of extracellular polymers: such extracellular polymers are typically responsible for tuning both the mechanical properties and the permeability of eukaryotic tissues. A very similar function is described here for a prokaryotic “tissue” where the biofilm matrix selectively traps toxic ions and – on top of that – even utilizes them to improve the mechanical performance of the biofilm layer. Thus, biofilm formation can be regarded as a well-tailored strategy that microorganisms use to survive in potentially harmful environments.54
Acknowledgements
We thank Masaaki Morikawa for providing the Bacillus subtilis B1 strain. This project was supported by the Deutsche Forschungsgemeinschaft through project B11 “Mechanics of bacterial biofilms” in the framework of SFB 863. Additional support from the Center for Nanoscience Munich (CeNS) and the International Graduate School of Science and Engineering (IGGSE) is gratefully acknowledged. The authors thank Konstantinia Bidmon for assistance with the bacterial cultures and Dionis Minev and Andreas Mader for conducting pilot experiments for this study. Marina Caldara's helpful comments on the manuscript are appreciated.References
- S. C. Booth, I. F. S. George, D. Zannoni, M. Cappelletti, G. E. Duggan, H. Ceri and R. J. Turner, Effect of aluminium and copper on biofilm development of Pseudomonas pseudoalcaligenes KF707 and P. fluorescens as a function of different media compositions, Metallomics, 2013, 5, 723–735 RSC.
- A. Bridier, R. Briandet, V. Thomas and F. Dubois-Brissonnet, Resistance of bacterial biofilms to disinfectants: a review, Biofouling, 2011, 27, 1017–1032 CrossRef CAS PubMed.
- J. J. Harrison, R. J. Turner and H. Ceri, High-throughput metal susceptibility testing of microbial biofilms, BMC Microbiol., 2005, 5, 53 CrossRef PubMed.
- J. Costerton, P. Stewart and E. Greenberg, Bacterial biofilms: A common cause of persistent infections, Science, 1999, 284, 1318–1322 CrossRef CAS.
- R. D. Monds and G. A. O'Toole, The developmental model of microbial biofilms: ten years of a paradigm up for review, Trends Microbiol., 2009, 17, 73–87 CrossRef CAS PubMed.
- G. O'Toole, H. B. Kaplan and R. Kolter, Biofilm formation as microbial development, Annu. Rev. Microbiol., 2000, 54, 49–79 CrossRef CAS PubMed.
- H. Vlamakis, Y. Chai, P. Beauregard, R. Losick and R. Kolter, Sticking together: building a biofilm the Bacillus subtilis way, Nat. Rev. Microbiol., 2013, 11, 157–168 CrossRef CAS PubMed.
- T. Bjarnsholt, The role of bacterial biofilms in chronic infections, APMIS, 2013, 121, 1–58 CrossRef PubMed.
- S. R. Shah, A. M. Tatara, R. N. D'Souza, A. G. Mikos and F. K. Kasper, Evolving strategies for preventing biofilm on implantable materials, Mater. Today, 2013, 16, 177–182 CrossRef CAS PubMed.
- R. M. Donlan, Biofilms: Microbial Life on Surfaces, Emerging Infect. Dis., 2002, 8, 881–890 CrossRef PubMed.
- B. Carpentier and O. Cerf, Biofilms and their consequences, with particular reference to hygiene in the food industry, J. Appl. Bacteriol., 1993, 75, 499–511 CrossRef CAS.
- S. Aggarwal and R. M. Hozalski, Effect of Strain Rate on the Mechanical Properties of Staphylococcus epidermidis Biofilms, Langmuir, 2012, 28, 2812–2816 CrossRef CAS PubMed.
- M. Böl, A. E. Ehret, A. Bolea Albero, J. Hellriegel and R. Krull, Recent advances in mechanical characterisation of biofilm and their significance for material modelling, Crit. Rev. Biotechnol., 2013, 33, 145–171 CrossRef PubMed.
- A. W. Cense, E. A. G. Peeters, B. Gottenbos, F. P. T. Baaijem, A. M. Nuijs and M. E. H. van Dongen, Mechanical properties and failure of Streptococcus mutans biofilms, studied using a microindentation device, J. Microbiol. Methods, 2006, 67, 463–472 CrossRef CAS PubMed.
- D. N. Hohne, J. G. Younger and M. J. Solomon, Flexible Microfluidic Device for Mechanical Property Characterization of Soft Viscoelastic Solids Such as Bacterial Biofilms, Langmuir, 2009, 25, 7743–7751 CrossRef CAS PubMed.
- P. C. Y. Lau, J. R. Dutcher, T. J. Beveridge and J. S. Lam, Absolute Quantitation of Bacterial Biofilm Adhesion and Viscoelasticity by Microbead Force Spectroscopy, Biophys. J., 2009, 96, 2935–2948 CrossRef CAS PubMed.
