Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Identification of redox-regulated components of arsenate (AsV) tolerance through thiourea supplementation in rice†
A. K.
Srivastava
*a,
S.
Srivastava‡
a,
S.
Mishra§
b,
S. F.
D'Souza
a and
P.
Suprasanna
a
aNuclear Agriculture and Biotechnology Division, Bhabha Atomic Research Centre, Mumbai 400085, India. E-mail: ashishbarc@gmail.com; Fax: +91-22-25505151; Tel: +91-22-25593870
bUFZ – Helmholtz Centre for Environmental Research, Department of Analytical Chemistry, Permoserstr. 15, D-04318 Leipzig, Germany
First published on 2nd June 2014
Abstract
Arsenic (As) is a ubiquitously present environmental carcinogen that enters into the human food chain through rice grains. In our previous research, the application of thiourea (TU; a non-physiological thiol based ROS scavenger) has been demonstrated to enhance salt and UV stress tolerance as well as the crop yield under field conditions. These effects were associated with the ability of TU to maintain plant redox homeostasis. Since As stress also induces redox imbalance, the present research was initiated to evaluate the efficiency of TU in regulating As tolerance/accumulation in rice. The supplementation of TU (75 μM) to AsV (25 μM) improved the root growth and also reduced the As concentration by 56% in the aerial parts, which could be attributed to significant downregulation of the Lsi2 transporter responsible for the translocation of As from root to shoot. The fact that these effects were not due to direct interaction between As and TU was confirmed from complexation studies using HPLC-(ICP-MS)-(ESI-MS). Short-term kinetic studies of GSH levels and the GSH/GSSG ratio confirmed the establishment of differential redox states in As and As + TU treated seedlings. The real-time RT-PCR based comparative expression profiling under As with/without TU treatment identified Sultr1;1 and Sultr1;2 as major redox-regulated sulfate transporters. Their specific induction in shoots coupled with enhanced root-to-shoot sulfate translocation (analyzed using 35S-sulfate as a radiotracer) was observed under TU supplementation. Furthermore, the level of thiolic metabolites (PC2 in roots and GSH and PC3 in shoots) and activities of sulfur metabolism enzymes (ATP sulfurylase and cysteine synthase in roots and 5′-adenylylsulfate reductase in shoot) were also increased with As + TU as compared to As treatment. Thus, this study utilizes the interaction between As and TU to identify the critical redox regulated components of As tolerance in rice.
Introduction
Arsenic (As) is a ubiquitously present environmental toxin and is recognized as a group-1 carcinogen by the International Agency for Research on Cancer (IARC). The health of nearly 150 million people worldwide from over 70 countries spanning six inhabited continents is threatened from As hazards. The major route of As contamination for humans is either through drinking water or crops and fodders,1 mainly rice.2 Thus, different strategies are being developed to obtain low grain arsenic rice, either through conventional breeding/varietal selection or by modern transgenics; however, these approaches will still take some time to come into use under field conditions. Under this milieu, the strategy with the most potential is supposed to be the management of agronomic practices to provide an immediate and sustainable solution to reduce As load in rice grains. Various approaches have been demonstrated to hold potential, e.g. growing rice with less irrigation,3 supply of silicate minerals4 and phosphorus5 and inoculation with arsenic-tolerant soil fungi6 and mycorrhiza.7Inorganic As is a prevalent form present in the environment, existing as arsenate (AsO43−, AsV) or arsenite (AsO33−, AsIII), depending upon the pH and redox potential of the environment.8 Although the mode of toxicity of the two forms of As is different, As toxicity, in general, is associated with the induction of sulfur deficiency, oxidative stress and alteration of redox states.9–11 Sulfur is an essential element for plant growth. There is a family of sulfate transporters (classified as groups-1 to 4) which takes up sulfur in the form of inorganic sulfate.12 Inside the plant, sulfate is first activated to adenosine-5′-phosphosulfate (APS) by ATP sulfurylase, and then reduced to sulfite by APS reductase (APR). Sulfite is reduced to sulfide, which is incorporated by cysteine synthase into O-acetylserine to form cysteine.13 The key enzyme of the sulfur assimilation pathway is APR, which is regulated by transcription factor Long Hypocotyl 5 (HY5) in a demand-driven and light-dependent manner.14 The major proportion of sulfur reduction takes place in shoot chloroplasts, which is supported by the light regulated nature of HY5.15 Glutathione (GSH; γ-Glu-Cys-Gly) and phytochelatins (PCs; GSH oligomers) are the important sulfur-containing compounds responsible for As complexation, vacuolar sequestration and maintenance of redox states.16–18 The importance of sulfur is also due to the fact that its supply affects As uptake, translocation and accumulation in rice plants.19,20 The relevance of redox the state in the regulation of As toxicity9 and for the activation of downstream signaling events is known.21 Thus, it was hypothesized that a plant’s As stress tolerance may be enhanced by avoiding redox imbalance. In our earlier research, we used thiourea (TU), as an external agent, to maintain the plant's redox balance under salt and UV stress.22,23 TU is a non-physiological thiol and its broad range ROS scavenging activity in biological systems is well documented.24 The positive effect of TU was also demonstrated in the enhancement of source-to-sink sucrose translocation,25 in identification of the signaling and effector components of salt tolerance26 and to improve the crop yield and oil content of Brassica.27 In the present work, the effect of the interaction between As and TU was utilized for the identification of redox regulatory mechanisms of As tolerance in rice. The efficacy of TU in reducing the As load was also assessed.
