Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selenoprotein P is the essential selenium transporter for bones†
Nicole
Pietschmann
ab,
Eddy
Rijntjes
a,
Antonia
Hoeg
ab,
Mette
Stoedter
a,
Ulrich
Schweizer
c,
Petra
Seemann
d and
Lutz
Schomburg
*a
aInstitute for Experimental Endocrinology, Charité-Universitätsmedizin Berlin, D-13353 Berlin, Germany. E-mail: lutz.schomburg@charite.de; Fax: +49 30 450524922; Tel: +49 30 450524289
bBerlin-Brandenburg School for Regenerative Therapies (BSRT), Charité-Universitätsmedizin Berlin, Germany
cInstitut für Biochemie und Molekularbiologie, Rheinische Friedrich-Wilhelms-Universität Bonn, D-53115 Bonn, Germany
dBerlin-Brandenburg Center for Regenerative Therapies, Charité-Universitätsmedizin Berlin, D-13353 Berlin, Germany
First published on 24th February 2014
Abstract
Selenium (Se) plays an important role in bone physiology as best reflected by Kashin–Beck disease, an endemic Se-dependent osteoarthritis. Bone development is delayed in children with mutations in SECIS binding protein 2 (SBP2), a central factor for selenoprotein biosynthesis. Circulating selenoprotein P (SePP) is positively associated with bone turnover in humans, yet its function for bone homeostasis is not known. We have analysed murine models of altered Se metabolism. Most of the known selenoprotein genes and factors needed for selenoprotein biosynthesis are expressed in bones. Bone Se is not associated with the mineral but exclusively with the organic matrix. Genetic ablation of Sepp-expression causes a drastic decline in serum (25-fold) but only a mild reduction in bone (2.5-fold) Se concentrations. Cell-specific expression of a SePP transgene in hepatocytes efficiently restores bone Se levels in Sepp-knockout mice. Of the two known SePP receptors, Lrp8 was detected in bones while Lrp2 was absent. Interestingly, Lrp8 mRNA concentrations were strongly increased in bones of Sepp-knockout mice likely in order to counteract the developing Se deficiency. Our data highlight SePP as the essential Se transporter to bones, and suggest a novel feedback mechanism for preferential uptake of Se in Se-deprived bones, thereby contributing to our understanding of hepatic osteodystrophy and the consistent bone phenotype observed in subjects with inherited selenoprotein biosynthesis mutations.
Introduction
The essential trace element selenium (Se) plays an important role in human health.1 A low Se status is related to a higher risk of e.g. cancer reported in the majority of experimental animal and human studies.2 Mortality has been shown to inversely correlate with blood Se concentrations in critical illness3 and renal cancer4 highlighting the outstanding importance of Se as an essential micronutrient. Se status, however, varies considerably between human populations worldwide as it depends on dietary Se, which in turn is decisively controlled by soils used for agricultural food production.5 The global dietary intakes range from 7 to 220 μg Se per day,6 and large parts of Europe, Asia and Africa must be considered as areas with insufficient Se supply from natural resources. In addition, the dietary preferences, the personal genotype and the general health state are important modifiers of Se metabolism and status.Upon reduced intake, Se concentrations decline in serum, liver, kidney and other tissues. Notably, the central nervous system and the endocrine organs are largely resistant to a limited Se supply. These organs retain their privileged Se status even in experimental models of severe Se deficiency.7 Such a hierarchical dependence on the Se status is observed on both the tissue level and with respect to different selenoproteins,8 ensuring that the most essential Se-dependent processes are maintained in privileged tissues even in times of severe Se deficiency.9
Se exerts its effects mainly in the form of selenocysteine (Sec), the 21st proteinogenic amino acid. Sec-dependent selenoproteins are encoded by a set of 25 human and 24 murine genes, respectively.10 Biosynthesis of selenoproteins requires the re-coding of a UGA stop-codon within the open reading frame of selenoprotein transcripts. The co-translational incorporation of Sec into growing selenoproteins depends on the interaction of a set of Sec-specific trans- and cis-acting factors including Sec-loaded tRNA[Ser]Sec, a Sec insertion sequence (SECIS) element in selenoprotein transcripts, which is recognized by the rate-limiting RNA-binding factor SECIS binding protein-2 (SBP2).11 In addition, two Se-binding proteins involved in intracellular Se metabolism have been described, i.e., the 56 kDa Se-binding protein-1 (SELENBP-1) and 14 kDa fatty acid-binding protein-1 (FABP-1).12 Within the cell, Se is liberated and reduced to selenide before becoming phosphorylated by the Se-dependent selenophosphate synthetase-2 (SEPHS2). In parallel, seryl-loaded tRNA[Ser]Sec is phosphorylated by phosphoseryl-tRNA kinase (PSTK). Combination of these two energy rich substrates to Sec-loaded tRNA[Ser]Sec is catalyzed by P-Ser-tRNA:Sec-tRNA synthase (SEPSECS). The Sec-specific elongation factor EFsec and nucleolin (NCL) finally contribute to the co-translational insertion of Sec along with additional translation factors.11