- A. Rochex, A. Massé, R. Escudié, J.-J. Godon and N. Bernet, Influence of abrasion on biofilm detachment: evidence for stratification of the biofilm, J. Ind. Microbiol., 2009, 36, 467–470 CrossRef CAS PubMed.
- S. S. Rogers, C. van der Walle and T. A. Waigh, Microrheology of Bacterial Biofilms In Vitro: Staphylococcus aureus and Pseudomonas aeruginosa, Langmuir, 2008, 24, 13549–13555 CrossRef CAS PubMed.
- L. C. Simões, M. Lemos, P. Araújo, A. M. Pereira and M. Simões, The effects of glutaraldehyde on the control of single and dual biofilms of Bacillus cereus and Pseudomonas fluorescens, Biofouling, 2011, 27, 337–346 CrossRef PubMed.
- Y. Zhou, A. Torres, L. Chen, Y. Kong, J. D. Cirillo and H. Liang, Fluid-shear method to evaluate bacterial adhesion to glass surfaces, J. Appl. Phys., 2012, 112, 014703 CrossRef PubMed.
- X. Chen and P. S. Stewart, Biofilm removal caused by chemical treatments, Water Res., 2000, 34, 4229–4233 CrossRef CAS.
- L. Pavlovsky, J. G. Younger and M. J. Solomon, In situ rheology of Staphylococcus epidermidis bacterial biofilms, Soft Matter, 2013, 9, 122–131 RSC.
- M. J. Chen, Z. Zhang and T. R. Bott, Direct measurement of the adhesive strength of biofilms in pipes by micromanipulation, Biotechnol. Tech., 1998, 12, 875–880 CrossRef CAS.
- X. Chen and P. Stewart, Role of electrostatic interactions in cohesion of bacterial biofilms, Appl. Microbiol. Biotechnol., 2002, 59, 718–720 CrossRef CAS PubMed.
- M. Wloka, H. Rehage, H.-C. Flemming and J. Wingender, Structure and rheological behaviour of the extracellular polymeric substance network of mucoid Pseudomonas aeruginosa biofilms, Biofilms, 2005, 2, 275–283 CrossRef.
- W. L. Jones, M. P. Sutton, L. McKittrick and P. S. Stewart, Chemical and antimicrobial treatments change the viscoelastic properties of bacterial biofilms, Biofouling, 2011, 27, 207–215 CrossRef CAS PubMed.
- O. Lieleg, M. Caldara, R. Baumgaertel and K. Ribbeck, Mechanical robustness of Pseudomonas aeruginosa biofilms, Soft Matter, 2011, 7, 3307–3314 RSC.
- P. Stoodley, L. Hall-Stoodley and H. Lappin-Scott, Microbial Growth In Biofilms, Pt B., Academic Press Inc, 525 B Street, Suite 1900, San Diego, CA 92101-4495 USA, 2001, vol. 337, pp. 306–318 Search PubMed.
- M. J. Chen, Z. Zhang and T. R. Bott, Effects of operating conditions on the adhesive strength of Pseudomonas fluorescens biofilms in tubes, Colloids Surf., B, 2005, 43, 61–71 CrossRef CAS PubMed.
- T. R. Garrett, M. Bhakoo and Z. Zhang, Characterisation of bacterial adhesion and removal in a flow chamber by micromanipulation measurements, Biotechnol. Lett., 2008, 30, 427–433 CrossRef CAS PubMed.
- P. Ramasamy and X. Zhang, Effects of shear stress on the secretion of extracellular polymeric substances in biofilms, Water Sci. Technol., 2005, 52, 217–223 CAS.
- C. Rupp, C. Fux and P. Stoodley, Viscoelasticity of Staphylococcus aureus biofilms in response to fluid shear allows resistance to detachment and facilitates rolling migration, Appl. Environ. Microbiol., 2005, 71, 2175–2178 CrossRef CAS PubMed.
- K. M. Thormann, R. M. Saville, S. Shukla and A. M. Spormann, Induction of Rapid Detachment in Shewanella oneidensis MR-1 Biofilms, J. Bacteriol., 2005, 187, 1014–1021 CrossRef CAS PubMed.
- P. Stoodley, A. Jacobsen, B. Dunsmore, B. Purevdorj, S. Wilson, H. Lappin-Scott and J. Costerton, The influence of fluid shear and AlCl3 on the material properties of Pseudomonas aeruginosa PAO1 and Desulfovibrio sp. EX265 biofilms, Water Sci. Technol., 2001, 43, 113–120 CAS.
- M. Simões, S. Cleto, M. O. Pereira and M. J. Vieira, Influence of biofilm composition on the resistance to detachment, Water Sci. Technol., 2007, 55, 473–480 CrossRef.