Materials and methods
Plant material, growth conditions and treatment
The study was performed on Oryza sativa var. IR64. Seeds were surface sterilized with 30% ethanol for 3 min and then washed thoroughly with distilled water to remove traces of ethanol. The seeds were then soaked in distilled water under shaking conditions (∼100 rpm) at 25 °C. The volume of water was adjusted so as to provide sufficient air to seeds while shaking. After 14–16 h of incubation, seeds were uniformly spread on a Petri plate and then allowed to germinate under dark conditions. A customized circular thermocol disc was made, which had the capacity to hold 18 seedlings. The 4 d old seedlings were fixed on these discs and then placed in a 1 L beaker with 800 ml of 1/2 Kimura solution supplemented with different treatments such as AsV (prepared using the salt Na2HAsO4), As + TU and TU. One separate set was maintained as a control. All the sets were transferred into a plant growth chamber (Sanyo, Japan) with a daily cycle of a 14 h photoperiod with a light intensity of 150 μE m−2 s−1, day/night temperatures of 25/22 °C and a relative humidity of 65–75%. After 12 d of growth, differential phenotype was recorded in terms of dry weight/seedlings and average root and shoot lengths. Dry weights were measured after drying the samples to a constant weight in an oven. A similar set-up was employed for the measurement of arsenic content, level of various thiols and activities of sulfur metabolism related enzymes. The roots and shoots were harvested and stored at −80 °C until analysis. The harvesting time was fixed at 1 PM for each batch of experiments. For the measurement of short-term 35S-sulfate uptake kinetics, the redox couple (GSH and GSSG) and real-time RT-PCR based expression profiling, seedlings were grown for 15 d under control conditions and then subjected to different treatments. For As + TU and TU, pre-treatment with TU was performed for 24 h. In order to study the light-dependent regulation, the treatments were given at 9 AM and then 1, 4 and 8 h harvesting of roots and shoots was performed, and samples were stored at −80 °C until analysis. The concentrations of AsV and TU were 25 μM and 75 μM, respectively.Arsenic measurement
For each treatment, seedlings were washed thoroughly in ice-cold milli-Q water to remove adsorbed As. The roots and shoots were then separated and oven-dried at 80–85 °C until a constant dry weight was obtained. The dried tissue (∼100 mg) was kept in 1 ml of concentrated HNO3 overnight at room temperature and then digested at 120 °C. The residue was then diluted in 10 ml of milli-Q water and subjected to As estimation using ICP-MS. The certified reference material (CRM) NIST 1568a rice flour from and blanks were included for quality assurance.In vitro complexation studies of arsenic with glutathione and thiourea
To check the complexation of As with thiourea, various combinations of As (4 to 40 mM, either AsIII or AsV) and thiourea (33 to 330 mM), with and without GSH (3.3 to 33 mM) were tested. The substances were dissolved in degassed water or 0.1% formic acid and allowed to react for 12–15 h under nitrogen. The complexes were analyzed by HPLC-(ICP-MS)-(ESI-MS).An HP1100 HPLC system (Agilent Technologies Böblingen, Germany) with an auto-sampler cooled to 4 °C was used. The separation was performed on a reverse-phase C18, Waters Atlantis column (150 mm × 4.6 mm × 5 μm, 100 Å) using a gradient of 0.1% (v/v) formic acid (A) and 0.1% formic acid in 20% (v/v) methanol (B) at a flow rate of 1 ml min−1. Post-column, the flow was split in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 into the ICP-MS and ESI-MS. The 6130 quadrapole LC/MS system (Agilent Technologies Böblingen, Germany) was used as a molecule-specific detector for postcolumn detection of the As complexes by their molecular ion peaks. The MSD was used in the positive ionization mode from m/z 50 to m/z 1000 with an API electrospray head. The settings chosen were: capillary voltage of 4000 V, nebulizer pressure of 40 psi, drying gas flow of 12 L min−1 at 350 °C, quadrupole temperature of 100 °C, and fragmenter voltage of 80 V. The ICP-MS 7500ce (Agilent Technologies Böblingen, Germany) was used for the element-specific detection of As. The instrument was equipped with a microconcentric nebulizer (flow rate < 100 μL min−1), a Peltier cooled spray chamber, and oxygen as an additional plasma gas. The instrument was used in the soft extraction mode. The instrument settings were checked daily for As sensitivity and optimized when necessary.
1 into the ICP-MS and ESI-MS. The 6130 quadrapole LC/MS system (Agilent Technologies Böblingen, Germany) was used as a molecule-specific detector for postcolumn detection of the As complexes by their molecular ion peaks. The MSD was used in the positive ionization mode from m/z 50 to m/z 1000 with an API electrospray head. The settings chosen were: capillary voltage of 4000 V, nebulizer pressure of 40 psi, drying gas flow of 12 L min−1 at 350 °C, quadrupole temperature of 100 °C, and fragmenter voltage of 80 V. The ICP-MS 7500ce (Agilent Technologies Böblingen, Germany) was used for the element-specific detection of As. The instrument was equipped with a microconcentric nebulizer (flow rate < 100 μL min−1), a Peltier cooled spray chamber, and oxygen as an additional plasma gas. The instrument was used in the soft extraction mode. The instrument settings were checked daily for As sensitivity and optimized when necessary.
Fluorescence HPLC based estimation of various thiols
For the measurement of various thiols, liquid nitrogen ground plant samples (∼400 mg) were extracted in buffer [diethylenetriamine pentaacetic acid (DTPA; 6.3 mM) and trifluoroacetic acid (TFA; 0.1% v/v)]. The extraction was performed on an equal volume basis and the supernatant was collected after centrifuging at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C. The supernatant (250 μL) was added to 615 μL of HEPES buffer [HEPES (200 mM), DTPA (6.3 mM; pH 8.2)]. To this mixture, 25 μL of tris(2-carboxyethyl)phosphine hydrochloride (TCEP; 20 mM; as a disulfur reductant) and 10 μL of N-acetyl-L-cysteine (0.5 mM; as an internal standard) were added and the final mix was pre-incubated at 45 °C for 10 min in a water bath. This step is required to ensure that all thiols are in a reduced state so that maximum derivatization can occur. For monobromobimane (mBBr) based derivatization, 10 μL of mBBr (50 mM) was added and the mix was incubated in the dark in a water bath for 30 min at 45 °C. The reaction was terminated by the addition of 100 μL of acetic acid (10 mM). The derivatized samples were filtered with 0.22 micron nylon syringe filters and then stored at −20 °C for HPLC analyses. The separation and analysis of various thiols (GSH, cysteine and PCs) was carried out on reverse-phase HPLC (Waters, USA) with a Purospher RP-18e column (Merck) using a gradient of solvent A (99.9% acetonitrile + 0.1% TFA) and B (89.9% water + 10% acetonitrile + 0.1% TFA) at a flow rate of 1 ml min−1 as described in Minocha et al.28 Fluorescence intensity with an excitation wavelength of 380 nm and an emission wavelength of 470 nm was recorded using a fluorescence detector (Waters 474). The chromatograms were recorded and analyzed using Empower software.
000g for 10 min at 4 °C. The supernatant (250 μL) was added to 615 μL of HEPES buffer [HEPES (200 mM), DTPA (6.3 mM; pH 8.2)]. To this mixture, 25 μL of tris(2-carboxyethyl)phosphine hydrochloride (TCEP; 20 mM; as a disulfur reductant) and 10 μL of N-acetyl-L-cysteine (0.5 mM; as an internal standard) were added and the final mix was pre-incubated at 45 °C for 10 min in a water bath. This step is required to ensure that all thiols are in a reduced state so that maximum derivatization can occur. For monobromobimane (mBBr) based derivatization, 10 μL of mBBr (50 mM) was added and the mix was incubated in the dark in a water bath for 30 min at 45 °C. The reaction was terminated by the addition of 100 μL of acetic acid (10 mM). The derivatized samples were filtered with 0.22 micron nylon syringe filters and then stored at −20 °C for HPLC analyses. The separation and analysis of various thiols (GSH, cysteine and PCs) was carried out on reverse-phase HPLC (Waters, USA) with a Purospher RP-18e column (Merck) using a gradient of solvent A (99.9% acetonitrile + 0.1% TFA) and B (89.9% water + 10% acetonitrile + 0.1% TFA) at a flow rate of 1 ml min−1 as described in Minocha et al.28 Fluorescence intensity with an excitation wavelength of 380 nm and an emission wavelength of 470 nm was recorded using a fluorescence detector (Waters 474). The chromatograms were recorded and analyzed using Empower software.
