Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A full conformational characterization of antiandrogen cortexolone-17α-propionate and related compounds through theoretical calculations and nuclear magnetic resonance spectroscopy
Patrizia
Ferraboschi
a,
Laura
Legnani
ab,
Giuseppe
Celasco
c,
Luigi
Moro
c,
Laura
Ragonesi
c and
Diego
Colombo
*a
aDipartimento di Biotecnologie Mediche e Medicina Traslazionale, Università di Milano, Via Saldini 50, 20133 Milano, Italy. E-mail: diego.colombo@unimi.it; Fax: +39-0250316036; Tel: +39-0250316039
bDipartimento di Chimica – Università degli Studi di Pavia, Via Taramelli 12, 27100 Pavia, Italy
cCosmo Research & Development S.p.A., Via C. Colombo 1, 20020 Lainate, MI, Italy
First published on 4th April 2014
Abstract
Cortexolone-17α-propionate is a topical antiandrogen under investigation for the treatment of androgen-related skin disorders. A full conformational characterization was realized, in comparison with other steroidal androgens and antiandrogens, by means of theoretical calculations at the B3LYP/6-31G(d) level supported by high-field NMR analyses. All of the studied molecules showed a good overlay; nevertheless, the different functional groups present in the skeleton of the molecules drive the individual biological profile.
Introduction
The skin's capability of synthesizing and converting androgens is well documented.1 The 5α-reductase-catalyzed transformation of testosterone 1 affords dihydrotestosterone (DHT, 2) (Fig. 1), the most active metabolite involved in many androgen-related skin disorders such as hirsutism, androgen alopecia and acne.1–5 The treatment of these disorders can be realized by either inhibiting the 5α-reductase or antagonizing the binding of testosterone and DHT at the androgen binding sites.6 Known androgen antagonists, such as finasteride 6 or cyproterone acetate 7, when systemically administered, show beneficial effects in the management of these skin disorders together with an interference with the hormonal environment in male and female patients.7 Several years ago we investigated8 the antiandrogenic activity of a family of 17α-esters of cortexolone (17α,21-dihydroxy-4-pregnene-3,20-dione 3), an intermediate of the glucocorticoid biosynthesis, devoid of endocrine function, with the exception of a weak glucocorticoid activity.9 Among the studied esters, 17α-propionate 4 showed a strong local antiandrogenic activity in the hamster flank organ test10 being, on the contrary, ineffective when subcutaneously injected by repeated administrations in animals even at very high doses.8 The systemic antiandrogenic activity of 17α-propionate of cortexolone was assessed by its ability to decrease the weight of the androgen-dependent organs (ventral prostate, seminal vesicles and preputial glands) stimulated by the injection of testosterone propionate (TP).8The absence of systemic antiandrogenic effects could be explained considering that the propionate, after percutaneous application, is quickly hydrolyzed by the skin and plasma esterases into the inactive parent cortexolone (3) getting through the 21-propionate 5.
The topical activity of compound 4 is higher than that of finasteride 6 and about equivalent to that of cyproterone acetate 7.
Taking into account the topical activity of propionate 4 coupled with the lack of systemic activity, we planned to compare this 17α-ester with the well-known antiandrogen cyproterone acetate 7 and with the natural androgens, testosterone 1 and dihydrotestosterone 2, from a conformational point of view.
In fact, the conformation of a biologically active compound plays a central role when it interacts with the target, for example, a receptor or an enzyme; among the possible conformations only one could be able to stimulate the biological response as we also reported in a previous work.11 The choice of the compounds to be compared with propionate 4 was driven by its antiandrogenic activity. In fact testosterone (1) and dihydrotestosterone (2) are antagonized both by compounds 4 and 7 with the same action mechanism that does not implicate the interference with the 5α-reductase. The conformational characterization was realized by means of theoretical calculations, validated by complete assignment of the 1H and 13C NMR signals, as in the case of our previous studies of steroidal compounds.11
Results and discussion
Biological activity of cortexolone-17α-propionate (4)
| Topical treatment (acetone 0.05 mL) | Daily doseb | Flank organ inhibitionc (%) |
|---|---|---|
| a The local antiandrogenic activity of 4 and other tested compounds 3, 6, and 7 was expressed as the percentage inhibition of the flank organ enlargement induced by the topical application of testosterone propionate (TP) alone. b μg per animal, antiandrogen + androgen. c *P < 0.05, **P < 0.01. | ||
| Cortexolone (3) + TP | 400 + 4 | 0 |
| Finasteride (6) + TP | 400 + 4 | 71* |
| Cyproterone acetate (7) + TP | 400 + 4 | 93** |
| Cortexolone-17α-propionate (4) + TP | 100 + 4 | 40* |
| Cortexolone-17α-propionate (4) + TP | 200 + 4 | 78* |
| Cortexolone-17α-propionate (4) + TP | 400 + 4 | 84** |
As concerns the mechanism of action, compound 4, compared to finasteride (6) the well-known inhibitor of 5α-reductase, did not inhibit the conversion of testosterone (1) to DHT (2) in reconstructed human epidermis (Cosmo R & D personal communication), thus resulting in the absence of inhibitory activity on the 5α-reductase, as shown by the studied [14C]-testosterone metabolism after 24 h transepidermal diffusion (Fig. 2).
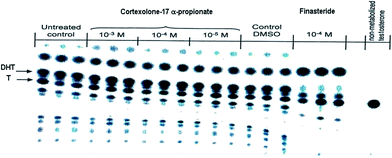 | ||
| Fig. 2 [14C]-testosterone (T, 1) metabolism and DHT (2) production in presence of cortexolone-17α-propionate (4) or finasteride (6). | ||
Additional experiments (Cosmo R & D personal communication) showed that in the binding affinity test to the androgen-receptor of human prostate cancer cells, compound 4 inhibited the specific binding of [3H] methyltrienolone (R1881) to the androgen receptor with a Ki value of 4.0 × 10−8, and an IC50 value of 5.0 × 10−8 M. As a consequence, compound 4 should be considered as an antiandrogen acting at the androgen-receptor level.
| Incubation time | Cortexolone-17α-propionate (4) (%) | Cortexolone (3) (%) |
|---|---|---|
| 0 | 100 | 0 |
| 5 min | 95–90 | 5–10 |
| 15 min | 95–90 | 5–10 |
| 30 min | 90–80 | 10–20 |
| 1 h | 80–60 | 20–40 |
| 2 h | 50 | 50 |
| 4 h | 40–20 | 60–80 |
| 8 h | 10–0 | 90–100 |
An analogous metabolic profile of 4 was observed with incubation in human plasma (Table 3).