Among the mammalian selenoproteins, selenoprotein P (SePP) is unique as it carries multiple Sec residues per protein and constitutes the majority of Se in blood.13 SePP decisively contributes to the hierarchical Se supply of brain and testis.14 Two receptors of the lipoprotein receptor related protein (LRP) family have been identified as SePP receptors, i.e., LRP2 (Megalin) and LRP8 (ApoER2).15,16 Serum Se and SePP concentrations correlate over a wide range of nutritional Se intake. Once Se supply is sufficient, SePP biosynthesis is saturated thereby becoming independent of further Se intake.17 The threshold at which maximal expression of SePP is observed is used to define the optimal nutritional intake range for Se.18
The physiological role of SePP was analysed in mouse models with modified Sepp expression.19 Homozygous Sepp knockout (Sepp−/−) mice display a Se-dependent phenotype with a tissue-specific Se deficit, a reduced growth rate, ataxia and male-specific infertility.20,21 The neurological phenotypes are replicated in Lrp8 knockout mice, highlighting the mutual dependence of brain Se homeostasis on Sepp and Lrp8.22,23
A central importance of Se for bones is becoming increasingly recognized.24 Upon low dietary Se intake, rodents develop a bone phenotype characterized by poorly developed cortical and trabecular mineralization.25 In humans, Se deficiency is associated with Kashin–Beck disease (KBD), an endemic degenerative osteoarthritis, which is preventable by Se supplementation.26 Subjects with inherited defects in SBP2 display reduced selenoprotein expression, a disturbed thyroid hormone pattern in blood and consistently a drastically delayed bone development and relatively short stature.27–29 Recently, we have reported that serum SePP concentrations are associated with markers of bone turnover and bone mineral density.30 With this study, we evaluated the importance of SePP for bone Se metabolism. Bones turned out to dynamically express the SePP receptor Lrp8 and to depend on liver-derived SePP for efficient Se uptake thereby representing a preferentially supplied target organ residing high in the hierarchy of tissue-specific Se supply, similar to brain and the classical endocrine organs. In hindsight, this metabolic privilege makes perfect sense, as bones are equally important as brain and the endocrine organs for growth and survival.
Materials and methods
Experimental animals
Animal maintenance was in accordance with the Guide for the Care and Use of Laboratory animals: 8th ed. and had been approved by the local authorities (LaGeSo Berlin, Germany). Sepp wildtype (Sepp+/+), heterozygous (Sepp+/−) and homozygous knockout (Sepp−/−) mice were raised and backcrossed on a C57/BL6 background as described.20 In addition, homozygous Sepp−/− mice expressing a human SePP transgene in liver only (Sepp−/−tg) were genotyped and reared as described.14 Mice were bred and raised on a rodent diet containing a defined amount of Se equal to the Recommended Daily Allowance (RDA) for mice (Altromin C1045, Lage, Germany, supplemented with Na-selenite to 0.15 ppm as described31). Mice were sacrificed at postnatal day 35–40 (P35–40) in order to isolate serum and tissues for further analyses.RNA extraction
Bones were prepared free of surrounding tissues and powdered with a steel ball in a teflon capsule using a Mikro-Dismembrator (B. Braun, Melsungen, Germany) under liquid nitrogen. Total RNA extractions from tibia and femur powder were performed using TRIzol® Reagent (PEQLAB, Erlangen, Germany) according to the manufacturer's instructions. RNA was further purified by one round of chromatography using RNeasy Mini columns (QIAGEN, Hilden, Germany).Quantitative reverse transcription-PCR (qRT-PCR)
RNA samples were reverse-transcribed using the iScript™ Select cDNA Synthesis Kit (BIO-RAD, Munich, Germany). qRT-PCR analyses were performed using the iCycler-System (BIO-RAD) and ABsolute QPCR SYBR Green Fluorescein Mix (Thermo Scientific, Schwerte, Germany). A melting curve analysis was performed at the end of each qRT-PCR analysis in order to verify specificity of the primer pairs and amplicons. All results were normalized to 18S rRNA concentrations and compared to Sepp+/+ wildtype mice as the reference condition. Appropriate primer pairs for all 24 murine selenoprotein genes and important factors for selenoprotein biosynthesis and Se metabolism were designed with the primer3plus database (Table S1, ESI†).32 Gene expression was considered as absent when specific amplicons were not obtained after 40 cycles of qRT-PCR (Table 1).| Gene | Sepp+/− | Sepp−/− | Gene | Sepp+/− | Sepp−/− |
|---|---|---|---|---|---|
| a Differences in relative expression levels are shown (n = 6, Dunnett's t test). Expression is normalized to 18S rRNA and compared to Sepp+/+ (ratio = 1.0). b Below detection limit (Ct > 40). | |||||
| Gpx1 | 1.6 | 1.0 | Seli | 1.5 | 5.4 |
| Gpx2 | 0.8 | 0.7* | Selk | 1.7 | 0.3** |
| Gpx3 | 1.1 | 0.7* | Selm | 1.4 | 0.7* |
| Gpx4 | 2.4 | 0.3* | Selo | 1.8* | 1.1 |
| Dio1 | —b | —b | Selt | 1.1 | 1.1 |
| Dio2 | 1.1 | 1.2 | Selv | —b | —b |
| Dio3 | 3.7 | 0.3 | Sels | 1.3 | 3.1 |
| Txrndl | 1.4 | 2.2 | Sbp2 | 1.5 | 1.3 |
| Txrnd2 | 1.4 | 1.7 | Selenbpl | 1.3 | 1.4 |
| Txrnd3 | 1.7* | 1.6 | Secp43 | 1.7 | 1.4 |
| Sephs2 | 1.5 | 1.5 | Eif4a3 | 1.5 | 1.9 |
| Sepp | 0.4* | 0.0*** | Fabpl | 0.8 | 2.1 |
| Selr | 1.2 | 0.7 | Ncl | 1.4 | 1.9 |
| Sepn | 1.4 | 0.9 | Lrp1 | 1.5 | 5.4*** |
| Sep15 | 2.0 | 0.8 | Lrp2 | —b | —b |
| Selw | 1.7 | 2.9 | Lrp8 | 1.5 | 6.0* |
| Selh | 2.0 | 1.2 | |||
Western blot analyses
Murine femurs were powdered under liquid nitrogen using a steel ball in 2 ml polypropylene reaction tubes for 1 min at 20/s in a TissueLyser (Qiagen). Homogenization buffer (0.25 M sucrose; 20 mM HEPES; 1 mM EDTA, pH 7.4) was added, vortexed and the homogenate was sonified. Cytosolic fractions were obtained after centrifugation for 10 min at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 4 °C. Pellets were resuspended and solubilized in RIPA buffer (50 mM Tris/HCl pH 8.0; 150 mM NaCl; 1% NP-40; 0.5% Na-deoxycholate; 0.1% SDS) for obtaining membrane fractions. After incubation on ice (30 min) and a centrifugation step (20 min at 20
000 × g, 4 °C. Pellets were resuspended and solubilized in RIPA buffer (50 mM Tris/HCl pH 8.0; 150 mM NaCl; 1% NP-40; 0.5% Na-deoxycholate; 0.1% SDS) for obtaining membrane fractions. After incubation on ice (30 min) and a centrifugation step (20 min at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g; 4 °C), fractions were collected and stored at −40 °C until analysis.
000 × g; 4 °C), fractions were collected and stored at −40 °C until analysis.
Protein concentrations of cytosolic fractions were quantified by a BIO-RAD Protein Assay using IgG as standard (BIO-RAD). A Pierce® BCA Protein Assay (Thermo Scientific) was used for the membrane fractions using BSA as standard. Samples containing 20 or 50 μg protein were size fractionated by SDS-PAGE and blotted by semi-dry transfer onto nitrocellulose membranes (Optitran, Schleicher & Schuell, Dassel, Germany). Rabbit polyclonal antibodies against murine Sepp,33 Lrp8 (kindly provided by Prof. Dr Herz, UT Dallas, TX, USA) and Lrp1 (abcam, Cambridge, UK) were used and quantified by ECL™ Western Blotting Detection Reagents (GE Healthcare, UK).
Selenium (Se) determination
Se concentrations were quantified by total reflection X-ray fluorescence (TXRF) spectroscopy using a PICOFOX S2 spectrometer (Bruker Nano GmbH, Berlin, Germany). Briefly, bones were weighed, decalcified in 2.5% nitric acid solution for 2–3 h and dissolved in 65% nitric acid for 1 h at 70 °C. The acidic homogenates were supplemented with Gallium (Ga) as an internal standard (1000 μg Ga l−1, f.c.). Alternatively, bones were treated over night at 56 °C with proteinase K (10 mM Tris pH 8.0; 100 mM NaCl; 50 mM EDTA pH 8.0; 0.5% SDS and 10 mg ml−1 proteinase K), and soluble material was separated from insoluble minerals and analysed as above. Serum was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with the Ga standard. Duplicates of samples (8 μl each) were placed on polished quartz glass sample carriers, air-dried and analysed. The intra-assay coefficient of variation was determined with a standard serum (Sero AS, Billingstad, Norway) and was less than 10% during the measurements. The limits of quantification were tested by serial dilutions of serum and bone homogenate samples. The measurements yielded linear dilution-dependent Se signals in samples with final concentrations down to only 1.0 μg Se l−1 serum and 0.01 μg Se g−1 bone homogenate, respectively.
2 with the Ga standard. Duplicates of samples (8 μl each) were placed on polished quartz glass sample carriers, air-dried and analysed. The intra-assay coefficient of variation was determined with a standard serum (Sero AS, Billingstad, Norway) and was less than 10% during the measurements. The limits of quantification were tested by serial dilutions of serum and bone homogenate samples. The measurements yielded linear dilution-dependent Se signals in samples with final concentrations down to only 1.0 μg Se l−1 serum and 0.01 μg Se g−1 bone homogenate, respectively.
In silico analyses
The expression profiles of murine selenoprotein genes and genes involved in selenoprotein metabolism were extracted from the BioGPS database (www.biogps.org) and differences between murine osteoblasts and osteoclasts were determined.Statistics
Statistical analyses were performed with GraphPad Prism v.4.0 (GraphPad Software Inc., San Diego, USA) and IBM SPSS Statistics 19 (IBM Corporation, Armonk, USA). Trace element concentrations are represented as means ± SD. Two groups were compared with Student's t-test, and multiple groups with One-way ANOVA and Bonferroni's Multiple Comparison Test. Numbers of animals tested are specified in the respective figure legends. Spearman's coefficient of correlation was determined to assess the association between Se levels of serum and bones. Dunnett's t test (two-tailed) was used to analyse differences in expression levels. Statistical significance was assigned if P < 0.05 (*), P < 0.01 (**) or P < 0.001 (***).Results
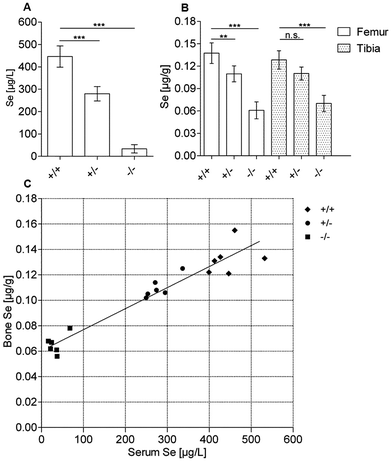
Bone Se status is resistant to low Se concentrations
Se concentrations in serum and peripheral tissues are known to decline in the absence of SePP. Accordingly, serum Se concentrations are reduced in Sepp+/− and marginal in Sepp−/− mice (Fig. 1A). In order to analyse bone Se status, femur and tibia from adult mice were chosen as these bones can easily be prepared, are of sufficient size for biochemical and trace element analyses and are important regulators of body size and mineral homeostasis. Se concentrations were comparable in both bone types (Fig. 1B). Comparing all mouse groups, i.e. females and males of the three different genotypes, serum and bone Se concentrations show a strong positive linear correlation (Fig. 1C). However, differences in serum Se levels are not linearly reflected in bones; while serum Se concentrations differ between Sepp+/+ and Sepp−/− mice by up to 25-fold, the respective Se values in bones differ by 2.5-fold only. This apparent resistance of bones towards strongly diminished circulating Sepp and Se concentrations indicates that bones are preferentially supplied tissues residing high in the hierarchy of Se target tissues.Se associates with bone proteins but not with minerals
Bones are composed of roughly 1/3 organic components and 2/3 inorganic minerals. In order to localize Se within these principal components, murine femur and tibia samples were either digested by proteinase K thereby removing the organic protein components and generating a rigid brittle mineral-rich scaffold, or were treated by 2.5% nitric acid thereby dissolving the inorganic minerals leaving behind a flexible protein-rich bone matrix (Fig. 2A and B). Upon trace element analyses, Se was associated exclusively with the protein-rich samples and not with the inorganic matrix (Fig. 2C) indicating that Se is metabolized in bones into organic structures, i.e. selenoproteins, and not inserted by chemical reactions into hydroxyapatite or other minerals. This is in contrast to other trace elements like e.g. fluorine, which becomes predominantly inserted into apatite. Length of bones is slightly reduced in Sepp−/− as compared to Sepp+/+ mice (Fig. 2D).Expression of selenoproteins and Se metabolism genes in bones
Next, mRNA expression patterns of selenoprotein genes together with central factors involved in selenoprotein biosynthesis and Se metabolism were analysed in bone samples of the different genotypes. An impressive set of 22 out of the maximally possible 24 selenoprotein genes were detected on the transcript level with the only notable exceptions of Dio1 and Selv (Table 1). These findings are compatible with former studies reporting a selective lack of Dio1 in bones,34 or Dio1 and Selv in chondrocytes.35 A respective in silico analysis of expressed genes yields compatible results and offers insights into cell-specific expression profiles in osteoblasts versus osteoclasts (Table S2, ESI†). Notably, expression of genes encoding the protective enzymes Txrnd1-3 and Gpx1,-2,-4 along with the Sepp receptor Lrp8 is higher in osteoclast than in osteoblasts, while the ER-function and quality-associated genes Sepn, Sep15, Sepw, Selk, Selm and Seps are more abundantly expressed in osteoblasts. This finding nicely conforms to the major catabolic and anabolic functions of osteoclasts and osteoblasts, respectively.Resistance of bone selenoprotein expression to Se deficiency