Measurement of activities of sulfur metabolism related enzymes
The liquid nitrogen ground plant samples (∼500 mg) were homogenized in extraction buffer (1 ml), squeezed through four layers of cheesecloth and then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min at 4 °C. The specific extraction buffer was used for each enzyme as described previously by Hartmann et al.29 The detailed methodology for the measurement of enzyme activity is given in the ESI,† S-1. The protein content in the sample was measured as per the protocol of Lowry et al.30
000g for 15 min at 4 °C. The specific extraction buffer was used for each enzyme as described previously by Hartmann et al.29 The detailed methodology for the measurement of enzyme activity is given in the ESI,† S-1. The protein content in the sample was measured as per the protocol of Lowry et al.30
Measurement of redox state in terms of GSH/GSSG ratio
The level of reduced (GSH) and oxidized (GSSG) glutathione was determined fluorometrically using o-phthaldialdehyde (OPT) as a fluorophore by following the protocol of Hissin and Hilf.31Short-term uptake kinetics using 35S-sulfate as a radiotracer
For 35S-sulfate radiotracer uptake kinetics, the hydroponic solutions of the seedlings given different treatments were supplemented independently with 35S-sulfate (2 MBq L−1). After 1, 4 and 8 h, the root and shoot parts were separately harvested and 35S-sulfate levels were measured by scintillation counting. For scintillation counting, seedlings were removed from the radioactive solution and then rinsed with the ice-cold non labeled nutrient solution (3 times for 20 s each). Root and shoot samples were weighed separately and then digested in 10 ml of HCl (1 N) at room temperature. After 7 d, 100 μL of digested extract was mixed with 5 ml of scintillation cocktail [naphthalene (30 g), PPO (2 g), ethylene glycol (100 ml), methanol (50 ml) were mixed and the volume made up to 500 ml with dioxane] and then counted on protocol 2 of a TRI-CARB 2100 TR liquid scintillation analyzer (Packard, Canberra), as described previously.32 The efficiency of the counter used was 95%.Primer design and real-time PCR based expression profiling of sulfate and arsenite transporter (low silicon 2; Lsi2)
All the primers used for real-time PCR were from the exon–intron boundary and designed using a web-based Quant-prime tool.33 The details of the primers are given in ESI,† S-2. The specificity of all primers was confirmed by sequence analysis of RT-PCR amplicons. The DNA-free total RNA was extracted using a mirVANA kit (AM1560, Ambion). The 260/280 and 260/230 ratios greater than 2 and the intactness of rRNA bands (28/18 s) in denaturing gel electrophoresis were considered as quality control of RNA to be used for further analysis. RNA (2 μg) was subjected to cDNA synthesis using Superscript III RT (18080-093; Invitrogen) following the manufacturer’s protocol. Real-time PCR was carried out using Rotor-Gene 6600 (Corbett Life Science; http://www.corbettlifescience.com). Reactions were set up by combining 10 μL of SyBr green PCR reaction mix (Sigma; S 4320) with 2.5 μL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 diluted cDNA templates, 1.5 μL each of forward and reverse primer (10 mM each), and 4.5 μL of PCR grade water (Sigma W 1754). For gene expression analyses, the reference gene (tubulin) and one target gene were analyzed per run, and reactions were carried out in triplicates for each sample. The following PCR protocols were followed: 95 °C for 15 min; 40 cycles at 94 °C for 20 s, 55 °C for 30 s, and 72 °C for 30 s followed by 72 °C for 10 min and melting curve analysis. The data of the Ct value (cycle threshold) were calculated for target/reference genes for each treatment and respective control, and then log2 expression fold difference was calculated using REST-384 version 2 software. For both up- and down-regulation, a 1.5-fold change was set as the cutoff for detecting significant changes in expression.
5 diluted cDNA templates, 1.5 μL each of forward and reverse primer (10 mM each), and 4.5 μL of PCR grade water (Sigma W 1754). For gene expression analyses, the reference gene (tubulin) and one target gene were analyzed per run, and reactions were carried out in triplicates for each sample. The following PCR protocols were followed: 95 °C for 15 min; 40 cycles at 94 °C for 20 s, 55 °C for 30 s, and 72 °C for 30 s followed by 72 °C for 10 min and melting curve analysis. The data of the Ct value (cycle threshold) were calculated for target/reference genes for each treatment and respective control, and then log2 expression fold difference was calculated using REST-384 version 2 software. For both up- and down-regulation, a 1.5-fold change was set as the cutoff for detecting significant changes in expression.
Statistical analysis
The experiments were carried out in a completely randomized design. All the experiments were repeated at least twice to check reproducibility. One-way analysis of variance (ANOVA) was performed on all the data to confirm the variability of data and the validity of results. Duncan's multiple range test (DMRT) was performed to determine the significant difference between treatments using the statistical software SPSS 17.0.Results
Thiourea supplementation partially alleviated arsenic stress
The post-germination phenotyping was performed under different treatments to evaluate the effectiveness of TU supplementation. The analysis revealed differential phenotype of seedlings subjected to As with/without TU treatments (Fig. 1A). There was a significant reduction in root and shoot lengths of 46 and 21%, respectively, under As stress as compared to the control. The supplementation of TU increased the root length (Fig. 1B) and the dry weight (Fig. 1C) by 42 and 13%, respectively, as compared to those of seedlings treated with As alone. No significant differences were observed in the length and dry weight of shoots between As and As + TU treated seedlings (Fig. 1B). The phenotype of the seedlings subjected to TU treatment alone was comparable to that of the control (Fig. 1A–D).Level of arsenic in different plant parts