| Incubation time | Cortexolone-17α-propionate (4) (%) | Cortexolone-21-propionate (5) (%) | Cortexolone (3) (%) |
|---|---|---|---|
| 0 | 99.6 | 0.5 | 0.0 |
| 30 min | 93.2 | 6.5 | 0.4 |
| 1 h | 85.2 | 13.2 | 1.7 |
| 2 h | 67.2 | 25.3 | 7.5 |
| 4 h | 30.3 | 33.8 | 35.9 |
| 6 h | 11.3 | 23.8 | 64.9 |
In rat skin homogenate the metabolic transformation of 4 to cortexolone (3) reached a peak (40–44.7%) within 8–16 h, and remained stable during the remnant incubation period of up to 24 h (Table 4).
| Incubation time | Cortexolone-17α-propionate (4) (%) | Cortexolone-21-propionate (5) (%) | Cortexolone (3) (%) |
|---|---|---|---|
| 0 | 99 | 0.5 | 0 |
| 5 min | 99 | 0.5 | 0 |
| 15 min | 99 | 0.5 | 0 |
| 30 min | 98.5 | 0.5 | 0.5 |
| 1 h | 89.5 | 5 | 5 |
| 2 h | 69.5 | 15 | 15 |
| 4 h | 59.5 | 10 | 30 |
| 8 h | 49.5 | 10 | 40 |
| 16 h | 44.75 | 10 | 44.75 |
| 24 h | 44.75 | 10 | 44.75 |
| Compound | Flank organ inhibitiona (%) |
|---|---|
| a Ability of the tested steroids (400 μg) to inhibit the enlargement of the hamster's organ flank produced by the administration of 4 μg of testosterone propionate. | |
| Cortexolone-21-propionate (5) | 29 |
| Cortexolone-17α,21-dipropionate (8) | 57 |
| 9,11-Dehydrocortexolone-17α-butyrate (9)12 | 85 |
Differently from the 17α-monoesters of cortexolone, 9,11-dehydrocortexolone-17α-butyrate (9) was found to show systemic activity in the rat after subcutaneous injection.12
Compound 9 was also discovered to be a potent inhibitor of gonadotropin hypersecretion, thus mimicking the activity profile of cyproterone acetate (7), which blocks the androgen-receptor interaction and simultaneously reduces serum testosterone through its antigonadotropic action.13,14 The presence of a double bond at position 9,11 of the cortexolone, modifying the spatial conformation of the steroids rings, could be responsible for the systemic and increased topical activity of 9.12
Conformational properties
The above observations prompted us to study the conformational properties of compound 4 and of the related compounds to establish and compare their preferred conformations. To this end, other known androgenic or antiandrogenic steroids were simultaneously analyzed including testosterone (1) and its most potent metabolite DHT (2) active in the skin, cortexolone (3) and its derivatives 8 and 9 and cyproterone acetate (7).| E rel (kcal mol−1) | % | τ A (°) | τ B (°) | τ C (°) | τ 1 (°) |
τ
1′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (°)
(°) |
τ 2 (°) | τ 3 (°) | Ring puckering coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A ring | B ring | C ring | D ring | |||||||||||||||||
| Q | ϕ 2 | θ | Q | ϕ 2 | θ | Q | ϕ 2 | θ | q 2 | ϕ 2 | ||||||||||
| a τ A: C10–C1–C2–C3. b τ B: C5–C6–C7–C8. c τ C: C9–C11–C12–C13. d τ 1: C16–C17–O–H for 1 and 2, C16–C17–C20–C21 for 3, 4, 7–9. e τ 1′: C17–C20–C21–O. f τ 2: C16–C17–O–H for 3, C16–C17–O–C17′ for 4, 7–9. g τ 3: C20–C21–O–C22. | ||||||||||||||||||||
| 1A | 0.00 | 59.8 | −54 | 54 | −54 | 172 | 0.44 | 16 | 54 | 0.54 | 167 | 7 | 0.54 | 272 | 6 | 0.46 | 187 | |||
| 1Ab | 0.27 | 37.7 | −54 | 54 | −54 | 64 | 0.44 | 16 | 54 | 0.54 | 167 | 7 | 0.57 | 271 | 6 | 0.46 | 187 | |||
| 1B | 1.88 | 2.5 | 55 | 55 | −54 | 172 | 0.44 | 203 | 125 | 0.58 | 347 | 5 | 0.58 | 294 | 5 | 0.46 | 187 | |||
| 1C | 6.05 | 0.0 | −48 | −51 | −54 | 172 | 0.45 | 347 | 54 | 0.72 | 261 | 84 | 0.59 | 335 | 5 | 0.47 | 188 | |||
| 1D | 11.13 | 0.0 | −54 | 53 | 45 | 174 | 0.45 | 12 | 55 | 0.56 | 12 | 55 | 0.73 | 324 | 79 | 0.49 | 193 | |||
| 2A | 0.00 | 62.8 | −51 | 54 | −54 | 173 | — | — | — | 0.54 | 285 | 10 | 0.57 | 320 | 4 | 0.57 | 275 | 5 | 0.47 | 188 |
| 2Ab | 0.31 | 36.9 | −51 | 54 | −54 | 64 | — | — | — | 0.54 | 285 | 10 | 0.58 | 319 | 4 | 0.57 | 275 | 5 | 0.46 | 187 |
| 2B | 3.15 | 0.3 | 28 | 55 | −54 | 173 | — | — | — | 0.77 | 265 | 85 | 0.56 | 210 | 4 | 0.57 | 276 | 5 | 0.46 | 188 |
