Dendrimer-linked, renewable and magnetic carbon nanotube aerogels†
Xuetong
Zhang
*ab,
Liang
Chen
a,
Tianyu
Yuan
a,
Huan
Huang
a,
Zhuyin
Sui
a,
Ran
Du
a,
Xin
Li
a,
Yun
Lu
a and
Qingwen
Li
b
aSchool of Materials Science & Engineering, Beijing Institute of Technology, Beijing, 100081, P. R. China. E-mail: zhangxtchina@yahoo.com; Tel: +86-10-68918591
bSuzhou Institute of Nano-Tech & Nano-Bionics, Chinese Academy of Science, Suzhou 215123, P. R. China
First published on 23rd September 2013
Abstract
Magnetic carbon nanotube aerogels with a repeated aerogel–sol–hydrogel–aerogel transition have been acquired by the special drying of gel-precursors made via assembling individual nanotubes with dendritic poly(amido amine) molecules in the presence of Fe3O4 nanoparticles, which has inspired us to synthesize renewable 3D porosints composed of organic, inorganic and their hybrid building blocks.
Aerogels, one of the extremely significant assembly states of various nanoobjects including zero-dimensional (0D) nanoparticles, one-dimensional (1D) nanowires and two-dimensional (2D) nanosheets, have attracted much interest due to their wide applications in the fields of liquid purification, gas storage, advanced catalysis, energy conversion, thermal insulation, optical sensors, etc.1–5 Most conventional aerogels, e.g., silica aerogels, organic resorcin–formaldehyde aerogels, inorganic metal oxide aerogels, etc., have been made from molecular precursors with irreversible hydrolysis and condensation reactions in sequence.6 Experience of these irreversible and unavoidable reactions has endowed the resulting aerogels with permanent networks, making it difficult for them to be easily decomposed back to raw materials for regeneration by a simple chemical or physical processing. This results in difficulty reclaiming the aerogel waste after use, and thus leads to further contamination of increasingly fragile environments. Therefore, it is significantly important and vitally urgent to develop a strategy for synthesizing novel aerogels with a renewable attribute.
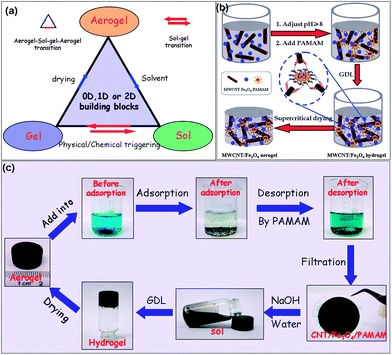
To synthesize the renewable aerogels, one has to consider developing gel precursors with the following characteristics, as shown in Fig. 1a: (1) no irreversible hydrolysis and condensation reactions have happened during the precursor syntheses, which suggests that low-dimensional nano-structured precursors instead of molecular ones could be used as the raw materials of the target aerogels, and (2) no permanent networks of the target aerogels have been formed during gelation, which suggests that wet gel precursors should possess a reversible sol–gel transition triggered by simply chemical or physical stimulation. If one can obtain gel precursors meeting the above requirements, aerogels with a renewable attribute would be easily obtained after an appropriate physical drying of the gel precursors. However, how to create such gel precursors is still a big challenge and thus, no renewable aerogels have been reported so far.
On the other hand, 1D carbon nanotubes (CNTs) have stimulated unprecedented attention in recent years as a result of their unique structure and outstanding applications in the fields of sensors,7 catalysis,8 drug delivery,9,10 electronic devices,11,12 energy storage,13,14etc. Assembling CNTs into macroscopic bulk materials, e.g. aerogels, with desired structures and striking performances is one of the most powerful strategies for expanding CNT structural features and creating novel physicochemical properties that are distinguished from their individuals. In the literature, CNT aerogels have been successfully made by the methods of (1) chemical vapor deposition,15,16 (2) blending with other materials, e.g., PVA, graphene, et al.,17–20 and (3) incorporating cross-linker molecules after CNT surface modifications.21–23 However, all these reported CNT aerogels are not renewable and have not been magnetically functionalized so far, and thus, their versatile applications have been greatly restricted.