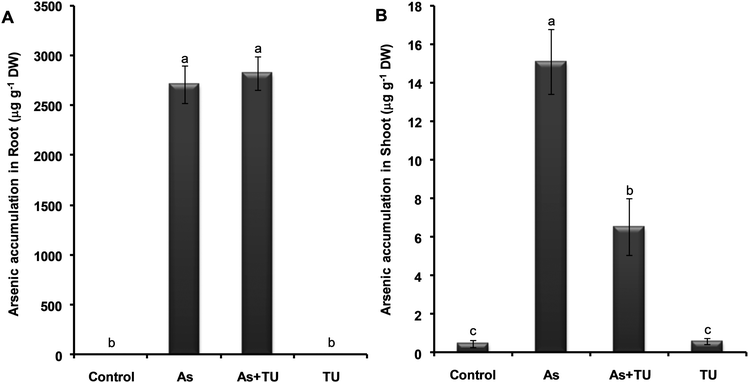
In roots, the concentration of As was not significantly different in As (2710 μg g−1 DW) and As + TU (2825 μg g−1 DW) treatments (Fig. 2A). However, TU supplementation significantly reduced the As concentration in the aerial parts of rice seedlings. The As + TU treated seedlings showed a 56% reduction in As concentration in the shoots as compared to that of treatment with As alone (Fig. 2B). By taking into account root and shoot dry weight data and As concentration, the total As content in the root and shoot (μg) was calculated. It was found that total root As content per plant increased from 4.07 μg with As alone to 5.65 μg for As + TU, while the total shoot As content per plant decreased significantly from 0.092 μg to 0.046 μg.Lack of complexation between arsenic and thiourea
To check the possibility of As complexation with TU, an in vitro experiment was performed and analyzed by HPLC coupled in parallel to ICP-MS as an element specific detector and ESI-MS as a molecule specific detector (Fig. 3). The complexes of As with TU and/or GSH which could form are: As-TU3, GS-As-TU2, GS2-As-TU, As-GS3. The reaction mixtures containing AsV in all combinations and AsIII without GSH showed only one peak in ICP-MS, corresponding to inorganic As. However, the reaction mixture containing AsIII, TU and GSH showed four As species in ICP-MS. ESI-MS showed strong a signal at m/z values of 75, 687, 865 and 994 corresponding to inorganic As, As+-GS2, GS2-As-CysGly + H+ and As-GS3 + H+ for the ICP-MS peaks. None of the peaks corresponding to As-TU complexes were detected in ESI-MS. Thiourea, reduced GSH and oxidized GSH were also detected by ESI-MS, showing signals at m/z 77, 308, and 613 respectively for [M + H]+.Thiourea treatment modulates the level of various thiols
Fluorescence HPLC based detection was performed for thiols such as cysteine and GSH (Fig. 4A) and phytochelatins (Fig. 4B). The levels of most of the thiols were significantly increased in both the roots and shoots under As and As + TU treatment. In roots, the cysteine, GSH and PC4 contents increased about 10-, 2.4- and 22-fold in both As and As + TU treatments, respectively, as compared to that of the control. This was in contrast to PC2, which specifically increased 56-fold under As + TU as compared to As treatment. No significant induction in the level of PC3 was observed under any treatment (Fig. 4B). In the shoot, the cysteine content was increased 1.15-fold for both As and As + TU treatments as compared to that of the control. In contrast, the GSH level increased 1.8- and 2.8-fold under As and As + TU treatment, respectively, as compared to that of the control. The level of PC3 was increased 2.63-fold in As + TU as compared to that of any other treatment. The level of PC2 was found to be same under As and As + TU treatments, while that of PC4 was increased for As (1.7-fold) but decreased for As + TU (0.5-fold) treatments, as compared to that of the control (Fig. 4B). For treatment with TU alone, no significant change in the level of any thiol was observed in roots (Fig. 4A), however in shoots, the cysteine, GSH and PC2 contents were significantly increased as compared to those of the control (Fig. 4A and B). To measure the extent of As chelation by thiols (GSH + PCs), molar ratios of –SH to As (analyzed in fresh samples) were calculated.34 The molar ratios of –SH to As were 0.109 and 0.122 for As and As + TU in roots. Hence, a maximum of about 3.6% and 4.1% As would be chelated by thiols in roots, assuming a stoichiometry of three SH to one As. In contrast, –SH to As molar ratios were very high in shoots for both As (27) and As + TU (76) treatment, suggesting an excess of thiols and that all As may be chelated.Activities of sulfur metabolism related enzymes
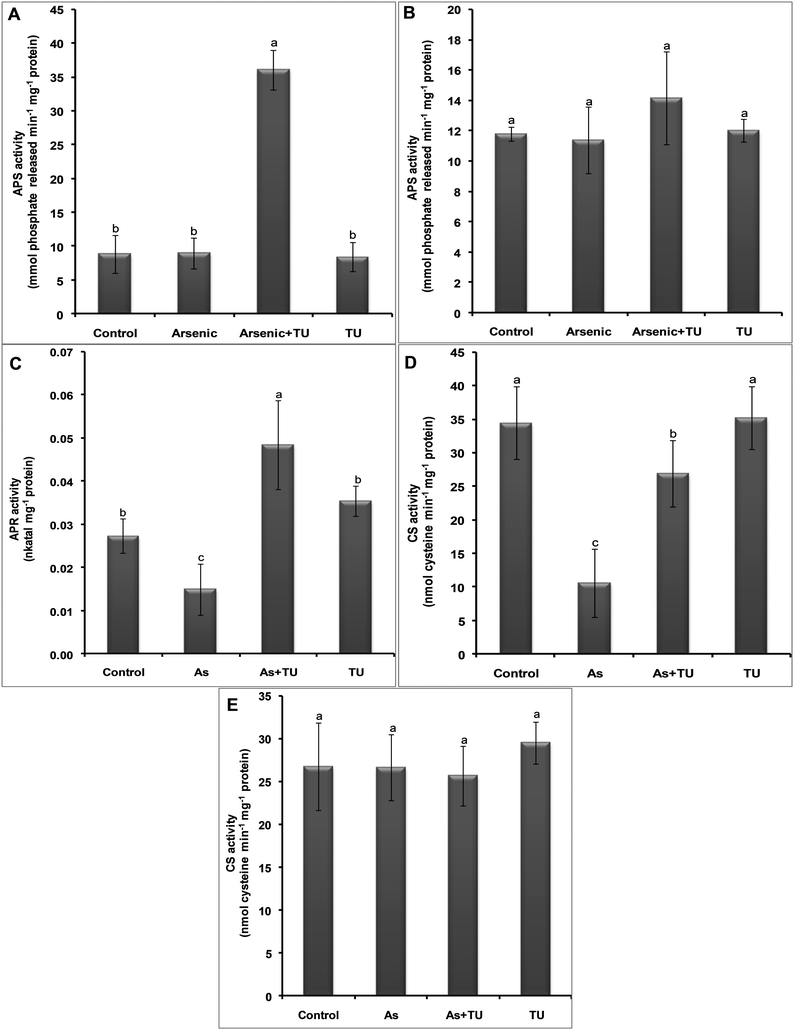
The activities of sulfur metabolism related enzymes such as ATP sulfurylase (APS), 5′-adenylylsulfate reductase (APR) and cysteine synthase (CS) were measured in the roots and shoots of seedlings subjected to different treatments. The APS activity was increased 4- and 1.19-fold in As + TU treated roots and shoots, respectively, as compared to that of the control. For treatments with As and TU alone, no significant differences in APS activity were observed in roots as well as in shoots (Fig. 5A and B). The APR activity in shoots was decreased and increased by 45% and 77% under As and As + TU treatments, respectively, compared with that of the control (Fig. 5C). No APR activity could be detected in roots. The light mediated regulation of APR activity might be responsible for its significantly low activity in roots, which could not be detected. The CS activity in roots was decreased by 70 and 20% under As and As + TU treatments, respectively, as compared to that of control and TU treatments (Fig. 5D). In shoots, no significant difference in CS activity was observed under any treatment (Fig. 5E).Thiourea mediates modulation of cellular redox state
In roots, under As stress, the GSH content decreased in a time-dependent manner and the maximum decrease of 32% was observed at 8 h. Under As + TU treatment and treatment with TU alone, the GSH level remained lower than that of the control until 4 h and a sharp increase was observed at 8 h (66% and 42% increase for As + TU and TU treatments, respectively, as compared to that of the control; Fig. 6A). In contrast with the GSH level, the GSH/GSSG ratio was found to be higher for all treatments compared to that of the control, with the maximum being at 8 h when the ratios were 1.35-, 2.26- and 2.1-fold higher for treatments with As, As + TU and TU alone, respectively (Fig. 6B).In shoots, no significant difference in the GSH level was seen until 4 h under any treatment. At 8 h, the GSH levels were increased 2.25-, 2- and 1.58-fold in treatment with As, As + TU and TU alone, respectively, as compared to that of the control (Fig. 6C). The response of the GSH/GSSG ratio was similar to that of the GSH level in all treatments (Fig. 6D).