- M. Simões, L. C. Simões and M. J. Vieira, Species association increases biofilm resistance to chemical and mechanical treatments, Water Res., 2009, 43, 229–237 CrossRef PubMed.
- E. Banin, K. M. Brady and E. P. Greenberg, Chelator-Induced Dispersal and Killing of Pseudomonas aeruginosa Cells in a Biofilm, Appl. Environ. Microbiol., 2006, 72, 2064–2069 CrossRef CAS PubMed.
- A. Lomander, P. Schreuders, E. Russek-Cohen and L. Ali, Evaluation of chlorines' impact on biofilms on scratched stainless steel surfaces, Bioresour. Technol., 2004, 94, 275–283 CrossRef CAS PubMed.
- M. Simões, M. Pereira and M. Vieira, Effect of mechanical stress on biofilms challenged by different chemicals, Water Res., 2005, 39, 5142–5152 CrossRef PubMed.
- H. A. Hong, R. Khaneja, N. M. K. Tam, A. Cazzato, S. Tan, M. Urdaci, A. Brisson, A. Gasbarrini, I. Barnes and S. M. Cutting, Bacillus subtilis isolated from the human gastrointestinal tract, Res. Microbiol., 2009, 160, 134–143 CrossRef CAS PubMed.
- M. Morikawa, Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species, J. Biosci. Bioeng., 2006, 101, 1–8 CrossRef CAS PubMed.
- J. C. Zweers, I. Barak, D. Becher, A. J. Driessen, M. Hecker, V. P. Kontinen, M. J. Saller, L. Vavrova and J. M. van Dijl, Towards the development of Bacillus subtilis as a cell factory for membrane proteins and protein complexes, Microb. Cell Fact., 2008, 7, 10 CrossRef PubMed.
- M. Morikawa, M. Ito and T. Imanaka, Isolation of a New Surfactin Producer Bacillus-Pumilus a-1, and Cloning and Nucleotide-Sequence of the Regulator Gene, Psf-1, J. Ferment. Bioeng., 1992, 74, 255–261 CrossRef CAS.
- S. C. Andrews, A. K. Robinson and F. Rodrĩguez-Quiñones, Bacterial iron homeostasis, FEMS Microbiol. Rev., 2003, 27, 215–237 CrossRef CAS.
- P. Stoodley, R. Cargo, C. Rupp, S. Wilson and I. Klapper, Biofilm material properties as related to shear-induced deformation and detachment phenomena, J. Ind. Microbiol., 2002, 29, 361–367 CrossRef CAS PubMed.
- P. Sarin, V. L. Snoeyink, J. Bebee, K. K. Jim, M. A. Beckett, W. M. Kriven and J. A. Clement, Iron release from corroded iron pipes in drinking water distribution systems: effect of dissolved oxygen, Water Res., 2004, 38, 1259–1269 CrossRef CAS PubMed.
- H.-C. Flemming and J. Wingender, The biofilm matrix, Nat. Rev. Microbiol., 2010, 8, 623–633 CAS.
- M. Morikawa, S. Kagihiro, M. Haruki, K. Takano, S. Branda, R. Kolter and S. Kanaya, Biofilm formation by a Bacillus subtilis strain that produces gamma-polyglutamate, Microbiology, 2006, 152, 2801–2807 CrossRef CAS PubMed.
- V. Körstgens, H. C. Flemming, J. Wingender and W. Borchard, Influence of calcium ions on the mechanical properties of a model biofilm of mucoid Pseudomonas aeruginosa, Water Sci. Technol., 2001, 43, 49–57 Search PubMed.
- E. R. Brindle, D. A. Miller and P. S. Stewart, Hydrodynamic Deformation and Removal of Staphylococcus epidermidis Biofilms Treated With Urea, Chlorhexidine, Iron Chloride, or DispersinB, Biotechnol. Bioeng., 2011, 108, 2968–2977 CrossRef CAS PubMed.
- I. W. Dawes, D. Kay and J. Mandelstam, Sporulation in Bacillus subtilis. Establishment of a time scale for the morphological events, J. Gen. Microbiol., 1969, 56, 171–179 CrossRef CAS PubMed.
- G. M. Teitzel and M. R. Parsek, Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa, Appl. Environ. Microbiol., 2003, 69, 2313–2320 CrossRef CAS.
- S. Langley and T. J. Beveridge, Metal binding by Pseudomonas aeruginosa PAO1 is influenced by growth of the cells as a biofilm, Can. J. Microbiol., 1999, 45, 616–622 CrossRef CAS.
- J. J. Harrison, H. Ceri and R. J. Turner, Multimetal resistance and tolerance in microbial biofilms, Nat. Rev. Microbiol., 2007, 5, 928–938 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4mt00049h |
| This journal is © The Royal Society of Chemistry 2014 |