Herein, we present a controllable route to make novel magnetically-functionalized CNT aerogels with a renewable attribute by crosslinking individual CNTs with dendritic polymers into 3D hydrogel networks in the presence of magnetic Fe3O4 nanoparticles, followed by the supercritical fluid drying or freeze drying of the obtained gels. The entrapment of magnetic nanoparticles in the nano-sized pores of the aerogel matrix has added new functionalities to the resulting CNT aerogels and improved the handing of the materials. The resulting CNT aerogels possess an adsorption capacity ca. 4 times higher than the hydrogel counterparts for the same organic dye. The selective adsorption of methylene blue (MB) from its mixture with Rhodamine B (RhB), as well as the controlled release of dyes has been observed from the CNT aerogels as the sorbent. The work presented here has also given unlimited inspiration of creation to produce more renewable 3D aerogels, e.g., an Fe-H3BTC aerogel with zero-dimensional building blocks, an agarose aerogel with one-dimensional building blocks and a graphene oxide aerogel with two-dimensional building blocks, and all these renewable aerogels might find wide applications in various emerging fields.
The synthesis of the dendrimer-linked magnetically-functionalized carbon nanotube aerogel with the renewable attribute is depicted in Fig. 1b. The MWCNTs employed here are acid treated multi-walled carbon nanotubes,24 the dendrimer employed here is an amino-terminated generation 5 poly(amido amine) (PAMAM),25 and the magnetic Fe3O4 colloidal-suspension (i.e. a sol) used here was synthesized according to the literature reported elsewhere.26 In the first instance, the pH value of the MWCNT aqueous mixture with the Fe3O4 sol (mass ratio of Fe3O4 to MWCNT 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) was adjusted to ca. 8.0 by using an appropriate amount of NaOH. After that, a certain amount of PAMAM (the optimum mass ratio of PAMAM to MWCNT is about 0.18) and glucono-δ-lactone (GDL) were added to above mixture, successively. With the help of the hydrolyzed product of the GDL to decrease the pH value of the mixture,27in situ protonized PAMAM molecules were connected with carboxyl groups attached to different MWCNTs via an electrostatic interaction. The mixture was left at ambient temperature (25 °C) for 24 h to achieve a uniform hydrogel. Finally, a free-standing magnetically-functionalized MWCNT aerogel monolith was obtained via either a supercritical CO2 drying or freeze drying of the obtained hydrogel precursor. In this case, if no PAMAM as the crosslinker existed in the mixture, no gelation would be observed, indicating that GDL cannot stimulate the gelation of MWCNTs. It merely acts as a hydrogel promoter and does not incorporate into the hydrogel 3D framework, which was confirmed by UV-vis spectroscopy (see the ESI, Fig. S1†). In addition, when a certain amount of NaOH was used to adjust the pH value of the original mixture, no less than an equimolar amount of GDL had to be used. In other words, the amount of the hydrolyzed product of the GDL should recover the pH value of the original mixture to form the uniform aerogel precursor.
100) was adjusted to ca. 8.0 by using an appropriate amount of NaOH. After that, a certain amount of PAMAM (the optimum mass ratio of PAMAM to MWCNT is about 0.18) and glucono-δ-lactone (GDL) were added to above mixture, successively. With the help of the hydrolyzed product of the GDL to decrease the pH value of the mixture,27in situ protonized PAMAM molecules were connected with carboxyl groups attached to different MWCNTs via an electrostatic interaction. The mixture was left at ambient temperature (25 °C) for 24 h to achieve a uniform hydrogel. Finally, a free-standing magnetically-functionalized MWCNT aerogel monolith was obtained via either a supercritical CO2 drying or freeze drying of the obtained hydrogel precursor. In this case, if no PAMAM as the crosslinker existed in the mixture, no gelation would be observed, indicating that GDL cannot stimulate the gelation of MWCNTs. It merely acts as a hydrogel promoter and does not incorporate into the hydrogel 3D framework, which was confirmed by UV-vis spectroscopy (see the ESI, Fig. S1†). In addition, when a certain amount of NaOH was used to adjust the pH value of the original mixture, no less than an equimolar amount of GDL had to be used. In other words, the amount of the hydrolyzed product of the GDL should recover the pH value of the original mixture to form the uniform aerogel precursor.