Differential translocation of sulfate from root to shoot: 35S-sulfate based radiotracer study
In roots, the 35S-sulfate progressively increased in a time dependent manner under all treatments. The control roots showed maximum uptake at 1 h with minimum uptake at 8 h. In TU-treated roots, the uptake was initially slow until 4 h, and then there was an increase in 35S-sulfate uptake at 8 h. In As and As + TU treatments, the level of 35S-sulfate was almost the same until 4 h. However at 8 h, the 35S-sulfate levels were increased by 1.91- and 1.12-fold, respectively under As and As + TU treatments as compared to the control (Fig. 7A).In shoots, initially at 1 h, the level of 35S-sulfate was almost same in control, As and TU alone treatments but was slightly higher in As + TU treatment. With increasing time, 35S-sulfate uptake increased in all treatments. However, the uptake of 35S-sulfate was lower in the control and As treatments as compared to TU alone and As + TU treatments, with the lowest uptake being in control treatments. At 8 h, the total 35S-sulfate levels in As + TU and treatment with TU alone were increased 3.42- and 2.96-fold, respectively, as compared to the control (Fig. 7B).
Expression profiling of different classes of sulfate (Sultrs) and AsIII (Lsi2) transporters in root and shoot
In roots, Sultr1;1, 1;2, 2;1 and 3;3 were up-regulated in both As and As + TU treatments, however, the level of regulation was comparatively higher for As than for As + TU treatment. Additionally, the higher level of expression was maintained until 8 h in As for Sultr1;1, 1;2 and 2;1 but not in As + TU. Additionally, few isoforms were regulated in a treatment-specific manner, viz., the up-regulation of Sultr1;3 in As + TU and Sultr3;4 in As, at 4 h and Sultr4;1 in As at 4 h and 8 h. In treatment with TU alone, the levels of most of the sulfate transporters were either significantly down-regulated or not significantly affected in roots for all time points, except for Sultr1;2 (at 1 h) and Sultr3;3 (at 8 h) which were 2.19- and 2.48-fold up-regulated, respectively. The expression of Lsi2 was not changed under any treatment until 4 h of treatment. At 8 h, Lsi2 was down-regulated 3- and 2.5-fold, respectively under As + TU and TU treatments, as compared to that of the control (Table 1A).| Arsenic | Arsenic + TU | TU | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 4 h | 8 h | 1 h | 4 h | 8 h | 1 h | 4 h | 8 h | |
| A | |||||||||
| Sultr1;1 | −1.43 | 4.467 | 3.859 | 1.135 | 3.733 | 1.306 | 0.95 | −3.427 | −3.058 |
| Sultr1;2 | −1.36 | 3.292 | 2.298 | 2.165 | 2.91 | 0.009 | 2.19 | 0.294 | −0.867 |
| Sultr1;3 | 0.34 | 1.332 | −0.032 | −0.325 | 1.985 | 0.419 | −0.22 | −0.931 | 0.158 |
| Sultr2;1 | 0 | 5.641 | 3.636 | 0.26 | 3.553 | 0.612 | −0.15 | −1.616 | −2.976 |
| Sultr2;2 | −1.16 | 0.972 | −0.207 | −1.82 | 0.03 | −0.411 | −1.145 | −1.901 | 0.113 |
| Sultr3;1 | 1.3 | −0.548 | −1.112 | 0.73 | −0.975 | −0.671 | 0.665 | −2.351 | −1.152 |
| Sultr3;2 | 0.21 | 0.842 | −0.277 | −1.16 | 0.525 | −0.381 | −2.41 | −1.516 | 1.283 |
| Sultr3;3 | 1.05 | 2.157 | −0.407 | −0.555 | 1.74 | 0.754 | −1.725 | −1.756 | 2.488 |
| Sultr3;4 | −0.405 | 2.087 | 0.243 | −0.9 | 0.99 | −2.361 | −1.375 | −0.926 | −2.127 |
| Sultr3;6 | 0.05 | 0.75 | 0.143 | −0.315 | −0.47 | −0.831 | −0.945 | −2.466 | −1.597 |
| Sultr4;1 | 0.94 | 2.587 | 2.273 | 0.545 | 1.435 | −0.356 | 0.885 | −2.001 | −1.112 |
| Lsi-2 | −0.11 | 0.552 | −1.481 | −0.725 | −0.94 | −3.051 | −0.385 | −1.271 | −2.472 |
| B | |||||||||
| Sultr1;1 | 3.88 | −2.379 | −0.451 | −2.585 | 1.214 | 6.893 | −6.21 | 11.597 | 8.17 |
| Sultr1;2 | 0.275 | −0.107 | −0.114 | −5.39 | 1.309 | 3.919 | −8.37 | 10.36 | 5.037 |
| Sultr1;3 | −0.45 | −1.142 | 0.346 | 1.835 | −0.506 | −1.541 | 1.775 | −1.32 | −1.643 |
| Sultr2;1 | 3.445 | 1.563 | 0.248 | 0.95 | 1.413 | 1.015 | 0.485 | 7 | 1.379 |
| Sultr2;2 | −0.465 | 0.553 | 1.086 | 2.385 | 0.759 | −0.271 | 1.715 | −0.135 | 1.067 |
| Sultr3;1 | −0.43 | −1.697 | −0.324 | 1.995 | −1.116 | 0.154 | 1.325 | −1.295 | −0.708 |
| Sultr3;2 | −2.01 | −0.507 | 0.661 | −0.335 | −0.831 | 0.044 | 0.72 | −1.46 | −0.163 |
| Sultr3;3 | −2.46 | −1.187 | 0.286 | 0.715 | −0.516 | −1.731 | 1.515 | −1.995 | −2.678 |
| Sultr3;4 | −0.505 | 0.568 | −0.324 | 0.115 | 0.239 | −0.576 | 0.755 | 1.695 | 1.512 |
| Sultr3;6 | 0.25 | −1.617 | 0.276 | 1.17 | −0.886 | 0.869 | 0.18 | −0.02 | 1.217 |
| Sultr4;1 | 0.335 | −1.787 | −0.194 | 1.97 | −0.951 | 0.354 | 1.06 | 0.87 | −0.283 |
In shoots, under As stress, Sultr1;1 and 1;2 were either down-regulated or remained level with the control except for Sultr1;1 at 1 h. This was in contrast to As + TU, where the down-regulation of Sultr1;1 and 1;2 was limited to 1 h; beyond which a time-dependent increase was observed in their expression. The levels at 8 h were 6.89- and 3.91-fold up-regulated for Sultr1;1 and 1;2, respectively. The profiles of the remaining Sultrs’ responses 1 h after treatment were also different between As with/without TU treatment. As treatment was associated with the induction of Sultr2;1 and down-regulation of Sultr3;2 and 3;3, while As + TU treatment caused up-regulation of Sultrs 1;3, 2;2, 3;1 and 4;1. In shoots treated with TU alone, the profiles of most of the Sultrs were comparable to that of As + TU; however, the extent of change was significantly greater. At 4 h, Sultrs 1;1, 1;2 and 2;1 were 1.21-,1.3-, and 1.41-fold higher in As + TU but 11.59-, 10.36-, and 7-fold higher under treatment with TU alone. One isoform showing a major difference in expression pattern between TU and As + TU was Sultr3;4, which was up-regulated in TU (ranging from 0.75- to 1.51-fold at different time points) but not in As + TU (ranging from −0.58- to 0.24-fold at different time points) (Table 1B).