In contrast to expectations, mRNA levels of most of the selenoproteins detected in bones were not strongly affected by the Sepp genotype, i.e., by a moderate or strong decline in serum Se concentrations. In line with the genotypes, Sepp mRNA was strongly reduced in heterozygous animals and not detectable in Sepp−/− mice (Table 1). Along with Sepp mRNA, significant differences were also seen in the expression of Txnrd3 and Selo between Sepp+/+ and Sepp+/− littermates. However, strong alterations can be noted in Sepp−/− knockout mice; transcript concentrations of Gpx2–4, Selk and Selm are significantly reduced, while Lrp1 and Lrp8 are up-regulated. Notably, Dio1, Selv and Lrp2 were not detectable in any of the bone RNA preparations.Sepp receptor Lrp8 is induced in Se-deficient bones
In accordance with the high number of selenoprotein transcripts, also all the essential factors for Se metabolism and selenoprotein biosynthesis are expressed in bones (Table 1). Of the well-established SePP receptors, Lrp8 was detectable and Lrp2 was absent; Lrp8 was strongly up-regulated in Sepp−/− mice. A similar regulation was observed for the third Lrp family member tested, i.e., Lrp1. Whether Lrp1 also contributes to Sepp uptake is currently a matter of debate. As SePP and Lrp8 have been established to form a central transporter–receptor pair for tissue-specific Se uptake,36 their protein expression was evaluated by Western Blot analysis (Fig. 3). Sepp is detectable in femurs of Sepp+/+ and Sepp+/− mice with bands migrating at approximately 47, 45 and 42 kDa. As expected, expression of Sepp is undetectable in Sepp−/− mice (Fig. 3A). Lrp8 is detected in bones of all three Sepp genotypes with isoforms migrating at >130 and >180 kDa (Fig. 3B). Lrp8 expression in bones is of almost similar strength as in testes which served as the positive control tissue.16 Bones are thus well equipped for Sepp-mediated Se uptake.In histochemical analyses, Sepp can be detected especially in mineralized bones while cartilage was negative (Fig. 3C and D).
Se is transported to bones by selenoprotein P (SePP)
Next, we tested whether bones can efficiently increase their Se status by using circulating SePP, i.e., whether Sepp transports Se from liver to bones. To this end, Sepp−/−tg mice expressing a human SePP transgene in hepatocytes only were analysed. Se concentrations in serum were only marginally affected by the transgene (Fig. 4A). In comparison, Se concentrations in femurs and tibia of Sepp−/−tg were efficiently increased by the transgene as compared to Sepp−/− mice (Fig. 4B). Notably, bone Se concentrations in Sepp−/−tg mice reached almost levels of heterozygous Sepp+/− mice despite the minor contribution of the transgene to serum Se. This finding indicates an efficient Se transport into bones by hepatically-derived SePP (Fig. 4C).Discussion
The scientific and medical interest into the importance of Se intake and Se status for general health and disease prevention has increased tremendously during the last few decades. Several experimental animal studies and human analyses have contributed to the current notion that this trace element is of high relevance for common human diseases including cancer, diabetes and inflammatory diseases, and that many subjects are insufficiently supplied by their diet.1 Severe Se deficiency is associated with growth defects and impaired mineralization in animal studies.20,21,25 A recent study reported on increased bone resorption and disturbed bone microarchitecture in Se-deficient mice.37 However, insights into the molecular role of specific selenoproteins in bone physiology are sparse. The available information has been reviewed recently highlighting some important knowledge gaps.24 Our identification of Sepp as the transporter of Se from liver into bone resolves an essential metabolic pathway.We decided to first establish a robust protocol for Se analysis from bones with the aim of separating inorganic minerals from the organic matrix. Our results indicate that in bones Se is exclusively associated with selenoproteins. Demineralization of bones is a suitable way of preparing the tissue for Se quantification and