More interestingly, a reversible gel–sol transition can be observed from the as-made dendrimer-linked magnetically functionalized MWCNT hydrogel. When a small amount of the NaOH solution was added to the PAMAM crosslinked MWCNT–Fe3O4 composite hydrogel after removal of abundant GDL and other impurities within the gel matrix, the gel turned into a sol as the strong electrostatic interaction between the carboxyl groups attached to the MWCNTs and terminated amino groups of the dendrimer had been diminished due to the deprotonized effect of the NaOH on the protonized amino groups. Thereafter, when an equimolar amount of the GDL solution was added to decrease the pH value of the mixture, the above-mentioned sol turned into a gel again due to restoration of the electrostatic interaction between the carboxyl groups and the terminated amino groups. The whole gel–sol–gel transition can be repeated at least three times (see Fig. S2†) if the doses of NaOH and GDL are controlled properly, indicating a reversible, pH-responsive gel–sol transition of the dendrimer crosslinked magnetic-nanoparticle-functionalized MWCNT hydrogel.
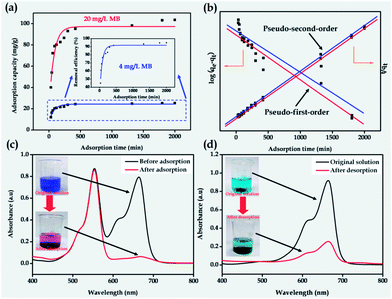
The corresponding magnetic carbon nanotube aerogels have shown the expected renewable attribute as illustrated in Fig. 1c. When part of the freshly-made MWCNT aerogel monolith was put into the MB solution, it was first broken down to much smaller pieces, due to the strong surface tension of the solvent molecules,6 and then the MB was adsorbed into the pores of the aerogels. The adsorption capacity at equilibrium of the MWCNT–Fe3O4 composite aerogel toward MB is 103 mg g−1 (see Fig. 2a), much higher than that of the original MWCNT aerogel (84 mg g−1) under the same conditions, which results from the enhancement of the specific surface area of the MWCNT–Fe3O4 composite aerogel in comparison with that of the original MWCNT aerogel (see below). In addition, the aerogel has ca. a 4 times higher adsorption capacity than its hydrogel precursor probably as no solvent (i.e. water in this case) molecules have occupied a large amount of the active sites in the pores of the aerogel matrix through hydrogen bonding before the adsorption of the dye molecules. What is more, the resulting MWCNT–Fe3O4 composite aerogel, similar to the original MWCNT aerogel, has the adsorption process of the MB fitted well with the pseudo-second-order kinetic model, as well as possessing a selective adsorption from the mixture of the MB with RhB and controllable desorption after the addition of the amino-terminated PAMAM (see Fig. 2b–d), similar to the behavior of the pure MWCNT aerogels (see Fig. S3†). By filtering the mixture of the desorbed MB and the aerogel sorbent, we could obtain a solid carbon nanotube/Fe3O4/PAMAM paper as shown in Fig. 1c. After dissolving this solid paper into the alkaline solution with mild sonication, we could obtain the uniform and stable sol. By addition of the GDL into the above sol, the MWCNT–Fe3O4 composite hydrogel could be obtained and served as the precursor to make the corresponding magnetically functionalized MWCNT aerogel again via the conventional supercritical drying or freeze drying. Such a cycle has strongly demonstrated that the MWCNT aerogel reported in this manuscript possesses the renewable attribute after use. To the best of our knowledge, the magnetically functionalized MWCNT aerogel reported herein is the first highly porous nanomaterial with a renewable characteristic among all the known functional nanoporous substances including zeolites, molecular sieves, metal–organic frameworks, conjugated micro-porous polymers, etc. The MWCNT aerogels functionalized with the Fe3O4 nanoparticles have endowed the resulting composite aerogels with a magnetic separation functionality when used as the sorbent (see Fig. S4†). The re-generation attribute has also endowed the magnetically functionalized MWCNT aerogels with great sustainability, in comparison with the MWCNT aerogels reported elsewhere.