Discussion
In an earlier study, TU supplementation has been demonstrated to impart salt tolerance through the maintenance of cellular energetics35 and redox homeostasis.22 Since these are also the major determinants of As stress tolerance in plants,9,11 the present study was performed to evaluate the efficiency of TU for ameliorating As-induced damage and to implicate the significance of redox homeostasis in As stress tolerance. Initially, post-germination phenotyping of rice was performed using a range of As concentrations (5–50 μM) on the basis of average root length and the IC50 value (25 μM) was calculated (data not shown). Then, a range of TU concentrations (10–200 μM) were tested along with 25 μM As (data not shown) and 75 μM TU was found to be optimum, which could partially revert the seedling phenotype (in terms of root length) (Fig. 1). The lack of complete phenotype reversal indicates that there are redox independent factors in As induced damage, and hence, redox homeostasis alone may not alleviate overall toxicity. The physiological thiol GSH has been evaluated in earlier studies for stress amelioration against As36 and cadmium.37 However, being a physiological thiol, it may also modulate a range of metabolic pathways in addition to the redox state. Such a possibility is comparatively low for TU, which is a non-physiological thiol, and hence the observed effects can be correlated to the redox state with a greater certainty. It has been confirmed in our earlier studies, using HyPer-transformed Arabidopsis lines (Srivastava et al., unpublished research) as well as through biochemical methods,22 that TU supplementation generates a reduced redox state. A TU-mediated shift in redox state towards the reducing direction might be responsible for partial stress amelioration against As stress. The As level was analyzed to test whether improved root growth in As + TU was associated with a decline in As. Surprisingly, As concentration in roots was not significantly affected. In fact, owing to the increase in root dry weight, the total root As content per plant in As + TU was even higher than for treatment with As alone. However, both As concentration and total shoot As content per plant were significantly reduced in shoots under As + TU treatment as compared to treatment with As alone (Fig. 2). This suggested that the loading of As into the xylem for root-to-shoot transport is affected under TU treatment. To test this hypothesis, the expression level of Lsi2 (a silicon or AsIII exporter) was analyzed in roots under different treatments. Owing to the localization of OsLsi2 on the proximal side of epidermal and endodermal cells, it is involved in the translocation of As from root to shoot.11 Although the present study deals with AsV, AsIII specific transporters were analyzed because, inside the plants, AsV has been shown to be rapidly converted into AsIII.38 Under As + TU treatment, Lsi2 expression was down-regulated in roots which might be responsible for decreasing the As levels in the shoots. These are interesting data which signify the redox state as an important regulator of As uptake and translocation in rice. This is further supported by the findings of Liu et al.39 and Duan et al.,40 where BSO (L-buthionine sulfoximine, a GSH biosynthesis inhibitor known for creating an oxidized redox environment) treatment has been demonstrated to enhance root-to-shoot or shoot-to-grain As translocation in Arabidopsis and rice. Since the application of TU under field conditions is already established, the present result of TU mediated reduction of root-to-shoot As translocation can have implications for reducing As load from rice grains.In spite of the decrease in As level, no significant differences in shoot growth were observed between As and As + TU treatment (Fig. 1). This might be either due to the short duration of the experiment or due to the differences in As concentration not being sufficient to produce visible differences in shoot growth. In contrast, root growth was improved under As + TU treatment compared with As treatment, despite the fact that As concentration was not significantly different between two treatments (Fig. 1A and B). There may be two possible reasons for this observation. Firstly, there might be improved tolerance against As toxicity through enhanced antioxidant potential. Such a mechanism has been suggested for TU supplemented Brassica juncea seedlings subjected to salt stress.22 Secondly, the level of free As might be variable between the two treatments, which may be achieved through efficient vacuolar sequestration of As mediated through some unknown redox-dependent transporter, or by As complexation either by TU itself due to presence of a thiol group (–SH) or by GSH and PCs. The possibility of As complexation with TU was evaluated in vitro using HPLC coupled with parallel ICP-MS and ESI-MS. The data obtained indicated that the formation of As-TU complexes was not feasible (Fig. 3) and was ruled out as one of the possible mechanisms for reducing free As levels in roots. The induction of in built tolerance mechanisms of As complexation via thiolic metabolites was then studied. Thiol metabolism is regarded as a major determinant of As tolerance41 as well as As accumulation in plants.39,40 The fluorescence HPLC based profiling of various thiols was performed in both roots and shoots (Fig. 4) and significant differences were observed for PC2 in roots and GSH, PC3 and PC4 in shoots between As and As + TU treatments. However, the molar ratio of total thiols (GSH + PC2 + PC3 + PC4)-to-As confirmed that the major portion of As would be present as a non-chelated form in the roots for both As and As + TU treatments. This indicated that a positive effect of TU on root growth was not dependent upon GSH/PCs mediated improved As complexation. This might be due to a preference for long-term As storage, as uncomplexed As, similar to what has been demonstrated for seaweeds.42 Thus, the possibility of a vacuolar transporter mediating the transport of uncomplexed As does exist, as discovered in the lower plant Pteris vittata.43 In contrast, thiols were present in excess in the shoot and all As might be present as complexed species in both treatments. The higher levels of GSH and PCs may play a role as redox buffers. This was also evident from the significant accumulation of cysteine, GSH and PC2 in treatment with TU alone. Although GSH is an established redox buffer,17 the role of PCs in redox balancing is only just emerging.18 Furthermore, the sulfur assimilation was also studied to explain the differential synthesis of GSH and PCs under different treatments. A significant increase was observed in the activities of APS and CS in roots (Fig. 5A and D) and APR in shoots (Fig. 5C) under As + TU treatment as compared to As treatment. This suggests that the regulation of these enzymes is also under redox control. The redox-dependent regulation of APR has previously been shown.14