speciation. However, our analyses did not provide spatial information on the distribution of Se in bones, which needs further analyses. The Se concentrations obtained are compatible with respective data from human bones (0.17 ± 0.04 μg g−1).38 In comparison to other tissues, Se concentrations in bones are in the same range as in brain or muscles (0.15–0.30 μg g−1), but significantly lower than in liver, kidneys or testes (0.7–1.5 μg g−1) of mice fed an adequate Se diet.39 However, upon limited Se supply or Sepp deficiency, liver and kidneys are depleted from their Se content, while brain and testes are relatively resistant.40 Our results indicate that bones belong to the preferentially supplied tissues, too. This notion is supported by the robust expression of Lrp8 mRNA and protein in bones as this receptor has been proven to be decisively involved in tissue-specific Sepp-mediated Se uptake.36 Together with the expression of Sepp and Sepp-receptors in bones, an efficient local biosynthesis and reuptake circuit can be envisaged similar to the “Sepp-cycle” suggested for the homeostatic mechanism maintaining brain Se concentrations against an adverse gradient in blood safeguarding high tissue Se levels in Se deficiency.41
Most of the biochemically characterized selenoproteins catalyse redox reactions, often linked to antioxidative protection, repair of oxidized proteins or quality control of secreted proteins.42 These functions may be of central importance for bone turnover in view of the important role of reactive oxygen species during bone remodelling and as endogenous signals for osteoclastogenesis and osteoblast activities.24 Besides the selenoproteins being implicated in these functions, the thyroid hormone activating selenoprotein Dio2 has been shown to decisively affect mineralization and bone quality.43 However, it is unlikely that bone Dio2 activity is strongly affected in our mice as the mRNA levels remained fairly constant and translation of Dio2 is hierarchically preferred during limited Se supply and impaired Sepp expression.44
To our knowledge, this is the first in-depth analysis of Se supply to bones. Changes in gene expression in response to reduced or missing Sepp expression are of relevance as SePP is a valid biomarker for Se status and functional polymorphisms in human SePP are described.45 The up-regulation of Lrp8 in Se deficiency may constitute a meaningful feedback mechanism for avoiding critically low bone selenoprotein expression levels endangering full bone functionality.
This hypothesis needs to be tested in future experiments. Notably, chronic liver disease negatively affects bone health, known as hepatic osteodystrophy, likely caused by insufficient IGF-1, vitamin D and vitamin K biosynthesis and transport.46 It needs to be tested, in how far impaired Se transport to bones via low SePP secretion from liver contributes to the increased osteoporosis and osteopenia risk in patients with chronic liver disease, and whether Se substitution is an effective adjuvant treatment option in patients with chronic liver disease.
Conclusions
Bones are hierarchically well-supplied SePP-target tissues largely resistant to low Se concentrations in blood. Bones express an almost complete set of selenoproteins and all the factors needed for selenoprotein biosynthesis. When exposed to strong SePP deficiency, bones react by up-regulating the gene expression of the SePP receptor Lrp8. As of now, this metabolic response to a low intracellular Se level is unique to bones. It remains to be seen whether such a dynamic adaptation of SePP uptake and intracellular Se metabolism represents a more general mechanism of SePP-target tissues in Se deficiency, contributing to the hierarchical supply of essential tissues like the brain and endocrine organs and safeguarding vital Se-dependent processes in times of Se shortage.Acknowledgements
We thank Katja Schreiber and Carola Geiler for excellent technical assistance, and Drs Joseph Köhrle, Peter J. Hofmann, Eva Wirth, Andrea Schütte and Niels-P Becker for inspiring discussions as well as experimental support. We are indebted to Prof. Dr J. Herz, UT Dallas, USA, for providing the Lrp8 antibody used in our analysis, and to the BioGPS team for doing a great job. This study was supported by BSRT, Deutsche Forschungsgemeinschaft (DFG; GraKo 1208, SCHO849/4-1) and Federal Ministry of Economics and Technology (BMWi; KF2263202CS2).References
- M. P. Rayman, Lancet, 2012, 379, 1256–1268 CrossRef CAS.
- C. D. Davis, P. A. Tsuji and J. A. Milner, Annu. Rev. Nutr., 2012, 32, 73–95 CrossRef CAS PubMed.
- M. W. Angstwurm, L. Engelmann, T. Zimmermann, C. Lehmann, C. H. Spes, P. Abel, R. Strauss, A. Meier-Hellmann, R. Insel, J. Radke, J. Schüttler and R. Gärtner, Crit. Care Med., 2007, 35, 118–126 CrossRef CAS PubMed.
- H. A. Meyer, T. Endermann, C. Stephan, M. Stoedter, T. Behrends, I. Wolff, K. Jung and L. Schomburg, PLoS One, 2012, 7, e46644 CAS.
- M. P. Rayman, Br. J. Nutr., 2008, 100, 254–268 CAS.
- G. F. Combs, Jr., Br. J. Nutr., 2001, 85, 517–547 CrossRef.
- D. Behne and A. Kyriakopoulos, Am. J. Clin. Nutr., 1993, 57, 310S–312S CAS.
- J. R. Arthur, G. Bermano, J. H. Mitchell and J. E. Hesketh, Biochem. Soc. Trans., 1996, 24, 384–388 CAS.
- L. Schomburg and U. Schweizer, Biochim. Biophys. Acta, 2009, 1790, 1453–1462 CrossRef CAS PubMed.
- G. V. Kryukov, S. Castellano, S. V. Novoselov, A. V. Lobanov, O. Zehtab, R. Guigo and V. N. Gladyshev, Science, 2003, 300, 1439–1443 CrossRef CAS PubMed.
- C. Allmang, L. Wurth and A. Krol, Biochim. Biophys. Acta, 2009, 1790, 1415–1423 CrossRef CAS PubMed.
- M. P. Bansal, C. J. Oborn, K. G. Danielson and D. Medina, Carcinogenesis, 1989, 10, 541–546 CrossRef CAS PubMed.
- R. F. Burk and K. E. Hill, Annu. Rev. Nutr., 2005, 25, 215–235 CrossRef CAS PubMed.
- K. Renko, M. Werner, I. Renner-Müller, T. G. Cooper, C. H. Yeung, B. Hollenbach, M. Scharpf, J. Köhrle, L. Schomburg and U. Schweizer, Biochem. J., 2008, 409, 741–749 CrossRef CAS PubMed.
- G. E. Olson, V. P. Winfrey, K. E. Hill and R. F. Burk, J. Biol. Chem., 2008, 283, 6854–6860 CrossRef CAS PubMed.
- G. E. Olson, V. P. Winfrey, S. K. Nagdas, K. E. Hill and R. F. Burk, J. Biol. Chem., 2007, 282, 12290–12297 CrossRef CAS PubMed.
- Y. Xia, K. E. Hill, P. Li, J. Xu, D. Zhou, A. K. Motley, L. Wang, D. W. Byrne and R. F. Burk, Am. J. Clin. Nutr., 2010, 92, 525–531 CrossRef CAS PubMed.
- J. Hoeflich, B. Hollenbach, T. Behrends, A. Hoeg, H. Stosnach and L. Schomburg, Br. J. Nutr., 2010, 104, 1601–1604 CrossRef CAS PubMed.
- M. Conrad and U. Schweizer, Antioxid. Redox Signaling, 2010, 12, 851–865 CrossRef CAS PubMed.
- L. Schomburg, U. Schweizer, B. Holtmann, L. Flohé, M. Sendtner and J. Köhrle, Biochem. J., 2003, 370, 397–402 CrossRef CAS PubMed.
- K. E. Hill, J. Zhou, W. J. McMahan, A. K. Motley, J. F. Atkins, R. F. Gesteland and R. F. Burk, J. Biol. Chem., 2003, 278, 13640–13646 CrossRef CAS PubMed.
- R. F. Burk, K. E. Hill, G. E. Olson, E. J. Weeber, A. K. Motley, V. P. Winfrey and L. M. Austin, J. Neurosci., 2007, 27, 6207–6211 CrossRef CAS PubMed.
- W. M. Valentine, T. W. Abel, K. E. Hill, L. M. Austin and R. F. Burk, J. Neuropathol. Exp. Neurol., 2008, 67, 68–77 CrossRef PubMed.
- H. Zeng, J. J. Cao and G. F. Jr. Combs, Nutrients, 2013, 5, 97–110 CrossRef CAS PubMed.
- R. Moreno-Reyes, D. Egrise, M. Boelaert, S. Goldman and S. Meuris, J. Nutr., 2006, 136, 595–600 CAS.
- R. Moreno-Reyes, C. Suetens, F. Mathieu, F. Begaux, D. Zhu, M. T. Rivera, M. Boelaert, J. Neve, N. Perlmutter and J. Vanderpas, N. Engl. J. Med., 1998, 339, 1112–1120 CrossRef CAS PubMed.
- A. M. Dumitrescu, X. H. Liao, M. S. Abdullah, J. Lado-Abeal, F. A. Majed, L. C. Moeller, G. Boran, L. Schomburg, R. E. Weiss and S. Refetoff, Nat. Genet., 2005, 37, 1247–1252 CrossRef CAS PubMed.
- C. Di Cosmo, N. McLellan, X. H. Liao, K. K. Khanna, R. E. Weiss, L. Papp and S. Refetoff, J. Clin. Endocrinol. Metab., 2009, 94, 4003–4009 CrossRef CAS PubMed.
- M. F. Azevedo, G. B. Barra, L. A. Naves, L. F. Ribeiro Velasco, P. Godoy Garcia Castro, L. C. de Castro, A. A. Amato, A. Miniard, D. Driscoll, L. Schomburg and F. de Assis Rocha Neves, J. Clin. Endocrinol. Metab., 2010, 95, 4066–4071 CrossRef CAS PubMed.
- A. Hoeg, A. Gogakos, E. Murphy, S. Mueller, J. Kohrle, D. M. Reid, C. C. Gluer, D. Felsenberg, C. Roux, R. Eastell, L. Schomburg and G. R. Williams, J. Clin. Endocrinol. Metab., 2012, 97, 4061–4070 CrossRef CAS PubMed.
- J. Chiu-Ugalde, F. Theilig, T. Behrends, J. Drebes, C. Sieland, P. Subbarayal, J. Kohrle, A. Hammes, L. Schomburg and U. Schweizer, Biochem. J., 2010, 431, 103–111 CrossRef CAS PubMed.
- A. Untergasser, H. Nijveen, X. Rao, T. Bisseling, R. Geurts and J. A. Leunissen, Nucleic Acids Res., 2007, 35, W71–W74 CrossRef PubMed.
- U. Schweizer, M. Michaelis, J. Köhrle and L. Schomburg, Biochem. J., 2004, 378, 21–26 CrossRef CAS PubMed.
- A. J. Williams, H. Robson, M. H. Kester, J. P. van Leeuwen, S. M. Shalet, T. J. Visser and G. R. Williams, Bone, 2008, 43, 126–134 CrossRef CAS PubMed.
- J. Yan, Y. Zheng, Z. Min, Q. Ning and S. Lu, Biometals, 2013, 26, 285–296 CrossRef CAS PubMed.
- S. Kurokawa, K. E. Hill, W. H. McDonald and R. F. Burk, J. Biol. Chem., 2012, 287, 28717–28726 CrossRef CAS PubMed.
- J. J. Cao, B. R. Gregoire and H. Zeng, J. Nutr., 2012, 142, 1526–1531 CrossRef CAS PubMed.
- C.-T. Wang, W.-T. Chang, K.-C. Huang and R.-T. Wang, Anal. Sci., 1997, 13, 497–500 CrossRef CAS.
- A. Nakayama, K. E. Hill, L. M. Austin, A. K. Motley and R. F. Burk, J. Nutr., 2007, 137, 690–693 CAS.
- G. Bermano, F. Nicol, J. A. Dyer, R. A. Sunde, G. J. Beckett, J. R. Arthur and J. E. Hesketh, Biochem. J., 1995, 311(pt 2), 425–430 CAS.
- L. Schomburg, U. Schweizer and J. Köhrle, Cell. Mol. Life Sci., 2004, 61, 1988–1995 CrossRef CAS PubMed.
- M. V. Kasaikina, D. L. Hatfield and V. N. Gladyshev, Biochim. Biophys. Acta, 2012, 1823, 1633–1642 CrossRef CAS PubMed.
- J. H. Bassett, A. Boyde, P. G. Howell, R. H. Bassett, T. M. Galliford, M. Archanco, H. Evans, M. A. Lawson, P. Croucher, D. L. St Germain, V. A. Galton and G. R. Williams, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7604–7609 CrossRef CAS PubMed.
- L. Schomburg, C. Riese, M. Michaelis, E. Griebert, M. O. Klein, R. Sapin, U. Schweizer and J. Köhrle, Endocrinology, 2006, 147, 1306–1313 CrossRef CAS PubMed.
- C. Meplan, L. K. Crosley, F. Nicol, G. J. Beckett, A. F. Howie, K. E. Hill, G. Horgan, J. C. Mathers, J. R. Arthur and J. E. Hesketh, FASEB J., 2007, 21, 3063–3074 CrossRef CAS PubMed.
- G. Lopez-Larramona, A. J. Lucendo, S. Gonzalez-Castillo and J. M. Tenias, World J. Hepatol., 2011, 3, 300–307 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4mt00003j |
| This journal is © The Royal Society of Chemistry 2014 |