| 2C | 11.83 | 0.0 | −45 | −42 | −54 | 173 | — | — | — | 0.56 | 270 | 19 | 0.71 | 274 | 77 | 0.60 | 344 | 5 | 0.47 | 188 |
| 2D | 11.50 | 0.0 | −49 | 55 | 45 | 174 | — | — | — | 0.54 | 278 | 12 | 0.59 | 216 | 5 | 0.73 | 215 | 78 | 0.50 | 193 |
| 3A | 0.63 | 24.8 | −54 | 54 | −54 | 146 | 175 | −45 | — | 0.44 | 17 | 54 | 0.54 | 165 | 6 | 0.57 | 270 | 4 | 0.48 | 187 |
| 3Ab | 0.00 | 71.7 | −54 | 54 | −54 | −24 | −169 | −159 | — | 0.44 | 17 | 54 | 0.54 | 169 | 6 | 0.57 | 271 | 5 | 0.48 | 188 |
| 3B | 2.58 | 0.9 | 55 | 55 | −55 | 147 | 175 | −44 | — | 0.44 | 203 | 125 | 0.58 | 350 | 5 | 0.58 | 302 | 3 | 0.48 | 188 |
| 3Bb | 1.96 | 2.6 | 55 | 55 | −54 | −24 | −169 | −158 | — | 0.44 | 203 | 125 | 0.58 | 349 | 5 | 0.58 | 299 | 4 | 0.48 | 188 |
| 3Cb | 6.14 | 0.0 | −48 | −51 | −54 | −24 | −169 | −158 | — | 0.45 | 347 | 54 | 0.72 | 261 | 84 | 0.59 | 339 | 5 | 0.48 | 189 |
| 3Db | 10.55 | 0.0 | −54 | 53 | 45 | −25 | −169 | −156 | — | 0.44 | 11 | 54 | 0.56 | 182 | 13 | 0.74 | 324 | 78 | 0.51 | 193 |
| 4A | 0.00 | 66.0 | −54 | 54 | −55 | 156 | 163 | −65 | — | 0.44 | 17 | 54 | 0.54 | 163 | 6 | 0.57 | 271 | 3 | 0.47 | 190 |
| 4Ab | 0.46 | 30.3 | −54 | 54 | −54 | −13 | −172 | −75 | — | 0.44 | 17 | 54 | 0.54 | 166 | 7 | 0.57 | 271 | 5 | 0.46 | 188 |
| 4B | 1.93 | 2.6 | 55 | 54 | −55 | 156 | 163 | −65 | — | 0.44 | 202 | 125 | 0.58 | 345 | 5 | 0.58 | 298 | 3 | 0.47 | 190 |
| 4Bb | 2.44 | 1.1 | 55 | 55 | −54 | −13 | −172 | −75 | — | 0.44 | 203 | 125 | 0.58 | 352 | 5 | 0.58 | 301 | 4 | 0.46 | 188 |
| 4C | 6.20 | 0.0 | −49 | −51 | −55 | 156 | 164 | −65 | — | 0.45 | 348 | 53 | 0.72 | 261 | 84 | 0.59 | 353 | 4 | 0.48 | 192 |
| 4D | 10.59 | 0.0 | −54 | 53 | 45 | 157 | 168 | −64 | — | 0.44 | 13 | 55 | 0.56 | 183 | 12 | 0.73 | 325 | 78 | 0.49 | 199 |
| 8A | 1.29 | 9.8 | −54 | 54 | −54 | 155 | 150 | −65 | 95 | 0.44 | 17 | 54 | 0.54 | 166 | 6 | 0.57 | 268 | 4 | 0.46 | 190 |
| 8Ab | 0.00 | 85.8 | −54 | 54 | −53 | −11 | −179 | −75 | 77 | 0.44 | 16 | 54 | 0.54 | 165 | 6 | 0.57 | 278 | 5 | 0.46 | 185 |
| 8B | 3.17 | 0.4 | 55 | 55 | −55 | 156 | 149 | −66 | 96 | 0.44 | 203 | 125 | 0.58 | 353 | 5 | 0.58 | 312 | 3 | 0.47 | 191 |
| 8Bb | 1.81 | 4.0 | 55 | 54 | −53 | −11 | −179 | −75 | 77 | 0.44 | 202 | 125 | 0.58 | 346 | 5 | 0.58 | 301 | 4 | 0.47 | 185 |
| 8Cb | 6.10 | 0.00 | −48 | −51 | −54 | −11 | −179 | −75 | 77 | 0.45 | 347 | 54 | 0.72 | 261 | 84 | 0.59 | 344 | 5 | 0.47 | 187 |
| 8Db | 10.25 | 0.00 | −54 | 53 | 43 | −12 | −179 | −74 | 77 | 0.46 | 22 | 49 | 0.56 | 183 | 13 | 0.73 | 323 | 78 | 0.49 | 192 |
| 9A | 0.00 | 55.0 | −55 | 56 | −16 | 154 | 162 | −66 | — | 0.44 | 15 | 54 | 0.50 | 117 | 12 | 0.51 | 266 | 52 | 0.45 | 187 |
| 9Ab | 0.25 | 36.0 | −55 | 56 | −15 | −13 | −172 | −76 | — | 0.44 | 14 | 54 | 0.50 | 116 | 12 | 0.51 | 267 | 52 | 0.45 | 183 |
| 9B | 1.34 | 5.7 | 56 | 54 | −16 | 156 | 163 | −65 | — | 0.45 | 203 | 125 | 0.55 | 17 | 10 | 0.51 | 267 | 51 | 0.45 | 184 |
| 9Bb | 1.68 | 3.2 | 56 | 54 | −16 | −13 | −172 | −76 | — | 0.45 | 203 | 125 | 0.55 | 20 | 10 | 0.51 | 267 | 51 | 0.44 | 183 |
| 9C | 3.77 | 0.1 | −50 | −57 | −14 | 157 | 162 | −76 | — | 0.46 | 348 | 54 | 0.70 | 264 | 89 | 0.50 | 272 | 49 | 0.45 | 186 |
| 7A | 0.00 | 82.0 | −6 | −1 | −54 | 156 | — | −67 | — | 0.30 | 301 | 81 | 0.49 | 259 | 51 | 0.58 | 316 | 3 | 0.47 | 191 |
| 7Ab | 0.90 | 18.0 | −6 | −1 | −54 | −10 | — | −76 | — | 0.30 | 301 | 81 | 0.49 | 259 | 51 | 0.59 | 314 | 4 | 0.46 | 188 |
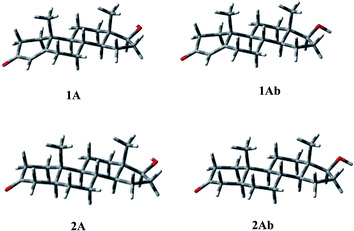
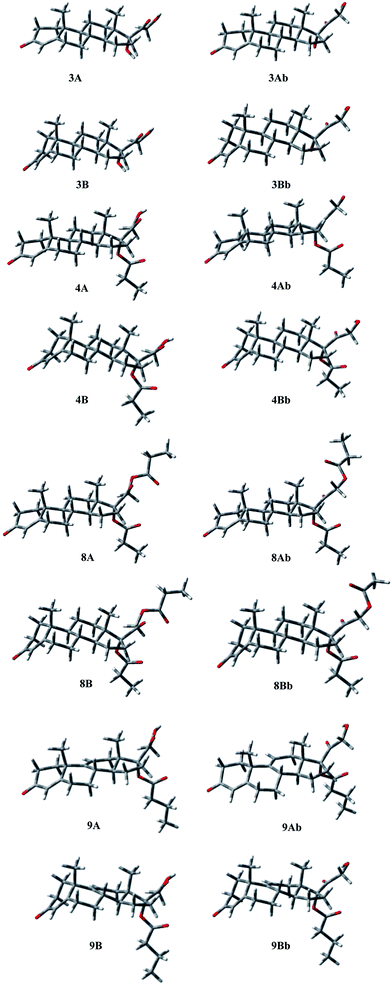
Some short contacts characterize the conformational preferences of the rings of 2: H-2ax/CH3-10 (2.81 Å), and H-4ax/CH3-10 (2.76 Å) for the A ring; H-6ax/CH3-10 (2.72 Å), H-8ax/CH3-10 (2.86 Å) for the B-ring conformation; H-11ax/CH3-10 (2.73 Å), H-11ax/CH3-13 (2.76 Å), and H-8ax/CH3-13 (2.78 Å) for the C ring; H-15ax/CH3-13 (2.90 Å) for ring D.