Although the chemical action of TU in the scavenging of a broad range of biological ROS is well established,24 to obtain a measure of the redox state kinetics of plants in the initial stages of As stress, GSH levels and GSH/GSSG ratios in rice seedlings were measured. The GSH/GSSG ratio was selected as it is considered the major determinant of the cellular redox state.17 In As + TU and TU alone treated roots, the GSH/GSSG ratio was significantly higher than that for As treatment at all time points. In contrast, a differential redox state in shoots was seen only at 8 h after treatment, wherein both the GSH level and the GSH/GSSG ratio were higher in all treatments as compared to the control (Fig. 6C and D). In order to correlate these changes of redox status with sulfur metabolism, measurements of sulfate uptake kinetics were performed under similar treatment conditions using 35S-sulfate as a radiotracer. The comparative analysis of the 35S-sulfate level in As and As + TU treatment confirmed that root-to-shoot translocation of sulfate, rather than its uptake, is the rate limiting step behind the As mediated induction of sulfur deficiency.44,45 Furthermore, the differential translocation observed under As with/without TU also confirmed that the process is redox regulated. In order to identify the associated candidate genes, the quantitative real-time PCR based comparative expression profiling of sulfate transporters was performed. In roots, the overall down-regulation of Sultrs in TU pretreated seedlings suggested their regulation in a demand driven manner.12 However, the expression of Sultr1;2, which is the major high-affinity sulfate transporter in plants, was increased at 1 h and not significantly down-regulated at 8 h under treatment with TU alone, which would have maintained the basal sulfate uptake. The plant's improved sulfur status under TU supplementation was also evident, as the comparatively higher and extended expression level of selected group-1 (Sultr1;1 and 1;2), -2 (Sultr2;1), -3 (Sultr3;3 and 3;4) and -4 (4;1) transporters was observed only in As treated roots and not under As + TU treatment. The expression profiling was correlated with radiotracer data, where the sulfate content in roots at 8 h under As was much higher than for any other treatment (Fig. 7A). The enhanced root-to-shoot sulfate translocation observed under As + TU and TU treatments was attributable to significant up-regulation of Sultrs 1;1 and 1;2 in shoots. These results suggested a tissue-specific function for Sultr1;1/1;2. In roots, they played a vital role in sulfate uptake, while in shoots they were responsible for sulfate unloading to facilitate the root-to-shoot translocation. Apart from redox regulation, these Sultrs were also found to be light-regulated, as their enhanced expression was observed only after 9 AM. Light-dependent regulation of Sultr1;2 has already been demonstrated.46 This is justified as maximum sulfate assimilation occurs only during the daytime. The early induction (1 h) of Sultr2;2 (low-affinity transporter), Sultr3;147 and Sultr4;1 (for vacuolar sulfate remobilization) transporters in the shoots upon As + TU treatment might have contributed towards the higher sulfate content observed even at the 1 h time point in comparison to other treatments. This was probably to compensate for the down-regulation of Sultr1;1 and Sultr1;2 at 1 h, and suggest transporters other than those of group 1 are not light-regulated, however this needs to be assessed further. The significantly different signatures of Sultrs observed in roots and shoots under As, As + TU and TU treatments suggest that their expression is co-ordinately regulated by the plant's sulfur demand, redox status and light. Recently, the regulatory role of a plant’s sulfur status48 and redox state49 has been established for the model plant Arabidopsis thaliana. To the best of our knowledge, this is the first study where spatial-, temporal-, and redox-regulation of Sultrs has been studied in rice.
In conclusion, this study implicates the importance of redox homeostasis for ameliorating As stress in rice through the use of TU, a non-physiological thiol based ROS scavenger. Under As stress, TU supplementation mediated the redox balance that led to the down-regulation of transporters for As translocation (Lsi2) leading to a reduction in As level from aerial parts. This was simultaneous with the up-regulation of sulfate transporters (Sultr1;1 and 1;2), enhanced root-to-shoot sulfate translocation and increased activities of sulfur assimilation related enzymes, which ultimately result in partial amelioration of the effects observed under As stress. Thus, the findings not only signify the importance of redox-regulatory mechanisms for enhancing a plant's tolerance against As stress and for reducing As load in rice grains, but also widen the range of TU application for ameliorating abiotic stress in crop plants.
Acknowledgements
The authors would like to express their sincere thanks to Dr S. P. Kale, Head, NABTD, BARC for the critical reading of the manuscript and Dr A. M. Badigannavar, NABTD, BARC for his help in statistical analysis.References
- H. Brammer and P. Ravenscroft, Environ. Int., 2009, 35, 647–654 CrossRef CAS PubMed.
- F. J. Zhao, S. P. McGrath and A. A. Meharg, Annu. Rev. Plant Biol., 2010, 61, 7.1–7.25 Search PubMed.
- A. Spanu, L. Daga, A. M. Orlandoni and G. Sanna, Environ. Sci. Technol., 2012, 46, 8333–8340 CrossRef CAS PubMed.
- A. L. Seyfferth and S. Fendorf, Environ. Sci. Technol., 2012, 46, 13176–13183 CrossRef CAS PubMed.
- A. S. M. H. M. Talukder, C. A. Meisner, M. A. R. Sarkar and M. S. Islam, Ecotoxicol. Environ. Saf., 2011, 74, 834–839 CrossRef CAS PubMed.
- S. Srivastava, P. C. Verma, V. Chaudhry, N. Singh, P. C. Abhilash, K. V. Kumar, N. Sharma and N. Singh, J. Hazard. Mater., 2013, 262, 1039–1047 CrossRef CAS PubMed.
- H. Li, Y. B. Man, Z. H. Ye, C. Wu, S. C. Wu and M. H. Wong, J. Hazard. Mater., 2013, 262, 1098–1104 CrossRef CAS PubMed.
- W. R. Cullen and K. J. Reimer, Chem. Rev., 1989, 89, 713–764 CrossRef CAS.
- S. Srivastava, P. Suprasanna and S. F. D'Souza, Protoplasma, 2011, 248, 805–815 CrossRef CAS PubMed.
- A. Rai, P. Tripathi, S. Dwivedi, S. Dubey, M. Shri, S. Kumar, P. K. Tripathi, R. Dave, A. Kumar, R. Singh, B. Adhikari, M. Bag, R. D. Tripathi, P. K. Trivedi, D. Chakrabarty and R. Tuli, Chemosphere, 2011, 82, 986–995 CrossRef CAS PubMed.
- P. M. Finnegan and W. Chen, Front. Physiol., 2012, 3, 182 CAS.
- J. C. Davidian and S. Kopriva, Mol. Plant, 2010, 3, 314–325 CrossRef CAS PubMed.
- H. Takahashi, S. Kopriva, M. Giordano, K. Saito and R. Hell, Annu. Rev. Plant Biol., 2011, 62, 157–184 CrossRef CAS PubMed.
- B. R. Lee, A. Koprivova and S. Kopriva, Plant J., 2011, 67, 1042–1054 CrossRef CAS PubMed.
- C. Kopriva, C. Buche, A. P. Fernandez, E. Schafer, E. Zwick and T. Kretsch, Plant Physiol., 2012, 160, 289–307 CrossRef PubMed.
- W. Y. Song, J. Park, D. G. Mendoza-Cózatl, M. Suter-Grotemeyer, D. Shima, S. Hörtensteiner, M. Geisler, B. Weder, P. A. Rea, D. Rentsch, J. I. Schroeder, Y. Lee and E. Martinoia, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21187–21192 CrossRef CAS PubMed.
- G. Noctor, A. Mhamdi, S. Chaouch, Y. Han, J. Neukermans, B. Marquez-Garcia, G. Queval and C. H. Foyer, Plant, Cell Environ., 2012, 35, 454–484 CrossRef CAS PubMed.
- E. Bianucci, J. Sobrino-Plata, R. O. Carpena-Ruiz, M. C. Tordable, A. Fabra, L. E. Hernández and S. Castro, Metallomics, 2012, 4, 1119–1124 RSC.
- Z. Y. Hu, Y. G. Zhu, M. Li, L. G. Zhang, Z. H. Cao and F. A. Smith, Environ. Pollut., 2007, 147, 387–393 CrossRef CAS PubMed.
- J. Zhang, Q. Z. Zhao, G. L. Duan and Y. C. Huang, Environ. Exp. Bot., 2011, 72, 34–40 CrossRef CAS PubMed.
- T. O. Jobe, D. Y. Sung, G. Akmakjian, A. Pham, E. A. Komives, D. G. Mendoza-Cózatl and J. I. Schroeder, Plant J., 2012, 70, 783–795 CrossRef CAS PubMed.
- A. K. Srivastava, S. Srivastava, S. Penna and S. F. D'Souza, Plant Physiol. Biochem., 2011, 49, 676–686 CrossRef CAS PubMed.
- M. Pandey, A. K. Srivastava, P. Suprasanna and S. F. D'Souza, J. Plant Interact., 2012, 7, 143–150 CrossRef CAS.
- M. J. Kelner, R. Bagnell and K. J. Welch, J. Biol. Chem., 1990, 265, 1306–1311 CAS.
- A. K. Srivastava, N. S. Nathawat, N. K. Ramaswamy, M. P. Sahu, G. Singh, J. S. Nair, P. Radha Krishna and S. F. D'Souza, Environ. Exp. Bot., 2008, 64, 250–255 CrossRef CAS PubMed.
- A. K. Srivastava, N. K. Ramaswamy, P. Suprasanna and S. F. D'Souza, Ann. Bot., 2010, 106, 663–674 CrossRef CAS PubMed.
- M. Pandey, A. K. Srivastava, S. F. D'Souza and P. Suprasanna, PLoS One, 2013, 8(9), e73921 CAS.
- R. Minocha, P. Thangavel, O. P. Dhankher and S. Long, J. Chromatogr. A, 2008, 1207, 72–83 CrossRef CAS PubMed.
- T. Hartmann, S. Mult, M. Suter, H. Rennenberg and C. Herschbach, J. Exp. Bot., 2000, 51, 1077–1088 CrossRef CAS.
- O. H. Lowry, N. J. Rosenbrough, A. L. Farr and R. J. Randal, J. Biol. Chem., 1951, 193, 265–275 CAS.
- P. J. Hissin and R. Hilf, Anal. Biochem., 1976, 74, 214–216 CrossRef CAS.
- S. T. Mehetre and S. P. Kale, Soil Sediment Contam., 2008, 17, 497–504 CrossRef CAS.
- S. Arvidsson, M. Kwasniewski, D. M. Riano-Pachon and B. Mueller-Roeber, BMC Bioinf., 2008, 9, 465 CrossRef PubMed.
- F. J. Zhao, J. R. Wang, J. H. A. Barker, H. Schat, P. M. Bleeker and S. P. McGrath, New Phytol., 2003, 159, 403–410 CrossRef CAS.
- A. K. Srivastava, N. K. Ramaswamy, R. Mukopadhyaya, M. G. ChiramalJincy and S. F. D'Souza, Ann. Bot., 2009, 103, 403–410 CrossRef CAS PubMed.
- M. Shri, S. Kumar, D. Chakrabarty, P. K. Trivedi, S. Mallick, P. Misra, D. Shukla, S. Mishra, S. Srivastava, R. D. Tripathi and R. Tuli, Ecotoxicol. Environ. Saf., 2009, 72, 1102–1110 CrossRef CAS PubMed.
- F. Chen, F. Wang, F. Wu, W. Mao, G. Zhang and M. Zhou, Plant Physiol. Biochem., 2010, 48, 663–672 CrossRef CAS PubMed.
- X. Y. Xu, S. P. McGrath and F. J. Zhao, New Phytol., 2007, 176, 590–599 CrossRef CAS PubMed.
- W. J. Liu, B. A. Wood, A. Raab, S. P. McGrath, F. J. Zhao and J. Feldmann, Plant Physiol., 2010, 152, 2211–2221 CrossRef CAS PubMed.
- G. L. Duan, Y. Hu, W. J. Liu, R. Kneer, F. J. Zhao and Y. G. Zhu, Environ. Exp. Bot., 2011, 71, 416–421 CAS.
- R. Dave, P. K. Singh, P. Tripathi, M. Shri, G. Dixit, S. Dwivedi, D. Chakrabarty, P. K. Trivedi, Y. K. Sharma, O. P. Dhankher, F. J. Corpas, J. B. Barroso and R. D. Tripathi, Arch. Environ. Contam. Toxicol., 2013, 64, 235–242 CrossRef CAS PubMed.
- B. A. Wood, S. Miyashita, T. Kaise, A. Raab, A. A. Meharg and J. Feldmann, Environ. Chem., 2011, 8, 30–43 CrossRef CAS.
- E. Indriolo, G. Na, D. Ellis, D. E. Salt and J. A. Banks, Plant Cell, 2010, 22, 2045–2057 CrossRef CAS PubMed.
- S. Srivastava, A. K. Srivastava, P. Suprasanna and S. F. D'Souza, J. Exp. Bot., 2013, 64, 303–315 CrossRef CAS PubMed.
- S. Srivastava, A. K. Srivastava, P. Suprasanna and S. F. D'Souza, J. Exp. Bot., 2009, 60, 419–3431 CrossRef PubMed.
- H. Rouached, M. Wirtz, R. Alary, R. Hell, A. B. Arpat, J. C. Davidian, P. Fourcroy and P. Berthomieu, Plant Physiol., 2008, 147, 897–911 CrossRef CAS PubMed.
- M. J. Cao, Z. Wang, M. Wirtz, R. Hell, D. J. Oliver and C. B. Xiang, Plant J., 2012, 73, 607–616 CrossRef PubMed.
- B. Zhang, R. Pasini, H. Dan, N. Joshi, Y. Zhao, T. Leustek and Z. L. Zheng, Plant J., 2014, 77, 185–197 CrossRef CAS PubMed.
- G. Jagadeeswaran, Y. F. Li and R. Sunkar, Plant J., 2014, 77, 85–96 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: S-1: Detailed methodology for the measurement of the activities of various enzymes. S-2: Details of the primers used for the quantitative real-time PCR of different sulfate transporters in rice. See DOI: 10.1039/c4mt00039k |
| ‡ Present address: Institute of Environment & Sustainable Development, Banaras Hindu University, Varanasi, India. |
| § Present address: Mathematisch-Naturwiss Sektion, Fachbereich Biologie, Universität Konstanz, Konstanz, Germany. |
| This journal is © The Royal Society of Chemistry 2014 |