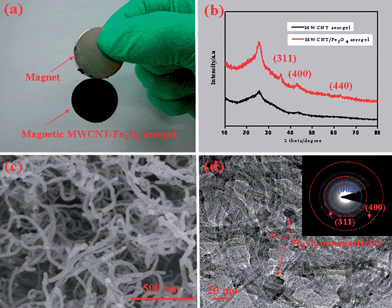
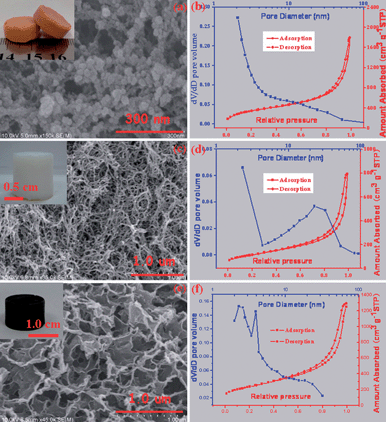
The morphology and porous structure of the as-prepared MWCNT hydrogel (as well as the aerogel) synthesized in the absence and presence of the magnetic sol were clarified by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD) and nitrogen sorption investigations. For the product synthesized in the absence of Fe3O4 nanoparticles, monolithic MWCNT aerogels with a uniform morphology, as illustrated in Fig. S5,† can be obtained via either the supercritical drying or freeze drying of their hydrogel precursors. High-magnification SEM images revealed that, for the aerogels processed with either the supercritical drying or freeze drying, the individual carbon nanotubes are interconnected mutually to form well-defined 3D porous networks. High-resolution TEM image (see Fig. S6†) have disclosed the interconnection of two hollow nanotubes linked by an amorphous and spherical dendrimer, indicative of the validity of the PAMAM crosslinked carbon nanotubes to form 3D gel networks, as shown in Fig. 1b. Nitrogen sorption investigations have indicated that either the freeze dried or supercritical dried MWCNT aerogels show a typical type IV isotherm characteristic with an adsorption hysteresis (see Fig. S7†), which indicates that many mesopores (pore diameter in the range 2–50 nm) existed in these aerogel samples.20 Furthermore, a Brunauer–Emmett–Teller (BET) analysis has shown that these aerogels have specific surface areas in the range 140–160 m2 g−1 and pore volumes in the range 0.3–0.7 cm3 g−1 (see Table S1†), consistent with the observation of the above electron microscopy. For the synthesis conducted in the presence of magnetic nanoparticles, the MWCNT–Fe3O4 composite aerogel could be successfully obtained and lifted with a magnet as shown in Fig. 3a, indicating the good magnetic behavior of the resulting composite aerogel. A rheological investigation (see Fig. S8†) has indicated that the existence of the magnetic Fe3O4 nanoparticles has not significantly influenced the 3D network formation of the 1D carbon nanotubes, as the G′ values are still higher than the G′′ values over the entire tested range. A diffraction hump appearing at 2θ = 25.6° can be detected from the MWCNT aerogel and the MWCNT–Fe3O4 composite aerogel as shown in Fig. 3b, which originates from the (002) plane of the MWCNT.28 In addition, there are another three peaks at 35.5, 43.0 and 62.6° observed from the resulting MWCNT–Fe3O4 composite aerogel, consistent with the (311), (400) and (440) planes of the standard XRD data for Fe3O4 (JCPDS no. 65-3107),29 suggesting that the Fe3O4 nanoparticles have been incorporated within the MWCNT aerogel matrix. The SEM image, as shown in Fig. 3c, has confirmed that the MWCNT–Fe3O4 composite aerogel possesses a 3D interconnected network with a randomly opened mesoporous structure. A high-resolution TEM image, as shown in Fig. 3d, has revealed the decoration of the individual MWCNTs with Fe3O4 nanoparticles in the diameter of several nanometers, and a selective area electron diffraction pattern (inset in Fig. 3d) has further confirmed the existence of the Fe3O4 nanoparticles in the MWCNT aerogel matrix.29 Nitrogen sorption measurements have revealed that the adsorption–desorption curves of the resulting functionalized MWCNT aerogels processed by either freeze drying or supercritical drying exhibit a type-IV isotherm with a H3 hysteresis loop (see Fig. S9†), implying the presence of many mesopores in the as-made MWCNT–Fe3O4 composite aerogels.20 The BET analysis has shown a specific surface area in the range 180–200 m2 g−1 (see Table S1†) for the MWCNT–Fe3O4 aerogels with the different drying methods, which are much higher than those of the original MWCNT aerogels. This ascribes to the idea that Fe3O4 nanoparticles can act as a spacer to hamper the aggregation of the individual MWCNTs.
Inspired by the dendrimer-linked magnetically-functionalized MWCNT aerogels reported herein, we have synthesized other aerogels with a renewable attribute also. First of all, we carefully selected three gel systems: (1) a Fe-H3BTC gel made by mixing Fe(NO3)3 with 1,3,5-benzenetricarboxylic acid (H3BTC) in ethanol,30 (2) an agarose gel made by the cooling of a hot agarose aqueous solution31 and (3) a graphene oxide gel made by assembling graphene oxide sheets with in situ released La3+ ions.25 These gels can be respectively assigned as an organic–inorganic hybrid, and organic and inorganic categories, and all of these gels show reversible gel–sol transitions (see Fig. S10†) without experiencing the irreversible hydrolysis and condensation reactions. All these gels have been transferred into the corresponding aerogels as shown in Fig. 4, after supercritical CO2 drying. The Fe-H3BTC aerogel is composed of 0D particles with a diameter of several tens of nanometers and abundant mesopores with diameter of several nanometers, and the BET surface area of the resulting Fe-H3BTC aerogel is as high as 1247 m2 g−1. The agarose aerogel is composed of 1D nanowires with a diameter of ca. 10 nanometers and various mesopores with double distributions, and the BET surface area of the resulting agarose aerogel is 265 m2 g−1. The graphene oxide aerogel is composed of 2D thin sheets and hierarchical pores (mesopores as well as macropores), and the BET surface area is up to 855 m2 g−1. The most important thing is that all these three aerogels show a reversible transition between the raw materials and products, indicative of the renewable attribute with the dimension-independence of their building blocks.
In conclusion, PAMAM-crosslinked magnetic MWCNT aerogels with an ultra-low density (12–14 mg cm−3) and a large BET surface area (182–196 m2 g−1) have been synthesized by the GDL-controlled assembly of individual nanotubes, in the presence of a Fe3O4 sol, into 3D hydrogel precursors with a subsequent supercritical CO2 drying or freeze drying. The resulting MWCNT–Fe3O4 composite aerogels have ca. a 4 times higher adsorption capacity than the hydrogel precursors for the same organic dye molecules. The chemical attribute has endowed the resulting magnetic MWCNT aerogels with a selective adsorption and controlled release towards organic dyes, and the reversible sol–gel transition has endowed the resulting magnetic MWCNT aerogels with a renewable characteristic. The mechanical and electrical properties of the resulting magnetic MWCNT aerogels are still under extremely difficult investigations due to the fragility of the samples. Inspired by the successful synthesis of the renewable magnetic MWCNT aerogels, we have also reported the Fe-H3BTC aerogel with 0D building blocks, agarose aerogel with 1D building blocks and graphene oxide aerogel with 2D building blocks consistently.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21373024), the 100 Talents Program of the Chinese Academy of Science, and the Innovation Program of the Beijing Institute of Technology.Notes and references
- S. Nardecchia, D. Carriazo, M. L. Ferrer, M. C. Gutiérrez and F. del Monte, Chem. Soc. Rev., 2013, 42, 794 RSC.
- L. Chen, B. Wei, X. T. Zhang and C. Li, Small, 2013, 9, 2331 CrossRef CAS PubMed.
- H. Huang, P. W. Chen, X. T. Zhang, Y. Lu and W. C. Zhan, Small, 2013, 9, 1397 CrossRef CAS PubMed.
- T. R. Cook, Y. R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef CAS PubMed.
- K. Zhang, D. Kopetzki, P. H. Seeberger, M. Antonietti and F. Vilela, Angew. Chem., Int. Ed., 2013, 52, 1432 CrossRef CAS PubMed.
- N. Hüsing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22 CrossRef.
- G. Peng, U. Tisch and H. Haick, Nano Lett., 2009, 9, 1362 CrossRef CAS PubMed.
- W. Zhang, J. K. Sprafke, M. L. Ma, E. Y. Tsui, S. A. Sydlik, G. C. Rutledge and T. M. Swager, J. Am. Chem. Soc., 2009, 131, 8446 CrossRef CAS PubMed.
- V. V. Chaban and O. V. Prezhdo, ACS Nano, 2011, 5, 5647 CrossRef CAS PubMed.
- G. Pastorin, W. Wu, S. Wieckowski, J. P. Briand, K. Kostarelos, M. Prato and A. Bianco, Chem. Commun., 2006, 1182 RSC.
- C. Biswas and Y. H. Lee, Adv. Funct. Mater., 2011, 21, 3806 CrossRef CAS.
- D. E. Johnston, M. F. Islam, A. G. Yodh and A. T. Johnson, Nat. Mater., 2005, 4, 589 CrossRef CAS PubMed.
- R. F. Zhang, Q. Wen, W. Z. Qian, D. S. Su, Q. Zhang and F. Wei, Adv. Mater., 2011, 23, 3387 CrossRef CAS PubMed.
- Z. Chen, D. Q. Zhang, X. L. Wang, X. L. Jia, F. Wei, H. X. Li and Y. F. Lu, Adv. Mater., 2012, 24, 2030 CrossRef CAS PubMed.
- X. C. Gui, J. Q. Wei, K. L. Wang, A. Y. Cao, H. W. Zhu, Y. Jia, Q. K. Shu and D. H. Wu, Adv. Mater., 2010, 22, 617 CrossRef CAS PubMed.
- X. C. Gui, A. Y. Cao, J. Q. Wei, H. B. Li, Y. Jia, Z. Li, L. L. Fan, K. L. Wang, H. W. Zhu and D. H. Wu, ACS Nano, 2010, 4, 2320 CrossRef CAS PubMed.
- B. B. Mateusz, D. E. Milkie, M. F. Islam, L. A. Hough, J. M. Kikkawa and A. G. Yodh, Adv. Mater., 2007, 19, 661 CrossRef.
- (a) K. H. Kim, M. Vural and M. F. Islam, Adv. Mater., 2011, 23, 2865 CrossRef CAS PubMed; (b) S. M. Jung, H. Y. Jung, M. S. Dresselhaus, Y. J. Jung and J. Kong, Sci. Rep., 2012, 2, 849 Search PubMed.
- Z. Y. Sui, Q. H. Meng, X. T. Zhang, R. Ma and B. Cao, J. Mater. Chem., 2012, 22, 8767 RSC.
- X. T. Zhang, J. R. Liu, B. Xu, Y. F. Su and Y. J. Luo, Carbon, 2011, 49, 1884 CrossRef CAS.
- T. Ogoshi, Y. Takashima, H. Yamaguchi and A. Harada, J. Am. Chem. Soc., 2007, 129, 4878 CrossRef CAS PubMed.
- J. H. Zou, J. H. Liu, A. S. Karakoti, A. Kumar, D. Joung, Q. Li, S. I. Khondaker, S. Seal and L. Zhai, ACS Nano, 2010, 4, 7293 CrossRef CAS PubMed.
- R. R. Kohlmeyer, M. Lor, J. Deng, H. Y. Liu and J. Chen, Carbon, 2011, 49, 2352 CrossRef CAS.
- X. T. Zhang, W. H. Song, P. J. F. Harris, G. R. Mitchell, T. T. T. Bui and A. F. Drake, Adv. Mater., 2007, 19, 1079 CrossRef CAS.
- H. Huang, S. Y. Lü, X. T. Zhang and Z. Q. Shao, Soft Matter, 2012, 8, 4609 RSC.
- W. F. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679 CrossRef CAS PubMed.
- Y. Pocker and E. Green, J. Am. Chem. Soc., 1973, 95, 113 CrossRef CAS PubMed.
- X. Zhang, J. Zhang and Z. F. Liu, Appl. Phys. A: Mater. Sci. Process., 2005, 80, 1813 CrossRef CAS.
- J. Su, M. Cao, L. Ren and C. Hu, J. Phys. Chem. C, 2011, 115, 14469 CAS.
- Q. Wei and S. L. James, Chem. Commun., 2005, 1555 RSC.
- X. T. Zhang, V. Chechik, D. K. Smith, P. H. Walton, A.-K. Duhme-Klair and Y. J. Luo, Synth. Met., 2009, 159, 2135 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details on the synthesis and characterization of the resulting magnetic carbon nanotube aerogels, porous attribute table and rheological curves of the resulting MWCNT and MWCNT–Fe3O4 aerogels, SEM as well as TEM images and sorption curves of the carbon nanotube aerogels, UV-vis spectra of the mixtures between GDL and PAMAM or between GDL and carboxylic carbon nanotube, digital photos showing sol–gel transitions of the carbon nanotube gel, Fe-H3BTC gel, agarose gel and graphene oxide gel, digital photo showing magnetic separation of the MWCNT–Fe3O4 composite aerogels used as the sorbent. See DOI: 10.1039/c3mh00076a |
| This journal is © The Royal Society of Chemistry 2014 |