The energy profiles for rotation around the C17–C20, and C17–O single bonds, defined by τ1 and τ2, were obtained and the preferred orientations determined. The C17–C20 bond showed a quite balanced distribution of its possible orientations, with the presence, for all compounds, of two significantly populated geometries that present τ1 ≈ 160, and τ1 ≈ −10, respectively. For 3 and 8 conformation Ab, with τ1 ≈ −10, is favoured by 0.63 and 1.29 kcal mol−1, respectively, whereas 4 and 9 prefer the other orientation by 0.46 and 0.25 kcal mol−1, respectively. Concerning the C17–O bond, in the case of 4, 8, and 9, a significant preference was observed for the orientation characterized by τ2 ≈ −60°, with the other higher in energy by 4–6 kcal mol−1, whereas in 3 the hydroxyl group bonded at C17 shows two orientations related to the value of τ1, being preferred τ2 = −45 for τ1 = 146, and τ2 = −159 for τ1 = −24. In compound 8, the hydroxyl at C21, present in 3, 4, and 9, is esterified, with the generation of a second propionate group. Considering the oxoethyl propionate bonded at C17 the torsional angle τ3 describes the relative orientation of the two carbonyl groups of the chain. The second one is perpendicular to the first (τ3 ≈ 90°), whatever the orientation of this latter (see τ1).
A careful analysis of the conformational freedom of the tetracyclic skeleton allowed us to determine the facility of inversion of the three hexacyclic rings. The A ring inversion from the 1α,2β-half-chair to the 1β,2α-half-chair conformation (3, 4, 8, 9A → 3, 4, 8, 9B) is the easiest among all the possible ring inversions. In the case of compounds 3, 4, and 8, the A ring inversion brings about a conformation less stable by about 2 kcal mol−1. For compound 9 the same inversion is easier, giving the conformation 9B with an energy value of 1.34 kcal mol−1. So the percentage contribution of the 1β,2α-half-chair conformation to the overall population is double in the case of 9 with respect to the others, although the 1α,2β-half-chair conformation remains widely preferred.
As regards B ring inversion, the obtained conformations C, presenting a relative energy of about 4–6 kcal mol−1, respectively, do not give any contribution to the overall population. The ring C inversion of 9 is not possible because of the presence of the double bond, whereas for 3, 4 and 8 a conformation (D) is obtained that is higher in energy than the global minimum by more than 10 kcal mol−1.
In 3, 4, 8 and 9 the preference of ring A for the 1α,2β-half-chair conformation is characterized by the short contact H-2ax/CH3-19 (2.90 Å). The contacts H-6ax/CH3-19 (2.91 Å), and H-8ax/CH3-19 (2.87 Å) confirm the B ring conformation; contacts H-8ax/CH3-18 (2.74 Å), H-11ax/CH3-19 (2.70 Å), and H-11ax/CH3-18 (2.73 Å) assure the C ring geometry, whereas contact H-15ax/CH3-18 (2.90 Å) gives the D ring conformation.
Finally, the different orientations of τ1 could be verified through contacts H-21b/H-12eq (2.48, 2.29, 2.36 Å, respectively, for 3, 4, and 8) for conformation A; H-21a,b/CH3-18 (2.63, 2.82, 2.90 Å), H-21a/H-16 (2.42, 2.25, 2.27 Å), and H-21b/H-16 (2.37, 2.43, 2.46 Å) for conformation Ab.
Analogously, conformation A of compound 9 presents the contacts: H-2ax/CH3-19 (2.85 Å) for ring A; H-6ax/CH3-19 (2.94 Å) for ring B; H-21a/H-12eq (2.39 Å) for τ1 = 156. The second orientation of τ1 could be verified through contacts: H-21b/CH3-18 (2.88 Å), H-21b/H-16 (2.24 Å), and H-21a/H-16 (2.48 Å).
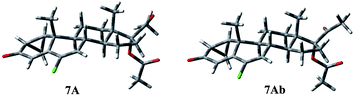
Compound 7 shows a rigid structure and the only degree of conformational freedom is the inversion of ring C. Rings A and B could not be inverted because of the presence of the cyclopropane ring and the double bond, respectively. Nevertheless, the C ring inversion gave conformations with a relative energy of about 13 kcal mol−1, giving no contribution to the overall population. So, only two geometries, 7A and 7Ab, are populated (Fig. 5). Conformation A of compound 7 presents the following contacts: H-11β/CH3-18 (2.24 Å), H-11β/CH3-19 (2.29 Å), H-8/CH3-19 (2.76 Å), and H-9/CHa-cPr (2.66 Å).
On the basis of crystallographic studies15 performed on dihydrotestosterone (2) complexed with the ligand-binding domain of the wild-type androgen receptor, both the carbonyl oxygen atom bonded at C3 and the hydroxyl group at C17 of this molecule actively contribute to the stabilization of the obtained complex. The A ring conformation influences the orientation of the carbonyl group that deeply affects the binding of the entire molecule.
A docking study performed on 7,16 into the homology model for the glucocorticoid receptor ligand binding domain, revealed that in the active site it assumes conformation A.
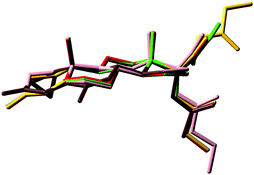
The superimposition of the heavy atoms of the tetracyclic system of the preferred conformations of compounds 3, 4 and 7–9 (Fig. 6) gave evidence that the presence of the hydroxyl group or the ester chain, bonded to C21 in compounds 3, 4, 8 and 9, does not affect the orientation of the substituent at C17.
Compounds 3, 4, 8 and 9 show a very good overlay, also concerning the O3 atoms that are perfectly coincident, in spite of the presence in 9 of a double bond on ring C. Conversely, the overlay shows that in 7 the O3 atom is differently oriented and diverges with the distance d(O–O) ≈ 1.0 Å.
NMR data
Complete 1H and 13C NMR signal assignments (Tables 7–9) of the spectra of compounds 3, 4 and 7–9 were achieved using a combination of 1D and 2D (COSY, HSQC and NOESY) experiments recorded in CDCl3 at 298 K. In general, starting from characteristic H-4, H-7 or H-11 olefinic protons it was possible to assign the resonances of all the other protons of the studied steroids on the basis of their 2D spectra. First of all, H-8 was assigned through COSY correlations from H-4 of compounds 3, 4, 8 and 9 or from H-7 of compound 7. Then, it was possible to discriminate between H-9 and H-14 (showing two very clear and distinctive HSQC cross-peaks accounting for C–H protons) on the basis of H-9/H-11 coupling, with H-11β being assigned on the basis of its NOESY cross-peak with 19-CH3 (previously distinguished from 18-CH3 that showed a NOESY correlation with the characteristic 21-protons). Also, H-1, and consequently H-2, resonances were assigned through the NOESY cross-peak of H-1β and 19-CH3. Finally, analysis of the 18- and 19-CH3 NOESY cross-peak network was especially useful for the assignment of the α- or β-configuration of geminal protons (see Table 7) of all the studied compounds. Even if some protons in the 1H NMR spectra resonated as complex multiplets (see Table 7), many signals were well resolved and their coupling could be measured. The obtained values are reported in Table 9 in comparison with the calculated constants of compounds 3, 4 and 7–9. For each populated conformer the 1H vicinal coupling constants were calculated with the electronegativity-modified Karplus relationship17 and were weight-averaged on the basis of the population percentages. The experimental and the calculated values resulted in close agreement. The following nuclear Overhauser effect (nOe) contacts were observed in the NOESY spectra of the studied compounds. Compound 3: CH3-19/H-1β, H-2β, H-6β, H-11β and H-8; CH3-18/H-11β, H-12β, H-15β, H-16β, H-21b and H-8; H-21a/H-12β and H-16β; H-21b/H-16 β; and H-7α/H-9 and H-14. Compounds 4 and 8: CH3-19/H-1β, H-2β, H-6β, H-11β and H-8; CH3-18/H-11β, H-12β, H-15β, H-16β, H-21a and H-8; H-21b/H-12β and H-16β; H-21a/H-16β; H-8/H-15β; and H-7α/H-9 and H-14. Compound 9: CH3-19/H-1β, H-2β, H-6β, H-11 (3.80 Å calcd) and H-8; CH3-18/H-11 (4.00 Å calcd), H-12β, H-15β, H-16β, H-21a and H-8; H-21b/H-12β and H-16β; H-21a/H-16β; H-8/H-15β; and H-7α/H-9 and H-14. Compound 7: CH3-19/H-1, H-11β and H-8; CH3-18/H-11β, H-12β, H-15β, H-16β, CH3-21 and H-8; CH3-21/H-12β and H-16β; H-8/H-15β; and H-9/CHa-cPr.| 1H | 3 | 4 | 7 | 8 | 9 |
|---|---|---|---|---|---|
| 1 | 1.70 | ||||
| 1α | 1.66 | 1.71 | — | 1.70 | 2.06–2.17 |
| 1β | 2.00 | 2.02 | — | 2.02 | 2.06–2.17 |
| 2 | 2.00 | ||||
| 2α | 2.32 | 2.32 | — | 2.34 | 2.43–2.50 |
| 2β | 2.39 | 2.41 | — | 2.40 | 2.43–2.50 |
| CHa (cPr) | — | — | 0.86 | — | — |
| CHb (cPr) | — | — | 1.26 | — | — |
| 4 | 5.70 | 5.72 | 6.16 | 5.72 | 5.74 |
| 6α | 2.26 | 2.27 | — | 2.27 | 2.35 |
| 6β | 2.38 | 2.38 | — | 2.38 | 2.56 |
| 7 | — | — | 6.20 | — | — |
| 7α | 1.08 | 1.10 | — | 1.08 | 1.16 |
| 7β | 1.85 | 1.85 | — | 1.84 | 2.01 |
| 8 | 1.59 | 1.60 | 2.31 | 1.62 | 2.22 |
| 9 | 0.95 | 1.00 | 1.45 | 0.99 | — |
| 11 | — | — | — | — | 5.52 |
| 11α | 1.62 | 1.65 | 1.94 | 1.65 | — |
| 11β | 1.38 | 1.40 | 1.55 | 1.45 | — |
| 12α | 1.72 | 1.89 | 2.03 | 1.88 | 2.75 |
| 12β | 1.40 | 1.54 | 1.61 | 1.74 | 1.76 |
| 14 | 1.70 | 1.67 | 1.96 | 1.67 | 1.85 |
| 15α | 1.80 | 1.76 | 1.89 | 1.72 | 1.94 |
| 15β | 1.37 | 1.35 | 1.44 | 1.34 | 1.45 |
| 16α | 1.57 | 1.85 | 1.82 | 1.84 | 1.97 |
| 16β | 2.66 | 2.81 | 2.98 | 2.82 | 2.80 |
| 17 (OCOCH2) | — | 2.34 | — | 2.35 | 2.28 |
| 17 (OCOCH2CH3) | — | 1.12 | — | 1.13 | — |
| 17 (OCOCH2CH2) | — | — | — | — | 1.62 |
| 17 (OCOCH2CH2CH3) | — | — | — | — | 0.93 |
| 17 (OCOCH3) | — | — | 2.09 | — | — |
| 17 OH | 2.45 | — | — | — | — |
| 18 (CH3) | 0.68 | 0.66 | 0.71 | 0.74 | 0.61 |
| 19 (CH3) | 1.16 | 1.17 | 1.21 | 1.16 | 1.32 |
| 21 (CH3) | — | — | 2.04 | — | — |
| 21a | 4.28 | 4.21 | — | 4.59 | 4.24 |
| 21b | 4.64 | 4.26 | — | 4.87 | 4.28 |
| 21 (OCOCH2) | — | — | — | 2.45 | — |
| 21 (OCOCH2CH3) | — | — | — | 1.15 | — |
| 21 OH | 3.09 | 3.03 | — | — | 3.04 |
| 13C | 3 | 4 | 7 | 8 | 9 |
|---|---|---|---|---|---|
| 1 | 35.68 | 35.70 | 26.07 | 35.67 | 33.83 |
| 2 | 33.87 | 33.91 | 25.22 | 33.92 | 34.22 |
| CH2 (cPr) | — | — | 12.29 | — | — |
| 3 | 199.58 | 199.26 | 197.94 | 199.40 | 199.05 |
| (199.04) | |||||
| 4 | 123.94 | 124.07 | 120.51 | 123.98 | 124.17 |
| 5 | 170.93 | 170.33 | 155.22 | 170.61 | 169.01 |
| 6 | 32.73 | 32.65 | 130.23 | 32.69 | 32.70 |
| 7 | 31.98 | 31.91 | 136.51 | 31.91 | 32.13 |
| 8 | 35.59 | 35.57 | 38.33 | 35.57 | 37.48 |
| 9 | 53.28 | 53.16 | 47.70 | 53.14 | 144.20 |
| 10 | 38.54 | 38.53 | 38.70 | 38.54 | 40.98 |
| 11 | 20.52 | 20.50 | 20.78 | 20.57 | 118.25 |
| 12 | 30.08 | 30.46 | 31.02 | 30.24 | 32.35 |
| 13 | 48.57 | 47.72 | 47.24 | 47.79 | 46.28 |
| 14 | 50.28 | 50.93 | 48.77 | 50.94 | 48.16 |
| 15 | 23.70 | 23.89 | 23.23 | 23.77 | 24.65 |
| 16 | 34.52 | 30.77 | 30.30 | 30.78 | 30.52 |
| 17 | 89.00 | 93.77 | 96.18 | 94.86 | 93.33 |
| 17 (OCO) | — | 174.05 | 170.55 | 174.20 | 173.24 |
| (174.02) | |||||
| 17 (OCOCH3) | — | — | 21.17 | — | — |
| 17 (OCOCH2) | — | 27.77 | — | 27.10 | 36.24 |
| (27.90) | |||||
| 17 (OCOCH2CH3) | — | 8.85 | — | 8.97 | — |
| (8.90) | |||||
| 17 (OCOCH2CH2) | — | — | — | — | 18.31 |
| 17 (OCOCH2 CH2CH3) | — | — | — | — | 13.57 |
| 18 | 14.99 | 14.29 | 14.19 | 13.75 | 14.31 |
| 19 | 17.36 | 17.39 | 22.83 | 17.35 | 26.20 |
| 20 | 212.33 | 206.21 | 203.61 | 199.40 | 206.22 |
| (199.04) | |||||
| 21 | 67.42 | 66.93 | 26.43 | 66.88 | 66.91 |
| 21 (OCO) | — | — | — | 174.20 | — |
| (174.02) | |||||
| 21 (OCOCH2) | — | — | — | 27.10 | — |
| (27.90) | |||||
| 21 (OCOCH2CH3) | — | — | — | 8.97 | — |
| (8.90) |
| J | 3 | 3 | 4 | 4 | 7 | 7 | 8 | 8 | 9 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Calc | Exp | Calc | Exp | Calc | Exp | Calc | Exp | Calc | |
| 1α,1β | 13.4 | 13.4 | — | 13.4 | n.d. | |||||
| 1α,2α | 4.5 | 4.0 | 5.0 | 3.9 | — | n.d. | 3.9 | n.d. | 3.9 | |
| 1α,2β | n.d | 13.5 | n.d | 13.5 | — | n.d. | 13.6 | n.d. | 13.6 | |
| 1β,2α | 3.2 | 2.9 | 3.3 | 2.9 | — | 2.7 | 2.9 | n.d. | 2.9 | |
| 1β,2β | 5.0 | 3.4 | 5.0 | 3.4 | — | 4.8 | 3.4 | n.d. | 3.5 | |
| 2β,2α | 16.7 | 16.7 | — | n.d. | n.d. | |||||
| 1,2 | — | — | 7.9 | 10.5 | — | — | ||||
| 1,CHa (cPr) | — | — | 6.4 | 9.5 | — | — | ||||
| 1,CHb (cPr) | — | — | 7.9 | 10.3 | — | — | ||||
| 2,CHa (cPr) | — | — | 4.5 | 8.2 | — | — | ||||
| 2,CHb (cPr) | — | — | 8.9 | 10.4 | — | — | ||||
| CHa,CHb(cPr) | — | — | 4.8 | — | — | |||||
| 6α,6β | 14.6 | 14.6 | — | 14.5 | 14.4 | |||||
| 6α,7α | 4.3 | 3.7 | 4.2 | 3.7 | — | 4.0 | 3.7 | 3.9 | 3.3 | |
| 6α,7β | 2.4 | 2.9 | 2.4 | 2.9 | — | 2.4 | 2.9 | 2.6 | 3.1 | |
| 6β,4 | n.d. | n.d | — | n.d. | 1.9 | |||||
| 6β,7α | 12.0 | 13.2 | 11.5 | 13.3 | — | 12.0 | 13.3 | 14.4 | 13.4 | |
| 6β,7β | n.d. | 3.8 | 5.0 | 3.8 | — | n.d. | 3.8 | 4.8 | 3.5 | |
| 7α,7β | 13.0 | 13.7 | — | 14.0 | 12.4 | |||||
| 7α,8 | 12.0 | 12.3 | 12.5 | 12.3 | — | 12.0 | 12.4 | 12.5 | 12.4 | |
| 7β,8 | n.d. | 3.4 | 3.3 | 3.2 | — | 3.3 | 3.2 | 4.7 | 3.2 | |
| 7,8 | — | — | 2.0 | 1.0 | — | — | ||||
| 8,9 | 10.8 | 12.1 | 11.0 | 12.3 | 9.8 | 12.9 | 10.8 | 12.9 | — | |
| 8,14 | n.d. | 12.1 | 11.0 | 12.3 | 10.0 | 10.8 | 10.8 | |||
| 8,12α | — | — | — | — | 2.9 | |||||
| 8,12β | — | — | — | — | 2.0 | |||||
| 11,8 | — | — | — | — | 2.0 | |||||
| 11,12α | — | — | — | — | 2.9 | 2.0 | ||||
| 11,12β | — | — | — | — | 5.9 | 6.4 | ||||
| 9,11α | 4.2 | 3.4 | 4.0 | 3.0 | 4.1 | — | ||||
| 9,11β | 11.8 | 12.3 | 12.5 | 12.8 | 13.0 | — | ||||
| 11α,12α | n.d. | 3.8 | 4.3 | 3.8 | n.d. | 4.3 | 4.0 | — | ||
| 11α,12β | n.d. | 2.8 | 2.9 | 2.9 | 2.7 | n.d. | 2.6 | — | ||
| 11β,12α | n.d. | 13.2 | 13.0 | 13.2 | 12.8 | 13.2 | 13.1 | — | ||
| 11β,12β | n.d. | 4.1 | 4.2 | 4.0 | 4.0 | 4.2 | 4.4 | — | ||
| 11α,11β | n.d. | 13.4 | 12.8 | 13.2 | — | |||||
| 12α,12β | n.d. | 13.0 | 12.6 | 13.0 | 17.0 | |||||
| 14,15α | n.d. | 5.8 | 7.0 | 5.4 | n.d. | n.d. | 7.7 | 6.1 | ||
| 14,15β | n.d. | 11.6 | 11.8 | 11.8 | 11.0 | 11.3 | 11.0 | 11.3 | ||
| 15α,16α | 9.4 | 11.0 | 9.4 | 11.8 | 9.2 | n.d. | 12.0 | 9.4 | 11.0 | |
| 15α,16β | 3.0 | 2.3 | 2.6 | 2.1 | 2.4 | 2.5 | 2.6 | 3.0 | 2.7 | |
| 15α,15β | n.d. | 11.8 | 11.6 | 11.3 | 17.0 | |||||
| 15β,16α | 6.2 | 5.4 | 6.5 | 5.7 | 6.0 | 6.5 | 4.7 | 5.0 | 4.9 | |
| 15β,16β | 11.5 | 12.0 | 11.8 | 11.8 | 10.2 | 11.3 | 12.0 | 12.5 | 12.0 | |
| 16α,16β | 14.8 | 16.0 | 15.7 | 15.7 | 15.2 | |||||
| 21a,21b | 19.8 | 18.2 | — | 16.5 | 18.3 | |||||
| 21a,OH | 4.5 | 4.9 | — | — | 4.9 | |||||
| 21b,OH | 4.5 | 4.9 | — | — | 4.8 |
These nOe data, such as the experimental values of the 1H vicinal coupling constants, supported the calculated preferred conformations. In particular, almost all these contacts correspond to distances of <3 Å as measured on the computed (Fig. 4 and 5) most populated conformations of compounds 3, 4 and 7–9.
Discussion
Cortexolone-17α-propionate (4) is a steroid endowed with a strong local antiandrogenic activity but it is devoid of systemic antiandrogenic activity and it does not affect the hypersecretion of gonadotropins. Cortexolone-17α-propionate (4) does not inhibit the conversion of testosterone (1) to DHT (2) in reconstructed human epidermis, thus resulting in being devoid of activity on the 5α-reductase. Nevertheless, compound 4 competes with the androgens at the androgen receptor level, and thus its strong antiandrogenic activity is attributable to this mechanism of action. As a consequence it is under investigation in the management of acne and alopecia.In the present work a conformational comparison of 4 with testosterone (1), active testosterone metabolite DHT (2) and antiandrogen cyproterone acetate (7) (similar to 4 for the action mechanism direct on the androgen receptor) was carried out by means of theoretical calculations, supported by their complete high-field NMR characterization. In addition, the comparison was extended to the related compounds cortexolone (3), devoid of activity, cortexolone-17α,21-dipropionate (8), less active, and Δ9-butyrate 9, active both topically and systemically.
The conformational characterization showed that all compounds are similar (see Fig. 6), minor differences being observed: cyproterone acetate (7) has the 3-carbonyl group differently oriented and the presence of the 9,11-double bond hampers the C ring inversion in compound 9. However the 3-carbonyl group orientation (7vs.4, Table 1) and the conformation of the C ring (9vs.4, Tables 1 and 5) do not influence the extent of the local antiandrogenic activity of the tested compounds. By contrast, the presence of the 17α-ester group, which was always oriented in the same way from the conformational study, seems to be mandatory for a good inhibitory activity (3vs.4, Table 1). In conclusion, the cortexolone series compounds 3–5 and 8, cyproterone acetate (7), and Δ9-17α-butyrate 9 share the same skeleton conformation but show different antiandrogenic activities due to the presence of an acyl chain linked to the 17α-hydroxyl functionality. The same skeleton conformation of the examined compounds is consistent with the capability of each of them to interact, even with different outcomes, with the androgen receptors. Furthermore, the absence of systemic activity of 4 could be explained by its metabolic fate, i.e. the esterases-catalyzed hydrolysis of the acyl chain, after the rapid migration from the 17- to the 21-position.
Experimental
Materials and methods
All the cortexolone derivatives were from an internal source. Others steroids, Sephadex LH-20 and all reagents and solvents were purchased from Sigma Aldrich, Milan (Italy); Extrelut® NT columns were obtained from Merck.Biological tests
Metabolism
Determination of 3, 4 and 5 by LC-MS and HPLC analyses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0.5 mL) and purified by gel chromatography on a Sephadex LH-20 column using cyclohexane–ethyl acetate (7
3, 0.5 mL) and purified by gel chromatography on a Sephadex LH-20 column using cyclohexane–ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluant; the first eluted 15 mL were collected and taken to dryness under a stream of nitrogen.
3) as the eluant; the first eluted 15 mL were collected and taken to dryness under a stream of nitrogen.
| Std | M | RT (min) | m/z |
|---|---|---|---|
| 3 | 346.21 | 3.48–3.89 | 345.0 [M − 1], 691.3 [2M − 1] |
| 4 | 402.24 | 5.38–5.67 | 803.1 [2M − 1] |
| 5 | 402.24 | 7.05–7.59 | 803.2 [2M − 1] |
Computational methods
All calculations were carried out using the Gaussian0318 program package. The conformational space of compounds 1–4 and 7–9 was explored through optimization of all the possible starting geometries which were optimized within the DFT approach at the B3LYP level with the 6-31G(d) basis set. All the degrees of conformational freedom were considered, in particular the possible existence of different conformations at the A, B, and C hexacyclic rings as well as the orientation of the groups bonded at C17. Several conformations were located for each compound and the corresponding percentage contributions to the overall population were determined using the Boltzmann equation. The geometry of the rings A–C is described through two kinds of descriptors, i.e., a significant torsion angle for each ring, τA, τB, τC, and the ring-puckering coordinates determined according to Cremer and Pople.19 Vibrational frequencies were computed at the same level as above in order to verify that the optimized structures were minima. The 1H vicinal coupling constants were calculated with the electronegativity-modified Karplus relationship17 and were weight-averaged on the basis of the population percentages.NMR spectroscopy
All NMR spectra were recorded at 298 K using a Bruker AVANCE-500 spectrometer operating at 500.13 and 125.76 MHz for 1H and 13C, respectively, using a 5 mm single-pulsed field gradient (z-PFG) broadband reverse probe. Chemical shifts are reported on the δ (ppm) scale and are relative to chloroform signals (7.24 for 1H and 77.0 ppm, central line, for 13C spectra respectively). Compounds 3, 4 and 7–9 (about 10 mg) were dissolved in CDCl3 (0.5 mL) under N2, and their assignments were given by a combination of 1D and 2D COSY, HSQC and NOESY experiments, using standard Bruker pulse programs. z-PFGs were used to obtain 1H–1H COSY and HSQC spectra. The pulse widths were 7.50 μs (90°) and 14.5 μs (90°) for 1H and 13C respectively. Typically, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768 data points were collected for one-dimensional spectra. Spectral widths were 11.45 ppm (5733 Hz) for 1H NMR (digital resolution: 0.17 Hz per point) and 259.84 ppm (32
768 data points were collected for one-dimensional spectra. Spectral widths were 11.45 ppm (5733 Hz) for 1H NMR (digital resolution: 0.17 Hz per point) and 259.84 ppm (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 Hz) for 13C NMR (digital resolution: 1.0 Hz per point). 2D experiments parameters were as follows. For 1H–1H correlations: relaxation delay 2.0 s, 1024 × 1024 data point matrices (512 experiments to 1024 zero filling in F1, 1024 in F2), 2 or 16 transients in each experiment for COSY and NOESY respectively, spectral width 6.0 ppm (3004.8 Hz). The NOESY spectra were generated with a mixing time of 1.0 s and acquired in the TPPI mode. There were no significant differences in the results obtained at different mixing times (0.5–1.5 s). For 13C–1H correlations (HSQC): relaxation delay 2.5 s, 1024 × 1024 data point matrices (512 experiments to 1024 zero filling in F1, 1024 in F2), 2 transients in each experiment, spectral width 6.0 ppm (3004.8 Hz) in the proton domain and 180.0 ppm (22
680 Hz) for 13C NMR (digital resolution: 1.0 Hz per point). 2D experiments parameters were as follows. For 1H–1H correlations: relaxation delay 2.0 s, 1024 × 1024 data point matrices (512 experiments to 1024 zero filling in F1, 1024 in F2), 2 or 16 transients in each experiment for COSY and NOESY respectively, spectral width 6.0 ppm (3004.8 Hz). The NOESY spectra were generated with a mixing time of 1.0 s and acquired in the TPPI mode. There were no significant differences in the results obtained at different mixing times (0.5–1.5 s). For 13C–1H correlations (HSQC): relaxation delay 2.5 s, 1024 × 1024 data point matrices (512 experiments to 1024 zero filling in F1, 1024 in F2), 2 transients in each experiment, spectral width 6.0 ppm (3004.8 Hz) in the proton domain and 180.0 ppm (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638.6 Hz) in the carbon domain. All 2D spectra were processed using the Bruker software package.
638.6 Hz) in the carbon domain. All 2D spectra were processed using the Bruker software package.
Acknowledgements
This work was financially supported by Università di Milano, by Università di Pavia and by Cosmo Research & Development S.p.A. The authors warmly thank Professor Lucio Toma for very helpful discussion.Notes and references
- W. Chen, D. Thiboutout and C. C. Zouboulis, J. Invest. Dermatol., 2002, 119, 992–1007 CrossRef CAS PubMed.
- C. R. Darley, J. D. Kirby, G. M. Besser, D. D. Munro, C. R. Edwards and L. H. Rees, Br. J. Dermatol., 1982, 106, 517–522 CrossRef CAS PubMed.
- J. H. Barth, Drugs, 1988, 35, 83–91 CrossRef CAS PubMed.
- G. Sansone and R. M. Reisner, J. Invest. Dermatol., 1971, 56, 366–372 CAS.
- V. H. Price, Arch. Dermatol., 1975, 111, 1496–1502 CrossRef CAS.
- C. Chen, X. Li, S. M. Singh and F. Labrie, J. Invest. Dermatol., 1998, 111, 273–278 CrossRef CAS PubMed.
- J. R. Brooks, R. L. Primia, C. Berman, D. A. Krupa, G. F. Reynolds and G. H. Rasmusson, Steroids, 1991, 56, 428–433 CrossRef CAS.
- G. Celasco, L. Moro, R. Bozzella, P. Ferraboschi, L. Bartorelli, C. Quattrocchi and F. Nicoletti, Arzneim.-Forsch./Drug Res., 2004, 54, 881–886 CAS.
- E. M. Glenn, S. L. Richardson and B. J. Bowman, Metabolism, 1959, 8, 265–285 CAS.
- W. Voigt and S. L. Hsia, Endocrinology, 1973, 92, 1216–1222 CrossRef CAS PubMed.
- (a) D. Colombo, P. Ferraboschi, P. Prestileo and L. Toma, J. Steroid Biochem. Mol. Biol., 2006, 98, 56–62 CrossRef CAS PubMed; (b) D. Colombo, P. Ferraboschi, L. Legnani, P. Prestileo and L. Toma, J. Steroid Biochem. Mol. Biol., 2007, 103, 163–169 CrossRef CAS PubMed.
- G. Celasco, L. Moro, R. Bozzella, P. Ferraboschi, L. Bartorelli, R. Di Marco, C. Quattrocchi and F. Nicoletti, Arzneim. Forsch., 2005, 55, 581–587 CAS.
- H. J. de Voogt, Prostate, 1992, 21(S4), 91–95 CrossRef.
- P. Reid, P. Kantoff and W. Oh, Invest. New Drugs, 1999, 17, 271–284 CrossRef CAS.
- J. S. Sack, K. F. Kish, C. Wang, R. M. Attar, S. E. Kiefer, Y. An, G. Y. Wu, J. E. Scheffler, M. E. Salvati, S. R. Krystek Jr, R. Weinmann and H. M. Einspahr, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4904–4909 CrossRef CAS PubMed.
- C. Honer, K. Nam, C. Fink, P. Marshall, G. Ksander, R. E. Chatelain, W. Cornell, R. Steele, R. Schweitzer and C. Schumacher, Mol. Pharmacol., 2003, 63, 1012–1020 CrossRef CAS.
- C. A. G. Haasnoot, F. A. A. M. de Leeuw and C. Altona, Tetrahedron, 1980, 36, 2783–2792 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision B.04, Gaussian Inc., Pittsburgh PA, 2003 Search PubMed.
- D. Cremer and J. A. Pople, J. Am. Chem. Soc., 1975, 97, 1354–1358 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |