A shape-memory scaffold for macroscale assembly of functional nanoscale building blocks†
Huai-Ling
Gao‡
a,
Yang
Lu‡
a,
Li-Bo
Mao
a,
Duo
An
a,
Liang
Xu
a,
Jun-Tong
Gu
b,
Fei
Long
c and
Shu-Hong
Yu
*a
aDivision of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at Microscale, Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, The People's Republic of China. E-mail: shyu@ustc.edu.cn; Fax: +86 0551-63603040
bCollege of Pharmacy, Anhui University of Chinese Medicine, Hefei 230031, P.R. China
cSchool of Life Science, University of Science and Technology of China, Hefei 230027, P.R. China
First published on 10th September 2013
Abstract
A shape-memory chitosan scaffold (CSS) fabricated by an ice-templated method can be used as a versatile host matrix for self-assembly of a wide range of functional nanoscale building blocks, and thus it can produce a family of functional three-dimensional (3D) macroscale assemblies, which show promising practical application potential in various fields.
Incorporating nanoscale components into serviceable macroscale devices is of significant importance for practical applications because the intriguing properties of the nanomaterials can be effectively transferred into macroscale assemblies.1–13 Numerous achievements have been made on macroscale 1D fibers,14,15 2D sheets,4,5,16,17 and 3D monoliths.2,8–12,18–23 However, the established methods to fabricate macroscale assemblies are confined to a few specific kinds of nanomaterials. The major limitation is that there is still a lack of efficient universal assembly strategies for various kinds of nanoscale building blocks, which severely hindered the possibility for their practical applications. Therefore, a universal, straightforward, low-cost, environmental and scalable assembly process is highly desirable from the industrial point of view. Compared with those non-deformable ones, assemblies with shape-memory property16–20 display more fascinating application prospects.
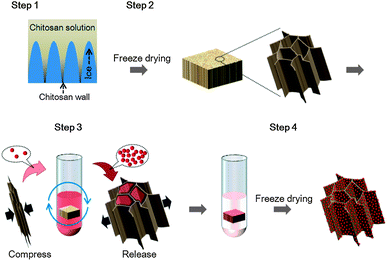
Herein, we present a shape-memory matrix-supported approach to fabricate a series of functional 3D macroscale assemblies from individual nanoscale building blocks, which has met the above-stated demands. Due to its excellent intrinsic properties, a chitosan scaffold (CSS) fabricated by the freeze casting process was chosen as the matrix to support and fix various kinds of nanoscale building blocks at the 3D macroscale level. Noteworthily, we demonstrate this universal strategy for assembling various types of functional nanocrystals into 3D macroscale assemblies with highly ordered porous microstructure (Fig. 1), which show promising potential for practical applications, but have been rarely reported.
Unidirectional solidification was applied during the freezing process24,25 to fabricate the highly ordered porous chitosan scaffold (CSSs) serving as a 3D macroscale matrix. The formation of such a highly ordered pore structure can be explained by the oriented growth of ice crystals in the chitosan solution.26 During the solidification process, the growing ice fingers push the chitosan chains into the inter-finger spaces where they form chitosan precipitate walls (Fig. 1).26,27
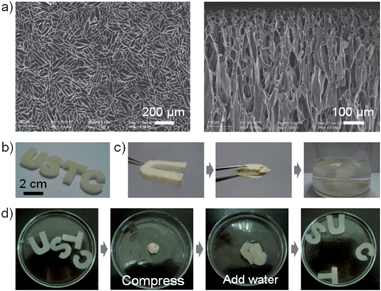
The microstructure of dried CSSs was revealed by Scanning Electron Microscopy (SEM), and a uniform network composed of co-aligned and interconnected pore channels was observed in parallel to the freezing direction (Fig. 2a). The size and shape of CSSs can be finely tuned by changing either the shape of the molds or the amount of chitosan solution (Fig. 2b). This simple process will make it feasible to fabricate a series of complex shapes of CSSs suited for different applications.
CSSs with channels of various pore diameters in the range of 20–200 μm can be finely tuned by changing the freezing temperature (ESI, Fig. S1a†). Moreover, the pore size can also be reduced by compression of CSS, and reach as low as a few micrometers (ESI, Fig. S1b†). Meanwhile, the thickness of the channel walls can be tuned by varying the concentration of chitosan solution (ESI, Fig. S1c and d†).
More remarkably, CSSs exhibit a perfect shape-memory property. In other words, they can be compressed and folded randomly no matter whether they are wet or dry, and no shape degradation was found even after many repeating cycles (Fig 2c and d and Movie S1 in the ESI†). During the repeating compression–release–compression cycles, CSSs underwent a reversible geometry change with recovery of their original shapes due to the release and absorption of water. This property was probably determined by the microstructure and strong water absorption of CSSs. When compressed, the internal channels deformed but the polymer walls were not damaged. Therefore, when water entered into the channels again, all of the channels began to expand, leading to the shape recovery of the macroscopic monoliths. In contrast, other polymer scaffolds made of collagen, sodium alginate and polyvinyl acetate were unstable in water due to their water-solubility, and thus no shape-memory behaviour was observed (ESI, Fig. S2†).
Porosities and water absorption of CSS were measured by the previously reported method,28 which revealed the ultra-high porosities larger than 97% and ultra-strong water absorption about 22.3 times of its weight (ESI, Table S1†).
To demonstrate the versatility of CSSs, different kinds of functional nanocrystals were assembled into this host matrix using a simple dip adsorption procedure (Fig. 1 and 3a). As nanoscale building blocks, zero dimensional (0D) nanoparticles including iron oxide nanoparticles (Fe3O4 NPs), gold nanoparticles (Au NPs), silver nanoparticles (Ag NPs), cadmium telluride quantum dots (CdTe QDs), one dimensional (1D) nanowires including silver nanowires (Ag NWs), carbon nanotubes (CNT), tellurium nanowires (Te NWs), as well as two dimensional (2D) nanosheets, such as reduced graphene stabilized by sodium polystyrene sulfonate (rGO-PSS), were adsorbed thoroughly by repeated compression–release–compression of CSSs in their aqueous solution at a certain concentration. As a result, a series of magnetic, photonic, and electrical 3D macroscale assemblies integrated with CSSs and different functional nanoscale building blocks were facilely fabricated (Fig. 3). Most importantly, the obtained macroscale assemblies still retain the shape-memory property and do not undergo structural collapse when they are subjected to cyclic compressional deformation (Fig. 3b and d). Unlike conventional porous scaffolds with isotropic porous structures, the dry assemblies exhibit anisotropic mechanical properties similar to CSSs, which were attributed to the unique unidirectional channel-like microstructure of CSSs. The compressive properties were studied quantitatively, which revealed a significant difference between mechanical properties parallel and perpendicular to the pore channel direction (ESI, Fig. S3†). The compressive strength in the longitudinal direction is much stronger than that in the transverse direction, while highly elastic compressibility was demonstrated in the transverse direction. Interestingly, after these assemblies were compressed into sheets, they can also get back to their primary shapes owing to their strong water absorption (Fig. 3c).
The efficient adsorption is driven by the electrostatic attraction between negatively charged nanocrystals and the positively charged channel surface of the chitosan scaffold rich in amino groups.29 In order to confirm the possible mechanism, the zeta potential of these nanocrystals was measured, and all nanocrystals used here were found to be negatively charged (ESI, Table S2†). On comparing positively charged gold nanorods (Au NRs) (+28.61 mV) with negatively charged poly (sodium-p-styrenesulfonate) coated gold nanorods (PSS–Au NRs), only PSS–Au NRs could be assembled efficiently into the CSS, indicating that the strong charge attraction is the key point to make CSSs so versatile for the assembly of different types of nanoscale building blocks (ESI, Fig. S4†).
Additionally, the shape-memory property is indispensable for an efficient integration of nanocrystals into the matrix. Namely, only CSSs with shape-memory property can continuously absorb nanocrystals in the repeated compressing and releasing process, during which nanocrystals entered into the micro-sized channels without a hitch and were efficiently absorbed onto the inner surface homogeneously. After sufficient absorption, nanoscale building blocks were assembled into highly ordered porous 3D macroscale assemblies. These obtained composite scaffolds are very stable in water for a very long time (even more than 6 months, ESI, Fig. S5†).
The XRD patterns of the as-prepared macroscale assemblies show that all of the diffraction peaks can be indexed as the corresponding nanocrystals (ESI, Fig. S6†), which confirmed the existence of different nanocrystals in this supported matrix. Regional spatial distribution of nanoscale building blocks in the CSS matrix was revealed from the SEM images, that is, nanocrystals with different sizes and shapes were all compactly and uniformly distributed on the internal channel surface (ESI, Fig. S7a†). Furthermore, in order to demonstrate the monolithic uniformity of the nanoscale building blocks in the whole scaffold at the macroscale level, CdTe QDs with different fluorescence emission spectra were integrated into CSSs and fluorescence section images of CSSs-QDs with different fluorescence demonstrate the uniform coating of individual nanocrystals onto the channel surface at the millimeter scale (ESI, Fig. S7c†). The integrating amount of the building blocks can be facilely tuned with the compression cycle times and the concentrations of nanocrystal solution. With increased absorption of Au NPs, the channel surface displayed an increasing dense distribution of Au NPs even to achieve an extremely high amount of assembled structures (ESI, Fig. S7b†), and the content of Au NPs (nanoparticles weight/scaffold weight) is quantified to be 24.06% by thermogravimetric analysis (TGA) (ESI, Fig. S8†). The maximum loading amounts of Fe3O4 NPs and Ag NWs were also quantified, which are 24.96% and 61.66%, respectively.
Integration of different types of functional nanoscale building blocks into this host matrix not only inherited the characteristics of the original CSSs, but also transferred the intrinsic functionalities of the nanoscale building blocks to the macroscopic assembled structures. With such a strategy, the functionalities of bioorganic components and inorganic nanocrystals can be facilely combined to work synergistically, which enable us to design and create a series of multifunctional 3D macroscopic entities.
Firstly, we demonstrated the sensitive magnetic response of magnetic CSS. In Fig. 4a, the dry magnetic scaffold showed an excellent magnetic response to overcome gravity and move with a household magnet. Moreover, the wet magnetic scaffold underwent an obvious and reversible deformation under magnetic actuation (Fig. 4b). These superior performances make it a good candidate to serve as a magnetic actuator.18,30 As a unique feature of noble metal nanoparticles resulting from surface plasmon resonance,31 the photothermal (PT) efficiency of CSS–Au NPs was also investigated. As shown in Fig. 4c, the water temperature for the CSS–Au NPs containing group increased rapidly, and even can reach the boiling point after only exposure to NIR for 8 minutes, compared with only a slight increase in bare CSS groups. In addition, as the amount of the Au NPs in CSS decreased, the heating rate was lower (Fig. 4c). The compact distribution of Au NPs on the channel surface of the scaffold should contribute to the excellent photothermal efficiency owing to the collective electromagnetic coupling effect (ESI, Fig. S7b, Fig. S8†).32,33 With respect to the biomedical application of this versatility system, we studied the antibacterial effect of CSS–Ag NPs. As shown in Fig. 4d, distinct inhibition zones were observed around the CSS–Ag NPs on the E. coli-inoculated surface of the LB agar plate after inoculation for 24 h. To further investigate the antibacterial property, CSS–Ag NPs presoaked in E. coli suspension at a high concentration were incubated on the surface of a pure LB agar plate for 6 h (Fig. 4e), and only a few colonies were found around the CSS–Ag NPs. In contrast, E. coli around the Ag-free scaffold was growing vigorously. The possible mechanism of the antibacterial effect was primarily regarded as the release of Ag+ ions from the composite matrix.34,35 These perfect performances make CSS–Au/Ag NPs good candidates as bio-functional scaffolds. As shown in Fig. 4f, a series of fluorescent 3D macroscale assemblies were fabricated by the integration of four different CdTe QDs with variable emission wavelengths into CSSs, which can be used in optical devices.
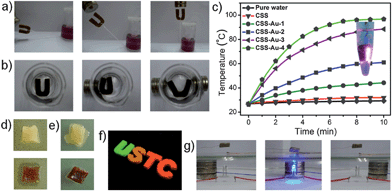 | ||
| Fig. 4 Multifunctionalities of the obtained macroscale assemblies. (a) Dried CSS–Fe3O4 NPs were suspended in midair in response to the movement of magnets. (b) Demonstration of the flexibility and magnetic response of the magnetic scaffold in water and it exhibits reversible and large deformation. (c) Effective heat-generation of CSS–Au NPs under the exposure of NIR. The inset photograph demonstrated the boiling water caused by effective heat-generation. CSS–Au-2, 3, 4 correspond to Fig. S7b† respectively and CSS–Au-1 (0.25 cm3) was obtained by absorbing 0.25 ml of Au NPs solution (1.0 mM). The inset shows the boiling water result for CSS–Au-4. (d) Obvious inhibition zone was displayed around CSS–Ag NPs (down) compared with that of Ag-free CSS (up). (e) CSS–Ag NPs presoaked with E. coli (down) were incubated into the solid culture medium, and effective antimicrobial property was observed in comparison with pure CSS (up). (f) Fluorescent scaffolds fabricated by assembling CdTe QDs. (g) Photographic images show that Fe3O4 NPs and Ag NWs co-assembled CSSs can switch the LED circuit through magnetic actuation. | ||
Furthermore, multifunctional macroscopic assemblies have also been achieved by the synergistic assembly of different kinds of nanoscale building blocks into the CSS matrix system. Here, as an example, we combined the magnetic actuation ability of Fe3O4 NPs with the conductivity of Ag NWs36 into one scaffold to construct a magnetically actuated switch. As illustrated in Fig. 4g, in response to an external small household magnet, LED light can be controlled reversibly with the assistance of the magnetic and electric dual-functional scaffold due to the magnetically actuated switching of the circuit. This special performance of ION–Ag dual functional CSS indicated abundant possibilities of various combinations and the broadened potential applications of these multifunctional assemblies. Other functional nanomaterials, such as carbon nanotubes37 graphene oxide,38–40 reduced graphene oxide41 and tellurium nanowires42 can also be individually or simultaneously assembled into various functional devices with promising applications in many fields. Therefore, a series of novel multifunctional assemblies at the macroscale can be prepared from various high-quantity nanoscale building blocks by using such a powerful scaffold as a host.
Conclusions
In conclusion, a universal, straightforward, low-cost, and scalable macroscale assembly technology has been presented for the fabrication of 3D macroscale multifunctional assemblies from various high-quantity nanoscale building blocks. Not only can the excellent properties of different individual nanoscale building blocks be successfully integrated into the versatile chitosan scaffolds, but also the shape-memory property was retained in the macroscale assembled scaffolds. Owing to these excellent performances, this macroscale assembly matrix holds great promise in the fabrication of advanced novel nanomaterial based macroscale devices for different demands.Acknowledgements
This work was supported by the National Basic Research Program of China (Grant 2010CB934700), the Ministry of Science and Technology of China (Grant 2012BAD32B05-4), the National Natural Science Foundation of China (Grants 91022032, 91227103, 21061160492), and the Chinese Academy of Sciences (Grant KJZD-EW-M01-1). Y. L. thanks the Fundamental Research Funds for the Central Universities (Grants WK2060190016), and the China Postdoctoral Science Foundation (2012M510160).Notes and references
- Y. Z. Long, M. Yu, B. Sun, C. Z. Gu and Z. Y. Fan, Chem. Soc. Rev., 2012, 41, 4560 RSC.
- B. Z. Tian, J. Liu, T. Dvir, L. H. Jin, J. H. Tsui, Q. Qing, Z. G. Suo, R. Langer, D. S. Kohane and C. M. Lieber, Nat. Mater., 2012, 11, 986 CrossRef CAS PubMed.
- K. Takei, T. Takahashi, J. C. Ho, H. Ko, A. G. Gillies, P. W. Leu, R. S. Fearing and A. Javey, Nat. Mater., 2010, 9, 821 CrossRef CAS PubMed.
- K. Y. Chun, Y. Oh, J. Rho, J. H. Ahn, Y. J. Kim, H. R. Choi and S. Baik, Nat. Nanotechnol., 2010, 5, 853 CrossRef CAS PubMed.
- J. W. Liu, H. W. Liang and S. H. Yu, Chem. Rev., 2012, 112, 4770 CrossRef CAS PubMed.
- M. C. McAlpine, H. Ahmad, D. W. Wang and J. R. Heath, Nat. Mater., 2007, 6, 379 CrossRef CAS PubMed.
- H. W. Liang, Q. F. Guan, L. F. Chen, Z. Zhu, W. J. Zhang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 5101 CrossRef CAS PubMed.
- H. B. Yao, G. Huang, C. H. Cui, X. H. Wang and S. H. Yu, Adv. Mater., 2011, 23, 3643 CrossRef CAS PubMed.
- Z. H. Tang, S. L. Shen, J. Zhuang and X. Wang, Angew. Chem., Int. Ed., 2010, 49, 4603 CrossRef CAS PubMed.
- J. U. Park, S. Nam, M. S. Lee and C. M. Lieber, Nat. Mater., 2012, 11, 120 CrossRef CAS PubMed.
- S. M. Zhang, M. A. Greenfield, A. Mata, L. C. Palmer, R. Bitton, J. R. Mantei, C. Aparicio, M. O. de la Cruz and S. I. Stupp, Nat. Mater., 2010, 9, 594 CrossRef CAS PubMed.
- Z. Q. Niu, J. Chen, H. H. Hng, J. Ma and X. D. Chen, Adv. Mater., 2012, 24, 4144 CrossRef CAS PubMed.
- S. Nardecchia, D. Carriazo, M. L. Ferrer, M. C. Gutierrez and F. del Monte, Chem. Soc. Rev., 2013, 42, 794 RSC.
- V. A. Davis, A. N. G. Parra-Vasquez, M. J. Green, P. K. Rai, N. Behabtu, V. Prieto, R. D. Booker, J. Schmidt, E. Kesselman, W. Zhou, H. Fan, W. W. Adams, R. H. Hauge, J. E. Fischer, Y. Cohen, Y. Talmon, R. E. Smalley and M. Pasquali, Nat. Nanotechnol., 2009, 4, 830 CrossRef CAS PubMed.
- H. P. Cong, X. C. Ren, P. Wang and S. H. Yu, Sci. Rep., 2012, 2, 613 CrossRef PubMed.
- C. X. Li, J. Adamcik and R. Mezzenga, Nat. Nanotechnol., 2012, 7, 421 CrossRef CAS PubMed.
- A. E. Aliev, J. Y. Oh, M. E. Kozlov, A. A. Kuznetsov, S. L. Fang, A. F. Fonseca, R. Ovalle, M. D. Lima, M. H. Haque, Y. N. Gartstein, M. Zhang, A. A. Zakhidov and R. H. Baughman, Science, 2009, 323, 1575 CrossRef CAS PubMed.
- R. T. Olsson, M. A. S. A. Samir, G. Salazar-Alvarez, L. Belova, V. Strom, L. A. Berglund, O. Ikkala, J. Nogues and U. W. Gedde, Nat. Nanotechnol., 2010, 5, 584 CrossRef CAS PubMed.
- K. H. Kim, Y. Oh and M. F. Islam, Nat. Nanotechnol., 2012, 7, 562 CrossRef CAS PubMed.
- X. C. Gui, J. Q. Wei, K. L. Wang, A. Y. Cao, H. W. Zhu, Y. Jia, Q. K. Shu and D. H. Wu, Adv. Mater., 2010, 22, 617 CrossRef CAS PubMed.
- C. Sanchez, P. Belleville, M. Popall and L. Nicole, Chem. Soc. Rev., 2011, 40, 696 RSC.
- H. W. Liang, Q. F. Guan, Z. Zhu, L. T. Song, H. B. Yao, X. Lei and S. H. Yu, NPG Asia Mater., 2012, 4, e19 CrossRef.
- C. Pang, G. Y. Lee, T. I. Kim, S. M. Kim, H. N. Kim, S. H. Ahn and K. Y. Suh, Nat. Mater., 2012, 11, 795 CrossRef CAS PubMed.
- S. Deville, E. Saiz, R. K. Nalla and A. P. Tomsia, Science, 2006, 311, 515 CrossRef CAS PubMed.
- S. Deville, E. Maire, G. Bernard-Granger, A. Lasalle, A. Bogner, C. Gauthier, J. Leloup and C. Guizard, Nat. Mater., 2009, 8, 966 CrossRef CAS PubMed.
- E. Munch, E. Saiz, A. P. Tomsia and S. Deville, J. Am. Ceram. Soc., 2009, 92, 1534 CrossRef CAS.
- S. Deville, E. Saiz and A. P. Tomsia, Acta Mater., 2007, 55, 1965 CrossRef CAS PubMed.
- W. C. Hsieh, C. P. Chang and S. M. Lin, Colloids Surf., B, 2007, 57, 250 CrossRef CAS PubMed.
- L. Shi, F. Carn, F. Boue, G. Mosser and E. Buhler, ACS. Macro Lett., 2012, 1, 857 CrossRef CAS.
- H. W. Liang, W. J. Zhang, Y. N. Ma, X. Cao, Q. F. Guan, W. P. Xu and S. H. Yu, ACS Nano, 2011, 5, 8148 CrossRef CAS PubMed.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759 RSC.
- S. A. Maier, P. G. Kik, H. A. Atwater, S. Meltzer, E. Harel, B. E. Koel and A. A. G. Requicha, Nat. Mater., 2003, 2, 229 CrossRef CAS PubMed.
- W. Rechberger, A. Hohenau, A. Leitner, J. R. Krenn, B. Lamprecht and F. R. Aussenegg, Opt. Commun., 2003, 220, 137 CrossRef CAS.
- A. Gupta, M. Maynes and S. Silver, Appl. Environ. Microbiol., 1998, 64, 5042 CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177 CrossRef CAS PubMed.
- C. Yang, H. W. Gu, W. Lin, M. M. Yuen, C. P. Wong, M. Y. Xiong and B. Gao, Adv. Mater., 2011, 23, 3052 CrossRef CAS PubMed.
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787 CrossRef CAS PubMed.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270 CrossRef CAS PubMed.
- J. W. Liu, J. Xu, H. W. Liang, K. Wang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 7420 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3mh00040k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |